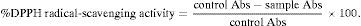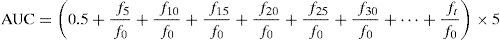Among the potential natural sources of bioactive compounds, those of the macroscopic fungi Phellinus spp. have been identified by previous researches. Phenolic compounds are among the major antioxidant and antimicrobial contributors due to their bioactive properties.
AimsThe goal of this study was to determine the total phenolic and flavonoid contents, and its relation with the antioxidant and antifungal activity of methanolic extracts of Phellinus gilvus, Phellinus rimosus and Phellinus badius, respectively.
MethodsThe collected and identified organisms of Phellinus spp. were treated with methanol and the generated aqueous extract was analyzed to quantified total phenolic compounds, total flavonoids, radical scavenging activity against DPPH, trolox equivalent antioxidant capacity, and oxygen absorbance capacity. The antifungal property of the extracts was evaluated against Alternaria alternata.
ResultsThe content of phenolic compounds was of 49.31, 46.51 and 44.7mg of gallic acid equivalents/g, for P. gilvus, P. rimosus and P. badius, respectively. The total flavonoid content followed the same pattern with values of 30.58, 28, and 26.48mg of quercetin equivalents/g for P. gilvus, P. rimosus and P. badius, respectively. The variation on the content of phenolic components was reflected on the antioxidant activity of every organism. The antioxidant activity ranked as follows: P. gilvus>P. rimosus>P. badius. The antifungal effect of the different extracts against A. alternata showed a significant effect, all of them, inhibiting the growth of this pathogen.
ConclusionsP. gilvus showed the best potential to inactivate free radicals, being all the tested fungi effective to inhibit A. alternata growth.
Investigaciones previas han revelado que los hongos de la especie Phellinus son una fuente potencial de sustancias bioactivas. Entre ellas, los fenoles son los principales antioxidantes y antimicrobianos que contribuyen a sus propiedades bioactivas.
ObjetivosEl objetivo del presente estudio fue determinar el contenido total de fenoles y flavonoides, y su relación con la actividad antioxidante y antifúngica de Phellinus gilvus, Phellinus rimosus y Phellinus badius, respectivamente, extraídos con metanol.
MétodosLos aislamientos de Phellinus fueron tratados con metanol para la extracción de la fracción acuosa. Mediante análisis espectroscópico del extracto acuoso generado se cuantificó el contenido total de fenoles y flavonoides, la actividad antioxidante frente a 2,2-difenil-1-picrilhidrazil (DPPH), la capacidad antioxidante expresada como equivalentes Trolox® (ET), y la capacidad de absorción del oxígeno reactivo. Las propiedades antifúngicas de los extractos se examinaron frente a Alternaria alternata.
ResultadosEl contenido de fenoles fue de 49,31, 46,51 y 44,7mg de equivalentes de ácido gálico/g, para P. gilvus, P rimosus y P. badius respectivamente. El contenido de flavonoides siguió el mismo patrón, con valores de 30,58, 28 y 26,48mg de equivalentes de quercetina/g, para P. gilvus, P. rimosus y P. badius, respectivamente. La variación en el contenido de fenoles se reflejó en la actividad antioxidante de cada hongo. La actividad antioxidante se clasificó del modo siguiente: P. gilvus>P. rimosus>P. badius. El efecto antifúngico de los diferentes extractos frente a A. alternata fue significativo, inhibiendo todos ellos el crecimiento de dicho patógeno.
ConclusionesP. gilvus manifestó la mayor capacidad antioxidante y los extractos de los tres hongos inhibieron el crecimiento de A. alternata.
