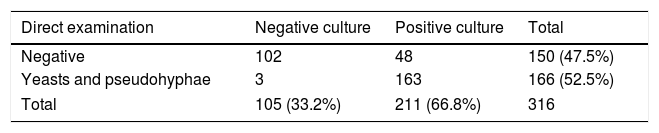Recurrent vulvovaginitis is a growing problem that affects millions of women worldwide. In many cases it is treated as vulvovaginal candidiasis, but there is not always microbiological confirmation.
AimsTo determine the etiology of vulvovaginitis in a group of patients.
MethodsThis is a cross-sectional study in which the data from the medical records of 316 adult patients who consulted for vulvovaginitis were analyzed. Eighty nine percent of the cases had already suffered previous episodes.
ResultsThe median age was 34 (265 patients were between 16 and 45 years old). Yeasts were isolated in culture from 211 (66.8%) patients, although pseudo-hyphae and yeasts were observed in only 166 samples (52.5%) in the direct microscopic examination. Multiple predisposing factors were found, among which the use of contraceptives or previous antibiotics stand out. Most of the patients (almost 90%) had been treated with antifungals, with or without microbiological confirmation. Candida albicans was isolated in 187 (88.6%) patients, followed by Candida glabrata in 6 (2.8%) patients. Association with bacterial vaginosis was found in 35.1% and with intermediate bacterial microbiota in 33.2% of the cases. A remarkably high proportion of C. albicans isolates resistant to fluconazole (80.1%) and itraconazole (58.8%) was found.
ConclusionsA microbiological analysis is essential to confirm the diagnosis of vulvovaginal candidiasis, whether simple, complicated, or recurrent. Identifying the isolated yeast species and determining its susceptibility to antifungal agents are particularly important.
Las vulvovaginitis recurrentes son un problema creciente que afecta a millones de mujeres en todo el mundo. En muchos casos, se tratan como candidiasis, aunque no siempre se tiene la confirmación microbiológica.
ObjetivosDeterminar la etiología de la vulvovaginitis en una muestra de pacientes.
MétodosEste es un estudio transversal donde se analizan los datos clínicos y microbiológicos de 316 pacientes adultas que consultaron por vulvovaginitis y que en un 89% de los casos ya habían cursado episodios previos.
ResultadosLa mediana de edad fue de 34 años (265 pacientes tenían hasta 45 años), en los cultivos de 211 (66,8%) pacientes crecieron levaduras, aunque solamente se visualizaron en el examen microscópico directo en 166 (52,5%) de los casos. Se encontraron múltiples factores predisponentes, entre los que se destaca el uso de anticonceptivos y la toma previa de antibióticos. La gran mayoría de ellas habían sido tratadas con antifúngicos (91% de las pacientes en las que se había aislado Candida y el 83% de los casos en que no se aisló), con o sin confirmación microbiológica. Candida albicans se aisló en 187 casos (88,6%) y Candida glabrata en seis (2,8%). Se encontró asociación con vaginosis bacteriana en el 35,1% de las pacientes y con microbiota bacteriana intermedia en el 33,2% de los casos. Hubo una considerable proporción de aislamientos de C. albicans resistentes a fluconazol (80,1%) y a itraconazol (58,8%).
ConclusionesEs imprescindible la realización de un análisis microbiológico para confirmar el diagnóstico de la candidiasis vulvovaginal, que puede ser simple, complicada o recidivante. Es muy importante la identificación de las especies de levaduras aisladas y la determinación de la sensibilidad a los antifúngicos.
Vulvovaginal candidiasis (VVC) is a common health condition worldwide. Numerous publications estimate that 75% of women suffer from this pathology at least once in their lives, and almost 50% will suffer more than one episode. It is also estimated that a small proportion, ranging from 4 to 9%, have at least four episodes per year (recurrent vulvovaginal candidiasis). According to a recent review study, these epidemiological data are not always well documented.37 Complicated vulvovaginal candidiasis is the term used to describe candidiasis associated with non-specific bacterial vaginosis and those cases produced by non-Candidaalbicans Candida species.1,21 On the other hand, 2019 British guidelines define this pathology as acute or recurrent VVC only.43
The fungal species most frequently isolated from vaginal exudate samples is C. albicans with a prevalence above 80% in most publications, although some describe this etiology in just over 50%.1,12,14,22,25,34,43,45,54 Other species are Candida glabrata complex, Candida krusei, Candida parapsilosis complex and, less frequently, Candida tropicalis, Candida guilliermondii and even Saccharomyces cerevisiae. Candida africana has also been isolated in cases of vulvovaginal candidiasis in Africa and in some European countries.32,38,39,51,52 This Candida species was not found in a study carried out in Argentina.30 Mixed cultures, generally of C. albicans with another species, frequently C. glabrata, can appear in 2–10% of the cultures.21,25,34
In vitro susceptibility show that there is an increase in antifungal resistance, especially to fluconazole.1,2,25,47 The objectives of this study are to analyze the prevalence of recurrent VVC in a group of women with symptoms of recurrent vulvovaginitis who consulted after being treated several times for this condition, and the prevalence of the Candida species isolated from their vaginal secretion as well as their antifungal susceptibility. The association of VVC with other causes of vulvovaginitis and some risk factors were also studied.
