Deoxycholate amphotericin B (DAB) is a nephrotoxic drug and the incidence of acute kidney injury (AKI) is high.
AimsThe aim of this study was to describe the incidence of AKI in patients under DAB therapy and determine risk factor to predict the AKI.
MethodsThe data of this retrospective study included previously hospitalized patients treated with intravenous DAB for at least five days. Clinical and laboratorial data were evaluated and AKI was classified in stages using Acute Kidney Injury Network criteria. Univariated test followed by a multivariable analysis was performed to determine risk factor and Kaplan–Meier survival estimates were calculated to evaluate the role of AKI in the outcome.
ResultsOne hundred six patients were included in the final analysis. AKI occurred in 51.9% and dialysis was necessary in 4.7%. The occurrence of AKI was not associated with any risk factor. The mortality of the patients was neither associated with AKI nor with dialysis. Other nephrotoxic drugs were not risk factors for AKI.
ConclusionsThe incidence of AKI in patients using DAB is high and we cannot predict the chance of AKI using clinical or laboratorial data.
La anfotericina B desoxicolato (DAB) es un fármaco nefrotóxico y la incidencia de insuficiencia renal aguda (IRA) asociada a su uso es alta.
ObjetivosEl objetivo del presente estudio fue describir la incidencia de IRA en pacientes tratados con DAB y determinar los factores de riesgo de predicción de IRA.
MétodosLos datos de este estudio retrospectivo incluyeron a pacientes hospitalizados previamente y tratados con DAB por vía intravenosa durante un mínimo de 5 días. Se valoraron los datos clínicos y de laboratorio, y se establecieron diferentes estadios de IRA según los criterios publicados por la Acute Kidney Injury Network. Se efectuó un análisis univariante seguido de otro multivariante para determinar los factores de riesgo; se calcularon estimaciones de supervivencia de Kaplan–Meier para evaluar el papel de la IRA en los desenlaces.
ResultadosEn el análisis final se incluyeron 106 pacientes. La IRA afectó al 51,9% de los pacientes y la diálisis fue necesaria en el 4,7%. La incidencia de IRA no se asoció con ningún factor de riesgo. La mortalidad de los pacientes no se asoció con IRA ni con la diálisis. Otros fármacos nefrotóxicos tampoco fueron factores de riesgo de insuficiencia renal aguda.
ConclusionesEn pacientes tratados con DAB la incidencia de IRA es alta y no es posible predecir el riesgo de padecer dicha enfermedad con una valoración de los datos clínicos o de laboratorio.
Deoxycholate amphotericin B (DAB) nephrotoxicity is frequent and severe. This side effect has been associated with higher mortality, increased length of stay and costs due to dialysis.8 Nowadays there are many options of less nephrotoxic antifungal drugs, such as echinocandins and liposomal formulation of amphotericin B.
Several approaches have been employed in order to try to decrease DAB's acute kidney injury (AKI) such as: association with lipid suspensions, continuous infusion, saline bolus before and after DAB.2,7 There are few well-known risk factors for AKI, such as cumulative dose of DAB and concomitant use of nephrotoxic drugs.5
To determine risk factors associated with AKI it is important to contraindicate DAB when there are unchangeable factors as age and previous diseases. It is also important to know changeable risk factors, mainly when there are no options of antifungal therapy, to modify the risk or optimize the possible condition. Considering this problem, this study evaluated risk factors for mortality and AKI in patients using DAB.
MethodsThis was a retrospective study carried out at Hospital Universitario Evangelico de Curitiba, a 700-bed tertiary-care hospital in Curitiba, a city located in Southern Brazil. The cases were obtained from pharmacy database from January 2007 to January 2011. Inclusion criteria were patients using at least five days of intravenous DAB. Exclusion criteria were lack of laboratory data to analyze renal function; <18 years old; DAB used in another vial as intravesical or oral for selective colonic decolonization, previous use of DAB.