The natural products and herbal medicine industries have become increasingly popular over the past three decades. The recognition of the value of traditional medical systems, particularly those of Asian origin, and the identification of indigenous medicinal plants and fungi with healing power, are factors that have significant influence in the expansion of the natural products industry.6,24 Furthermore, there is a constant search for new and effective natural drugs, which has been driven by the number of pathogenic organisms reported to have multi-resistance against many of the therapeutic products that are currently available on the market.21,24
Nowadays, fungus kingdom has become very attractive as a functional food and as a good source for the development of drugs and nutraceuticals.6,10 The number of mushrooms species on earth is estimated to be larger than 100,000, suggesting that only 15–20% are known.6 Even among the known species, the proportion of well-investigated mushrooms, as to their contents in secondary metabolites, and antioxidant and antimicrobial properties, is very low.7 This fact, together with the knowledge about the great potential of fungi for production of bioactive metabolites (e.g. penicillin from Penicillium notatum, ergotamin from Claviceps purpurea, ciclosporin from Tolypocladium inflatum), in addition to their use in ethnomedicinal, reveals mushrooms as a vast source of bioactive compounds.6,10
Among the potential natural sources of bioactive compounds, those of the macroscopic fungi Phellinus spp. have been identified in previous studies on the phyla Basidiomycota where they are located.7 Phenolic compounds are among the major contributors to the bioactive properties of Phellinus; however, variation could occur among species and regions of growth.7 Fractions rich in phenolics have been extracted from Phellinus baumii using various solvents like methanol and hot water. These extracts exhibited good inhibition rates, of about 80–90%, by hydroxyl radical scavenging activity, hydrogen peroxide scavenging activity, effect of reducing power on metallic compound formation and antioxidant activity.18Phellinus linteus extract showed strong anti-angiogenic activity related to its antioxidant activity.27 Ethyl acetate extract of Phellinus rimosus exhibited significant in vitro antioxidant activity. However, in all probability, genetically related species share the same “biochemical” composition, implying that they could show the same properties.7 With this in mind, a greater number of medicinal fungi could emerge, and this would give people more options and consequently reduce the cost of medicinal mushrooms.6,10,30 Previous review discussed the relevance of testing different medicinal functions of Phellinus sensu lato, among these properties, antioxidant and antibiotic properties are the less studied in Phellinus.7 Therefore the study of the bioactive properties of Phellinus spp. from different locations is needed. In this context, 11 Phellinus species were found in the Ajos-Bavispe natural protected area in Sonora, Mexico, three of them were more commonly collected and studied in the present survey: Phellinus badius (Berk. ex Cooke) G., Phellinus gilvus (Schwein.) Pat., and P. rimosus (Berk.) Pilát. In this context, the objective of the present work was to determine the antioxidant and antifungal activity of methanolic extracts of P. badius, P. gilvus and P. rimosus, respectively, collected in Sonora, Mexico.
Materials and methodsCollection and identification of Phellinus spp.P. badius, P. gilvus and P. rimosus were collected at the National Forest Reserve and Wildlife Refuge Ajos-Bavispe, located at Sonora, Mexico. Sampling was performed seasonally from fall 2004 to summer 2005. Phellinus specimens were collected and conserved following conventional mycological techniques. Taxonomic identification was reached according to Gilbertson and Ryvarden8 and Larsen & Cobb-Poulle.14 Three major species were identified as P. badius, P. gilvus and P. rimosus. Ten organisms with an approximate weight of 100g per organism per species were selected and grouped. Mycelia from each organism of each species were mixed, freeze dried and vacuum stored at −35°C until extraction.
Preparation of bioactive extracts from Phellinus spp.Methanolic extracts of Phellinus spp. were obtained as follows: 1g of freeze-dried sample was mixed in 15mL of 80% (v/v) methanol, and homogenized with an Ultra-Turrax T 25 basic (IKA® WERKE, Germany), thereafter the homogenate was sonicated for 15min and centrifuged at 10,000rpm at 5°C for 15min. The sample was vacuum-filtered through Whatman No. 1 filter paper. This procedure was repeated twice to ensure the maximum extraction of bioactive compounds using 20mL of methanol. The extracts were collected and made up to final volume of 50mL. The final concentration of the extract was 0.02g/mL, which was used for total phenolics, total flavonoids and antioxidant activity determinations.
Total phenolic and flavonoid contentsConcentrations of total phenolic compounds were measured by the methods described by Singleton and Rossi26 with some modifications. Extracts (50μL) were mixed with 3mL of H2O, and 250μL of Folin–Ciocalteu's phenol reagent 1N. After 8min of equilibration time, 750μL of Na2CO3 (20%) and 950μL of H2O were added to the extracts. After incubation for 30min at room temperature, the absorbance was read at 765nm with an UV–vis spectrophotometer (Cary, model 50 Bio, Varian, Italy). Concentration of total phenol compounds was calculated using a standard curve of gallic acid equivalents (GAE) and expressed as milligrams per g of dry weight.
Flavonoid content was determined based on the methods described by Zhishen et al.32 modified as required. Flavonoids were measured in 1mL of each methanolic extract and it was mixed with 5% NaNO2, 10% AlCl3 and 1mol/L NaOH and measured spectrophotometrically at 415nm using quercetin as standard. The results were expressed as mg of quercetin equivalents (QE) per g of dry weight.