MethodsA cross-sectional retrospective study on medical records from patients who came to a private Mycology Center for a presumptive diagnosis of recurrent vaginal candidiasis between June 2008 and December 2019 was carried out. All Candida isolates were identified and their susceptibility profile was performed at the Mycology Unit of the Hospital de Infecciosas F. J. Muñiz in Buenos Aires city. Demographic data, clinical characteristics and previous treatments registered in the clinical records were analyzed. It was a microbiological-based study. All patients were anonymized, so no ethical approval was required.
Data from the mycological studies conducted at the institution were recorded. These examinations were performed by taking vaginal discharge material with two swabs: one was used for culture in Sabouraud and CHROMAgar Candida media (CHROMagar™, France) and the second for microscopic examination in fresh preparations and in smears for Gram and Giemsa stains. Wet mount preparations were used to observe the presence of yeasts and pseudo-hyphae, and Gram staining was used to study the type of accompanying microbiota: Gram-positive bacilli compatible with Lactobacillus, intermediate microbiota (Gram-positive bacteria), GAM complex (Gardnerella vaginalis, anaerobic bacteria and Mobiluncus) or the absence of bacterial microbiota. Giemsa staining was performed to see the presence of Trichomonas vaginalis.19 The results of the microscopic examinations and those of the cultures were recorded.
The identification of the isolated yeasts was carried out by conventional and molecular methods in the Mycology Unit. The macroscopic morphology of the colonies and their color in the chromogenic medium were considered. The identification of the species was completed by studying the micromorphological characteristics on 1% milk agar with Tween 80, cornmeal agar with Tween 80, and the use of API ID 32C system (bioMérieux, Marci L’Etoile, France).19,35 PCR-multiplex technique was used for the definitive identification of the species of C. albicans, and the C. parapsilosis and C. glabrata complexes.3,32,39–41
Susceptibility to fluconazole and itraconazole was studied by determining the minimum inhibitory concentration by broth microdilution procedure according to CLSI document M27-4th ed., and the cut-off points set out in CLSI document M-60 were considered.8,9