Clinical data were evaluated: age, gender, indication of DAB, previous admission, mortality, concomitant nephrotoxic drugs (intravenous vancomycin, intravenous contrast, loop diuretics, polymyxin, non-steroidal anti-inflammatory drugs and aminoglycosides), current or previous admission on ICU. Current conditions, invasive procedures and previous disease were also evaluated: burn, mechanical ventilation, central venous line, bladder catheter, HIV infection, diabetes mellitus, chronic renal failure, cardiac failure, chronic obstructive pulmonary disease, essential hypertension, cancer, cirrhosis, trauma, elective or emergency surgery. The total number of nephrotoxic drugs for each patient was analyzed and the median compared considering cumulative risk.
Acute kidney injury was classified using Acute Kidney Injury Network (AKIN) criteria.6 Stage 1 is the classification for patients with increase in the creatinine level 50% or ≥0.3mg/dL of the basal value. Stage 2 is the classification for patients who show an increase in creatinine level 100% of the basal level. Stage 3 is the classification for patients who show an increase of 200% of the basal level or dialysis. Stage 3 was also classified when creatinine was ≥4mg/dL with acute rise of ≥0.5mg/dL.
Study end pointsThe acute renal injury was the main end point based on the classification of AKIN. Dialysis was the second end-point followed by in-hospital mortality. Patients with AKI were compared with those with normal renal function during the treatment with DAB to determine risk factors. A second analysis was performed comparing patients who died during hospitalization with those who survived to determine any association with AKI and/or dialysis.
Statistical analysisContinuous data were expressed as mean±standard deviation (SD) or median with ranges. Frequencies were expressed as percentages. Dichotomous variables were compared using χ2 test and Mann–Whitney test was used for continuous variables. Significance level was set at 0.05. Variables with P<0.10 in the univariated analysis were included in the multivariable analysis. Multivariate analysis was performed using binary logistic regression model. Odd ratios (OR) with 95% confidence intervals (95% CI) were calculated for each variable. Variables in which 95% CI did not include 1.0 were maintained in the final model.
Kaplan–Meier survival estimates were calculated to evaluate the role of AKI in the outcome, and the difference was assessed using the log-rank test. Significance was determined when P value was lower than 0.05.
All data were recorded using the software Excel (Microsoft, New York, USA) and the statistical analysis was performed using the software SPSS 11.5 (SPSS, Chicago, USA). Kaplan–Meier survival estimates were determined with GraphPad Prism 4.0 (GraphPad, La Jolla, USA).
ResultsA total of 137 patients used DAB in the period evaluated. Fifteen patients were <18 years old, ten patients were excluded due to lack of creatinine data and six patients received less than five days of therapy. One hundred six patients were included in the final analysis.
The mean age of the patients was 47.87±17.61 and most of them were male (66%) by undefined reason. The main indication for DAB was the empirical treatment of possible candidemia (69%). In 29% of the cases a fungemia was confirmed and two patients (2%) included in the analysis used DAB for the treatment of mucocutaneous leishmaniasis.
Most patients had previous admission on intensive care unit, however, the initiation of the DAB was mainly outside the critical care area (87%). The percentage of HIV infection in the study was high (15%) due to association of cases of fungemia by Cryptococcus neoformans. Other clinical findings of the patients receiving DAB are described in Table 1.