Evaluation of antioxidant capacityDPPHThis assay is based on the measurement of the scavenging ability on antioxidants toward the stable radical DPPH.9 A 3.9mL aliquot of a 0.0634mM of DPPH solution, in methanol was added to 0.1mL of each extract and shaken vigorously. Tubes were placed at 27°C for 30min. A control reaction was prepared as above without extract, and methanol was used for the baseline correction. Changes in the absorbance of samples were measured at 515nm. Radical scavenging activity was expressed at the inhibition percentage and was calculated using the following equation:
Trolox equivalent antioxidant capacityThis assay is based on the ability of the antioxidants to scavenge the blue-green ABTS+ radical compared to the scavenging ability of the water-soluble vitamin E analog trolox.22 The ABTS+ radical cation was generated by the interaction of 5mL of 7mM ABTS solution and 88μL of 0.139mM K2S2O8 solution. After the addition of 2970μL of ABTS+ solution to 30μL of methanolic extracts (0.2g/mL) or trolox standards (0 to 20μM range), the absorbance was monitored exactly 1 and 6min after the initial mixing. The percentage of absorbance inhibition at 734nm was calculated and plotted as a function of that obtained for the extracts and the standard reference (trolox). The final TEAC value was calculated by using a regression equation between the trolox concentration and the inhibition percentage and expressed as trolox equivalents (μmolTE) per g of dry weight.
Oxygen radical absorbance capacityThis assay measures the antioxidant ability of different components based on the decline in fluorescence of fluorescein (FL) induced by AAPH, a peroxyl radical generator.9 The reaction mixture contained 1.65mL of 0.75mM phosphate buffer (pH 7), 100μL of 0.106μM FL, 150μL of 0.8M AAPH, and 100μL of Phellinus methanolic extracts. Phosphate buffer was used as a blank for ORAC. FL, phosphate buffer, and samples were pre-incubated at 37°C for 15min. The reaction was started by the addition of AAPH, and the fluorescence was measured and recorded every 5min until the fluorescence of the last reading declined to less than 5% with respect to the initial reading. One blank and a maximum of 12 samples were analyzed at the same time. The excitation and emission wavelength was set at 484 and 515nm, respectively. All fluorescence measurements were performed on a Perkin-Elmer LS 55 spectrofluorometer (Norwalk, CT, U.S.A.). Each extract measurement was repeated 4 times. The final ORAC values were calculated by using a regression equation between the trolox concentration and the net area under the FL decay curve. ORAC values were expressed as trolox equivalents (μmolTE) per g dry weight. The area under the curve (AUC) was calculated according to the following equation:
where f0 is the initial fluorescence reading at 0min and ft is the fluorescence reading at time i.Evaluation of antifungal activityThe antifungal potential of methanolic extracts of Phellinus spp. was tested against Alternaria alternata using the agar dilution method. For this 0.05, 0.025, 0.0125, 0.00625g/mL of methanolic extracts of the tested Phellinus were added to Petri dishes containing potato dextrose agar, and then inoculated in the center with the fungi. The efficiency of the treatments was evaluated at 5 days of incubation at 25°C. Controls were pure agar inoculated with the fungi and without exposure to any extract, no effect of the solvent on the fungal growth was observed. Mycelial area was recorded (cm2) in triplicate by the digital analysis of the picture of every plate using the UTHSCSA ImageTool version 3.0 software.31
Statistical analysisThis experiment was based on a completely randomized design with equal replications. Analysis of variance for the treatments was done using Number Cruncher Statistical System version 6.0 software.11 Mean comparisons of the studied parameters among treatments were done using the least significant difference test at a 5% level (P<0.05).