For the statistical analysis all results were recorded in an Excel spreadsheet and data from continuous variables were expressed as average or median, as appropriate. The categorical variables were expressed as percentages; χ2 or Fisher exact tests were employed to evidence statistical differences between groups. Data analysis was performed with the Statistix 8® program and statistical significance was set at p<0.05.
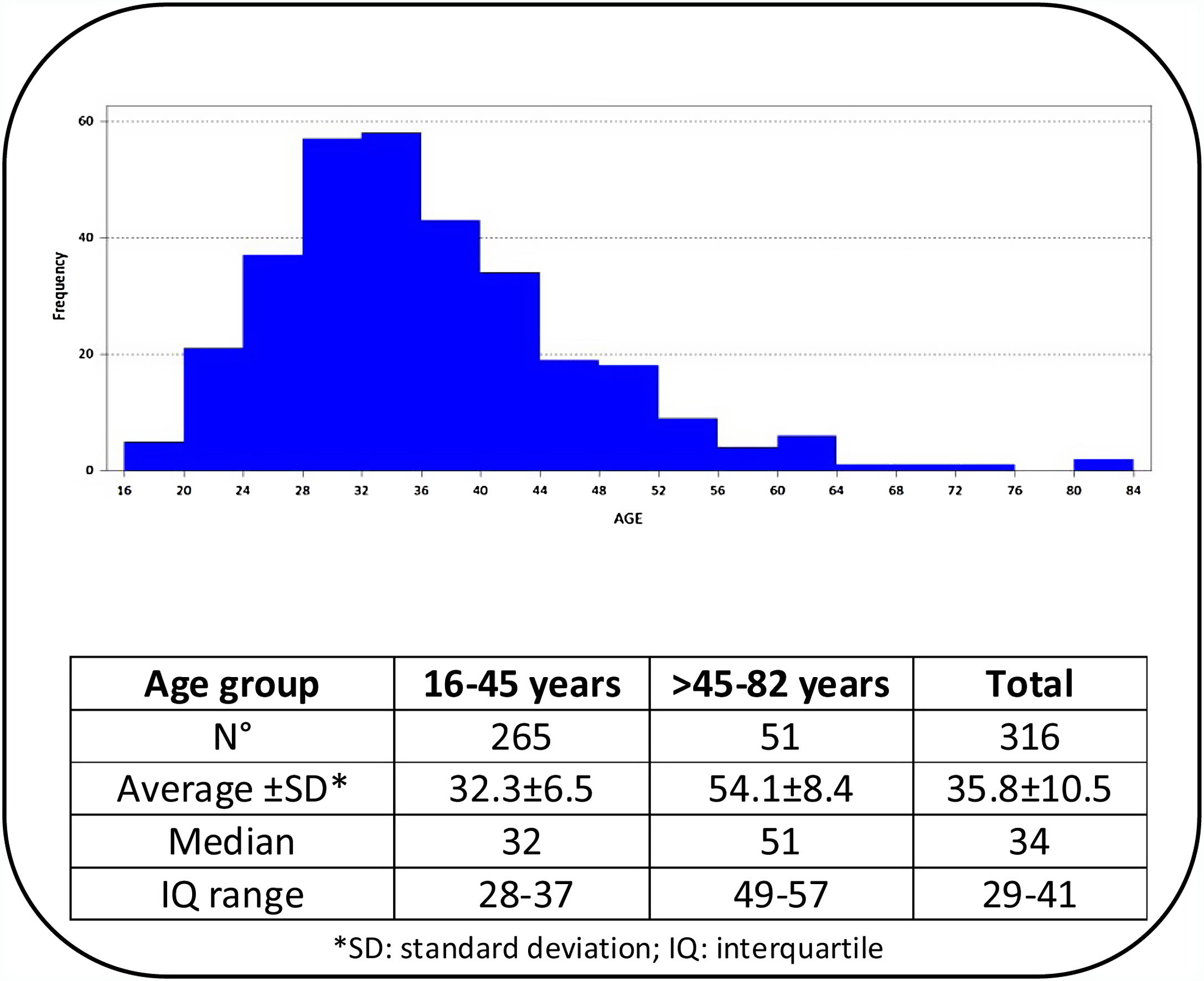
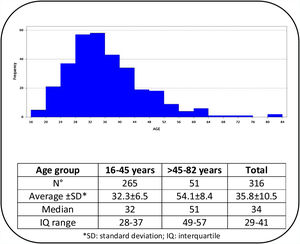
ResultsOut of 329 patients, 13 cases were excluded because their mycological exams could not be performed. The age of the 316 patients included ranged from 16 to 82 years, with a median of 34 years. According to age distribution, the patients were grouped into two age categories (≤45 years and >45 years) and the corresponding data are shown in Fig. 1. The results of the 316 mycological examinations and cultures are presented in Table 1. As shown in this table, only 52.5% of the direct microscopic examinations showed the presence of yeasts or pseudo-hyphae, and in 66.8% of the patients Candida was isolated in culture. Women under 45 years presented positive cultures in 69.8% of the cases, while in those above that age Candida grew in 51%. This difference was statistically significant (p=0.0101).
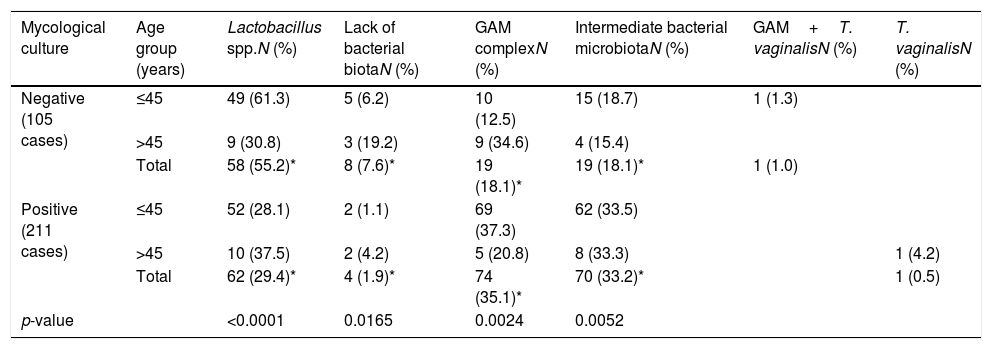
Table 2 shows the relationship between the type of accompanying microbiota and the result of the mycological cultures in both age groups. The distribution of the accompanying bacterial microbiota was statistically significant when comparing both groups (p<0.0001). The percentage of normal bacterial microbiota was 25.9% higher (CI: 14.5%–0.37.2%) in the 105 samples with negative Candida cultures (p<0.0001). In patients over 45 years the distribution of the bacterial microbiota was similar between those cases who presented positive Candida cultures and those without yeast growth (p=0.4466). In the group of younger women the proportion of Lactobacillus in the vaginal samples with negative cultures for Candida (61.3%) was higher than in those where yeasts were isolated (28.1%) (CI: 20.7%–45.7%, p<0.0001). In younger women a statistically significant difference was found between vaginal samples with intermediate bacterial microbiota (33.5%) or GAM complex (37.3%) associated with positive fungal cultures and those with the same bacterial microbiota (18.7% and 12.5%, respectively) and negative mycological cultures (p=0.0181; for intermediate bacterial microbiota, and p=0.0001 for GAM complex). T. vaginalis was found in only one case among the youngest group, and in another patient from the older group.