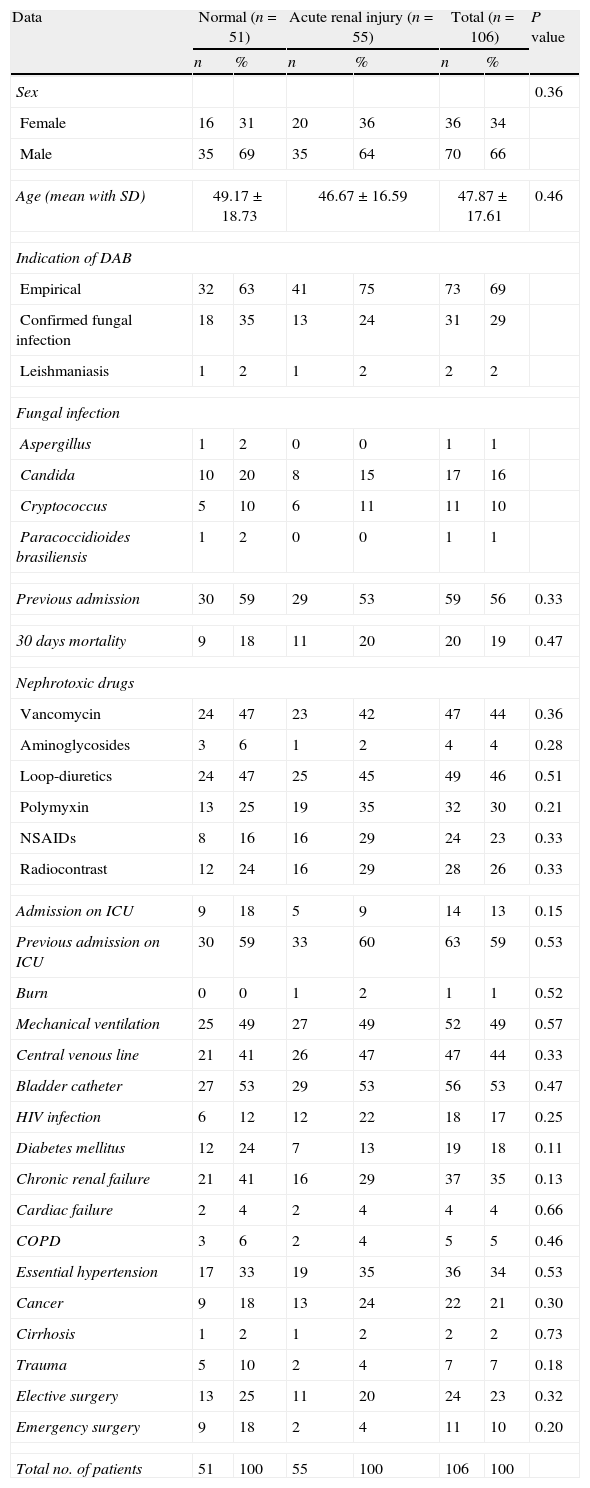
Data about patients treated with DAB. Risk factor are listed and comparison between patients who developed any level of acute renal injury during the treatment with DAB. P value was considered significant when P<0.05.
| Data | Normal (n=51) | Acute renal injury (n=55) | Total (n=106) | P value | |||
| n | % | n | % | n | % | ||
| Sex | 0.36 | ||||||
| Female | 16 | 31 | 20 | 36 | 36 | 34 | |
| Male | 35 | 69 | 35 | 64 | 70 | 66 | |
| Age (mean with SD) | 49.17±18.73 | 46.67±16.59 | 47.87±17.61 | 0.46 | |||
| Indication of DAB | |||||||
| Empirical | 32 | 63 | 41 | 75 | 73 | 69 | |
| Confirmed fungal infection | 18 | 35 | 13 | 24 | 31 | 29 | |
| Leishmaniasis | 1 | 2 | 1 | 2 | 2 | 2 | |
| Fungal infection | |||||||
| Aspergillus | 1 | 2 | 0 | 0 | 1 | 1 | |
| Candida | 10 | 20 | 8 | 15 | 17 | 16 | |
| Cryptococcus | 5 | 10 | 6 | 11 | 11 | 10 | |
| Paracoccidioides brasiliensis | 1 | 2 | 0 | 0 | 1 | 1 | |
| Previous admission | 30 | 59 | 29 | 53 | 59 | 56 | 0.33 |
| 30 days mortality | 9 | 18 | 11 | 20 | 20 | 19 | 0.47 |
| Nephrotoxic drugs | |||||||
| Vancomycin | 24 | 47 | 23 | 42 | 47 | 44 | 0.36 |
| Aminoglycosides | 3 | 6 | 1 | 2 | 4 | 4 | 0.28 |
| Loop-diuretics | 24 | 47 | 25 | 45 | 49 | 46 | 0.51 |
| Polymyxin | 13 | 25 | 19 | 35 | 32 | 30 | 0.21 |
| NSAIDs | 8 | 16 | 16 | 29 | 24 | 23 | 0.33 |
| Radiocontrast | 12 | 24 | 16 | 29 | 28 | 26 | 0.33 |
| Admission on ICU | 9 | 18 | 5 | 9 | 14 | 13 | 0.15 |
| Previous admission on ICU | 30 | 59 | 33 | 60 | 63 | 59 | 0.53 |
| Burn | 0 | 0 | 1 | 2 | 1 | 1 | 0.52 |