Results and discussionIdentification of Phellinus spp.Eleven macrofungus Phellinus species were found in the Ajos-Bavispe natural protected area, three of them were more commonly collected and studied in the present study. P. badius (Berk. ex Cooke) G. Cunn. was found in subtropical scrub and mezquital (plants from the genus Prosopis). It has a circumglobal distribution in the tropics and subtropical regions.8P. gilvus (Schwein.) Pat. was collected in pine-oak wood, oak wood and mezquital (plants from the genus Prosopis). It is a common fungus on living and dead hardwoods in many genera, and it is more common to be found on oaks. P. rimosus (Berk.) Pilát was observed in pine-oak forest, subtropical scrub and mezquital (plants from the genus Prosopis). This macrofungus has been commonly collected in the Sonoran mycobiota.19
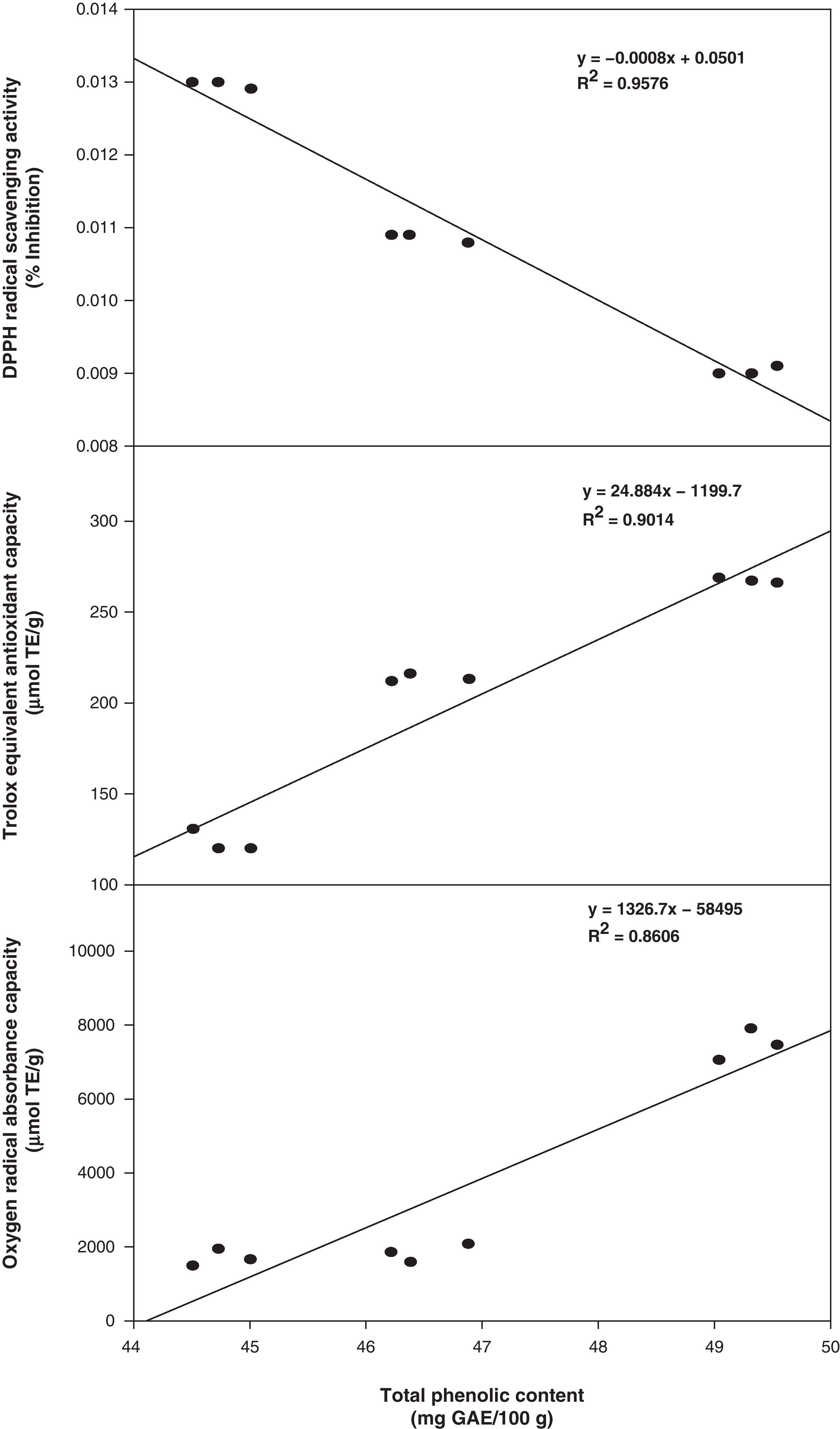
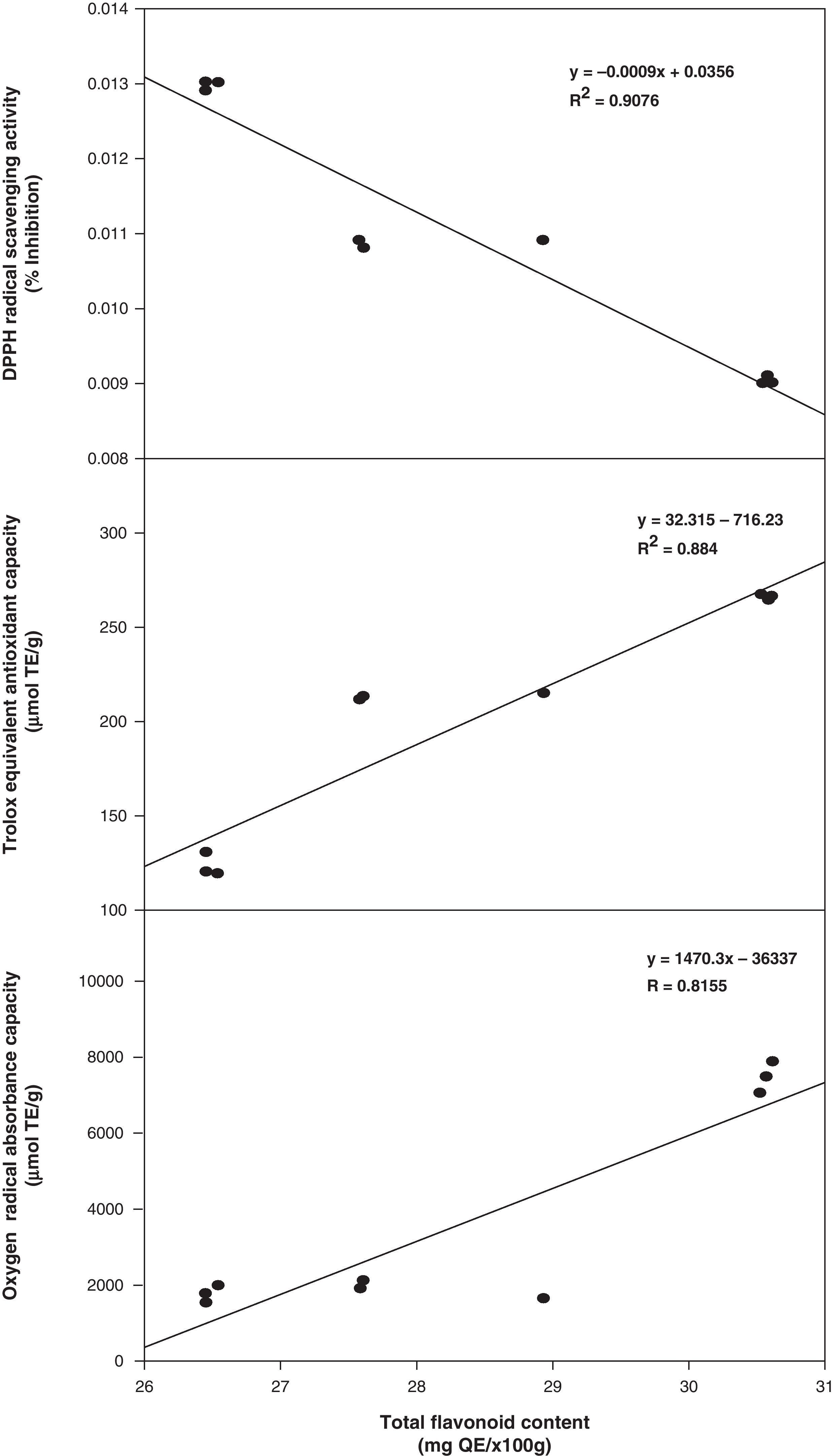
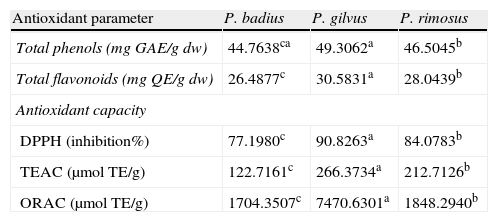
Total phenolics, flavonoids and antioxidant capacity analysisTable 1 shows the contents of total phenolic compounds expressed as gallic acid equivalents determined for P. badius, P. gilvus and P. rimosus, respectively. A significant difference (P<0.05) was observed among P. gilvus with the highest total phenolic content (49.31mgGAE/g), followed by P. rimosus (46.51mgGAE/g) and P. badius (44.76mgGAE/g). Similarly, the total flavonoid content of Phellinus methanolic extracts showed the same behavior already described for phenolic compounds (Table 1), where P. gilvus presented the highest flavonoids content (30.58mgQE/g), followed by P. rimosus (28mgQE/g) and P. badius (26.48mgQE/g).