| Mechanical ventilation | 25 | 49 | 27 | 49 | 52 | 49 | 0.57 |
| Central venous line | 21 | 41 | 26 | 47 | 47 | 44 | 0.33 |
| Bladder catheter | 27 | 53 | 29 | 53 | 56 | 53 | 0.47 |
| HIV infection | 6 | 12 | 12 | 22 | 18 | 17 | 0.25 |
| Diabetes mellitus | 12 | 24 | 7 | 13 | 19 | 18 | 0.11 |
| Chronic renal failure | 21 | 41 | 16 | 29 | 37 | 35 | 0.13 |
| Cardiac failure | 2 | 4 | 2 | 4 | 4 | 4 | 0.66 |
| COPD | 3 | 6 | 2 | 4 | 5 | 5 | 0.46 |
| Essential hypertension | 17 | 33 | 19 | 35 | 36 | 34 | 0.53 |
| Cancer | 9 | 18 | 13 | 24 | 22 | 21 | 0.30 |
| Cirrhosis | 1 | 2 | 1 | 2 | 2 | 2 | 0.73 |
| Trauma | 5 | 10 | 2 | 4 | 7 | 7 | 0.18 |
| Elective surgery | 13 | 25 | 11 | 20 | 24 | 23 | 0.32 |
| Emergency surgery | 9 | 18 | 2 | 4 | 11 | 10 | 0.20 |
| Total no. of patients | 51 | 100 | 55 | 100 | 106 | 100 | |
SD=standard deviation; DAB=deoxycholate amphotericin B; ICU=intensive care unit; HIV=human immunodeficiency virus; COPD=chronic obstructive pulmonary disease.
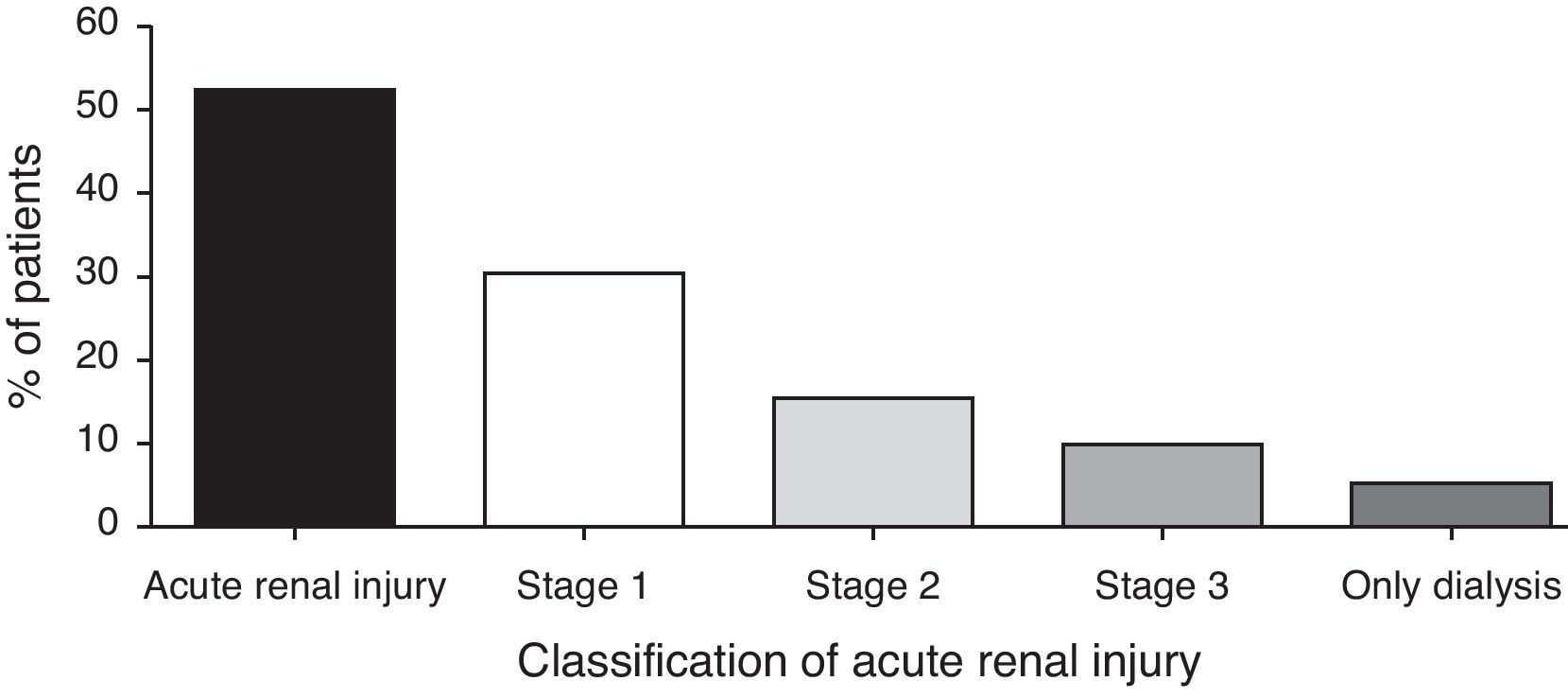
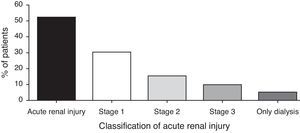
Acute kidney injury occurred in 51.9%. The median day of AKI was 6.5±4.6 day. The incidence of AKI was lower as the severity of AKIN criteria increased, and dialysis was necessary in 4.7% (Fig. 1). The AKI AKIN 1 occurred in 30 patients, AKIN 2 in 16 patients and AKIN 3 in 10 patients. The occurrence of AKI was not associated with any risk factor detailed in Table 1. The association was tried with any severity of AKI. The cumulative number of nephrotoxic drugs was not associated with AKI. Other nephrotoxic drugs were not risk factor for AKI. We compared risk factors for all AKIN classification and there was no significant finding.
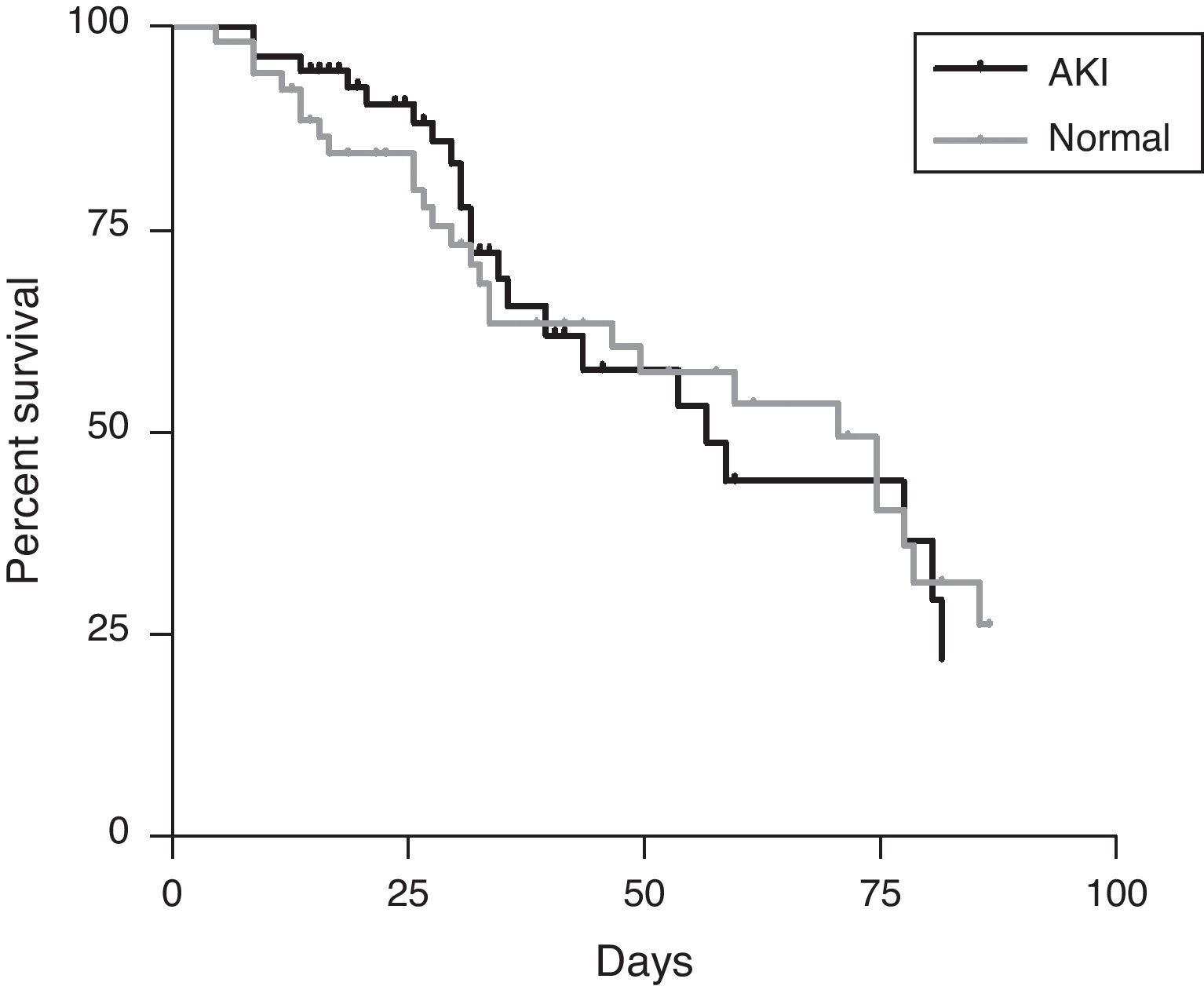
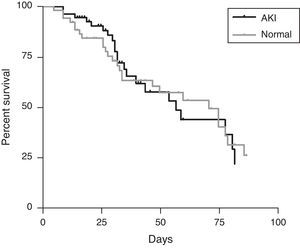
The mortality of the patients was neither associated with AKI nor with dialysis, which can be demonstrated in the mortality curve similarity between the group of AKI and with normal renal function during therapy with DAB (Fig. 2). The mortality of each AKIN classification was 17% (AKIN 1), 31.3% (AKIN 2) and 14.3% (AKIN 3), without statistical significance.
DiscussionSome studies have shown a clear association of AKI with some risk factors, mainly nephrotoxic drugs.5 The mechanism of AKI in patients under DAB therapy is well established.1 Glomerular lesion due to basal membrane thickening and tubular lesion with generalized degeneration of ascending limb of the loop of Henle are observed.4 We evaluated the previous use of nephrotoxic drugs, including vancomycin, aminoglycosides, radiocontrast, loop diuretics, polymyxin and non-steroidal anti-inflammatory drugs. However, they were not risk factors for AKI. Furthermore, critical patients admitted to the intensive care unit showed similar rates of AKI in comparison with non-critical patients. The infusion of saline solutions was not evaluated, but the overload and bolus infusions have been previously evaluated on the literature and showed similar azotemia levels.2,5 In a letter, Girmenia et al. report a 8% of AKI in patients using DAB with careful saline infusion as well as concomitant electrolyte reposition (potassium and magnesium).3 Accumulative dose of DAB was not associated with AKI in our study, probably because the kidney injury was early (<7 days).
Acute kidney injury is an independent risk factor of mortality for patients admitted in the hospital, no matter which the etiology is.9 In patients under DAB therapy the mortality rate was similar with patients without azotemia as demonstrated in Fig. 2. Regardless of the previous findings above, the incidence of AKI was high, occurring in more than 50% of patients independently of the dose. This study has several biases. The number of patient with end-stage renal disease is high in both groups, and the number of associated comorbidities in this group is high. The criteria of AKI in patients with chronic renal failure are established in the RIFLE and AKIN criteria, but we know that these criteria fail in some patients independent of the drug, including volume depletion. The indication of amphotericin instead of other antifungal drugs is not described, as severity of infection, which could reflect a higher rate of AKI.
The conclusion of this epidemiological study is that DAB therapy is highly nephrotoxic and it was not possible to predict those patients who developed AKI in the population analyzed, based on clinical findings. Cost-benefits studies in developing countries should be done to determine the real utility of this drug in an era of less nephrotoxic drugs.
Conflict of interestThe authors declare that they have no conflict of interest.