Total phenolics, flavonoids and antioxidant capacity of methanolic extracts from Phellinus spp.
| Antioxidant parameter | P. badius | P. gilvus | P. rimosus |
| Total phenols (mgGAE/gdw) | 44.7638ca | 49.3062a | 46.5045b |
| Total flavonoids (mgQE/gdw) | 26.4877c | 30.5831a | 28.0439b |
| Antioxidant capacity | |||
| DPPH (inhibition%) | 77.1980c | 90.8263a | 84.0783b |
| TEAC (μmolTE/g) | 122.7161c | 266.3734a | 212.7126b |
| ORAC (μmolTE/g) | 1704.3507c | 7470.6301a | 1848.2940b |
*Different superscripts among rows indicate significant differences among means (P<0.05).
Comparison with other kind of macrofungus reported in previous publications showed that Phellinus spp. from Sonora contains higher levels of phenolic compounds. The phenolic content of Leucopaxillus giganteus, Sarcodon imbricatus, and Agaricus arvensis was 6.29, 3.76, and 2.83mg/g, respectively.3 However, Phellinus baumii samples presented significantly higher levels of phenolic compounds (338μg/mL).18 In contrast, other species of fungus such as Endoptychum arizonycum and Disciseda verrucosa presented lower values of phenolic compounds (9.81 and 16.1mg/g, respectively; unpublished data). Total phenolic and flavonoid contents of different solvent extracts of Phellinus igniarius ranked from 6 to 23mg/g of extract, and 0.05 to 4.26mg/g, respectively.17 Clearly the phenolic content of P. badius, P. rimosus and P. gilvus are higher than those published values in other macrofungus species.
Phenolic compounds are one of the most widely occurring groups of phytochemicals which are of considerable physiological and morphological importance in fungi and plants.12,20 These compounds play an important role providing protection against pathogens and predators.20 In humans, phenolic compounds can exhibit a wide range of physiological properties, such as anti-allergenic, anti-artherogenic, anti-inflammatory, antimicrobial, antioxidant, anti-thrombotic, cardioprotective and vasodilatory effects.12,13 The beneficial effects derived from phenolic compounds have been attributed to their antioxidant activity.12
Antioxidant capacity analysisAll the macrofungus methanol extracts showed high DPPH radical scavenging activity (Table 1) as expected considering the total phenolic content. The highest antioxidant scavenging effect (90.8%) was showed by the P. gilvus extract, followed by P. rimosus extract (84%) and P. badius extract (77.0%) at a concentration of 0.02g/mL. The antiradical activity evaluated as the efficient concentration of each extract to inactivate the 50% of the DPPH radical (EC50) was 0.009g/mL for P. gilvus, 0.01g/mL for P. rimosus and 0.013g/mL for P. badius. The EC50 values also indicated that the antioxidant activity of the P. gilvus extract was higher than that of P. rimosus. The antiradical scavenging activity might be attributed to the replacement of hydroxyl groups in the aromatic ring systems of the phenolic compounds as a result of their hydrogen donating ability.4
Analysis of ABTS radical scavenging activity of the methanolic extracts of Phellinus spp. expressed as trolox equivalent antioxidant capacity (TEAC) (Table 1) showed the same pattern than that of DPPH assay. P. gilvus extract presented significantly (P<0.05) higher antioxidant activity (266μmolTE/g), followed by P. rimosus (212μmolTE/g), and P. badius (122μmolTE/g). The oxygen radical absorbance capacity (ORAC) values confirmed these results (Table 1), being P. gilvus (7470μmolTE/g) the more potent antioxidant extract, followed by P. rimosus (1848μmolTE/g) and P. badius (1704.35μmolTE/g). All the tested extracts were significantly different (P<0.05) among them.
The methanolic extract of Agrocybe cylindracea strain B exhibited a DPPH scavenging ability of 93.8% at 0.005g/mL.28 Tsai et al.29 found that scavenging abilities of three ethanolic extracts were 94.9%, 89.2%, and 88.8% at 0.005g/mL for Agaricus blazei, A. cylindracea, and Boletus edulis, respectively. Furthermore, ethanolic extracts of Pleurotus citrinopileatus15 and Hypsizgus marmoreus16 showed scavenging abilities of 94.9% and 59.7% at 0.005g/mL, respectively. Our results suggest that the scavenging ability of P. gilvus was higher than that of P. rimosus and P. badius; however, an effect of the solvent extraction method could explain the differences when compared with other studies.
The antioxidant activity of phenolic compounds is due to their ability to scavenge free radicals, donate hydrogen atoms or electron, or chelate metal cations. The structure of phenolic compounds is a key determinant of their radical scavenging and metal chelating activity. The position and number of hydroxyl groups in the phenolics and flavonoids determine the capacity of the molecules to donate an electron and stabilize free radicals. Therefore, antioxidants have the ability to scavenge free radicals and prevent diseases caused by these reactive species. Antioxidants from Phellinus sensu lato are composed of hispidin and its derivatives.13 Also, the catechol moiety is responsible for inhibiting radical generation by chelating metal ions.13 Therefore, these phenolic compounds could have synergistic effects and be responsible of the high antioxidant capacity of the studied Phellinus extracts.
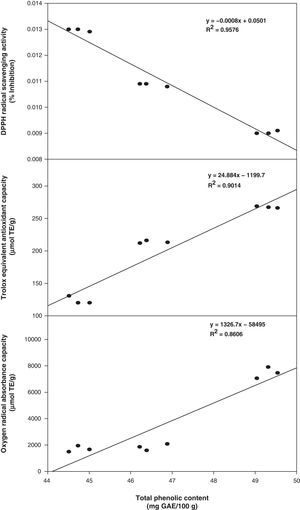
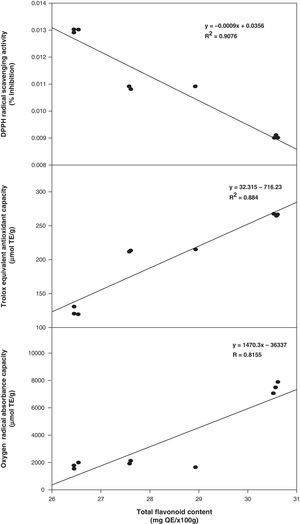
In this study, total antioxidant component contents (phenolic and flavonoids) were positively correlated with the values of antioxidant activity. The correlation coefficients (R2) of total phenolic compounds versus DPPH, TEAC, and ORAC were 0.9576, 0.9014, and 0.8606, respectively (Fig. 1). However, the correlation coefficients of total flavonoids versus DPPH, TEAC, and ORAC were slightly lower at 0.9214, 0.8840 and 0.8155, respectively (Fig. 2). In addition, an excellent correlation between contents of total phenolics and antioxidant activity was also obtained and pointed out that the antioxidant activity of phenolics was mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators and single oxygen quenchers.23 Consequently, total phenols in the three methanolic extracts from Phellinus spp. were possibly the major components that contribute in higher extent to their antioxidant activities.
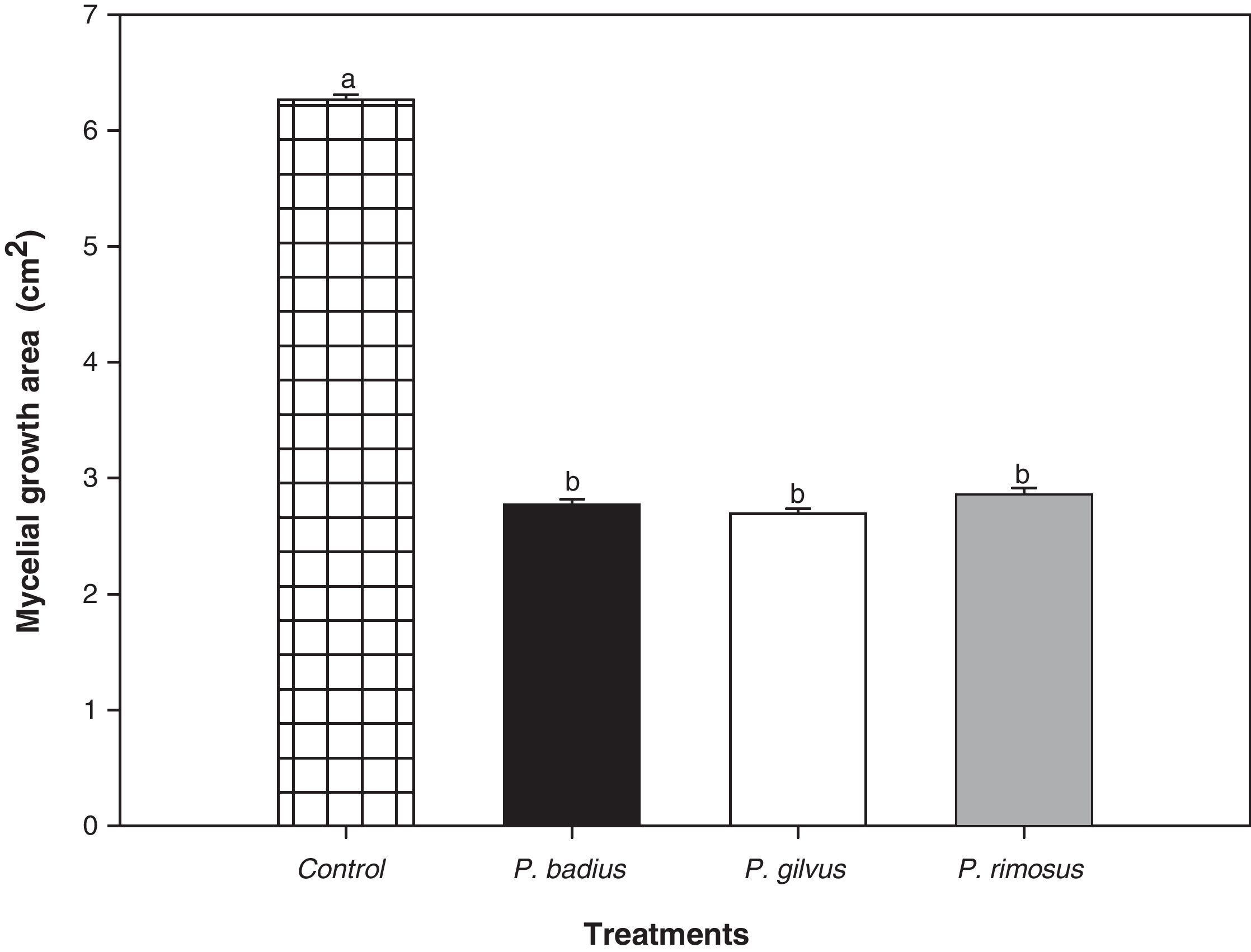
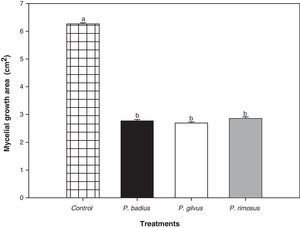
The antifungal potential of methanolic extracts of P. badius, P. gilvus and P. rimosus was determined (Fig. 3). Their antifungal potential was tested against A. alternata, a mold highly related to plant food spoilage. The three tested macrofungus extracts were effective to inhibit the growth of A. alternata, however, no significant differences among them were observed. The antifungal activity of all the extracts did not show a concentration-dependent behavior and no significant (P>0.05) effect of concentration was observed.
The antimicrobial activities of a variety of naturally occurring phenolic compounds from fungi have been studied.2 These compounds can play an important role in tissue's protection against pathogenic agents, penetrating the cell membrane of microorganisms, causing lysis.12,13 Phenolic compounds from spices such as gingeron, zingerone, and capsaicin have been found to inhibit the germination of bacterial spores.5 Polyphenols contained in green tea (Camellia sinensis) are effective against Vibrio cholerae O1, Streptococcus mutans, and Shigella.25 The antimicrobial activity of other Phellinus extracts has not been widely evaluated, only two reports from antibiotic activity of Phellinus fastuosus and Phellinus ribis were found in the literature.7 In this context, the mixture of phytochemical constituents in methanolic extracts of Phellinus spp. can be responsible for their antioxidant and antifungal activity.1
ConclusionsMethanolic extracts from mycelia of macrofungus P. gilvus, P. rimosus and P. badius isolated from Sonora, Mexico, revealed considerable antioxidant and antifungal properties as evidenced by their high total phenolics and flavonoid contents as well as their antioxidant capacity values. However, P. gilvus exhibited the highest antioxidant potential. The presence of Phellinus methanolic extracts was effective in the growth inhibition of A. alternata.
Conflict of interestThe authors have no conflict of interest to declare.
Special appreciation to the Mexican Council of Science and Technology (CONACYT project 103105) for financial support to carry out this research.