Bacterial and parasitic microbiota in vaginal discharge according to age (≤45 years and >45 years) and the mycological culture.
| Mycological culture | Age group (years) | Lactobacillus spp.N (%) | Lack of bacterial biotaN (%) | GAM complexN (%) | Intermediate bacterial microbiotaN (%) | GAM+T. vaginalisN (%) | T. vaginalisN (%) |
|---|---|---|---|---|---|---|---|
| Negative (105 cases) | ≤45 | 49 (61.3) | 5 (6.2) | 10 (12.5) | 15 (18.7) | 1 (1.3) | |
| >45 | 9 (30.8) | 3 (19.2) | 9 (34.6) | 4 (15.4) | |||
| Total | 58 (55.2)* | 8 (7.6)* | 19 (18.1)* | 19 (18.1)* | 1 (1.0) | ||
| Positive (211 cases) | ≤45 | 52 (28.1) | 2 (1.1) | 69 (37.3) | 62 (33.5) | ||
| >45 | 10 (37.5) | 2 (4.2) | 5 (20.8) | 8 (33.3) | 1 (4.2) | ||
| Total | 62 (29.4)* | 4 (1.9)* | 74 (35.1)* | 70 (33.2)* | 1 (0.5) | ||
| p-value | <0.0001 | 0.0165 | 0.0024 | 0.0052 |
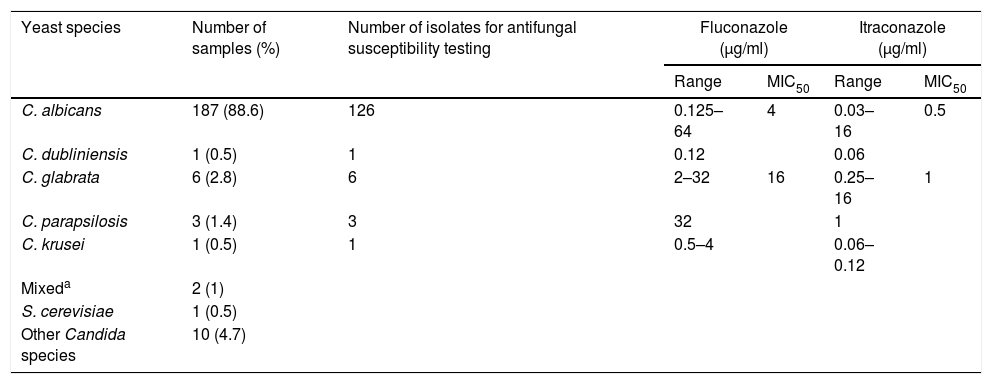
Only 29.4% of VVC were uncomplicated cases, and in 68.3% Candida was associated with intermediate microbiota (33.2%) or GAM complex (35.1%). The species distribution of the 211 yeast cultures and the antifungal susceptibility data for fluconazole and itraconazole of 137 of the isolates are presented in Table 3. Eighty eight of the 105 (83.8%) patients with negative cultures had previously suffered other vulvovaginal episode, and 86 of them had been treated with local or systemic antifungals, in some cases without mycological confirmation. The same situation occurred in 192/211 (91%) women with Candida growth in culture.
Yeast species isolated from 211 patients and antifungal susceptibility to fluconazole and itraconazole of 137 Candida isolates.
| Yeast species | Number of samples (%) | Number of isolates for antifungal susceptibility testing | Fluconazole (μg/ml) | Itraconazole (μg/ml) | ||
|---|---|---|---|---|---|---|
| Range | MIC50 | Range | MIC50 | |||
| C. albicans | 187 (88.6) | 126 | 0.125–64 | 4 | 0.03–16 | 0.5 |
| C. dubliniensis | 1 (0.5) | 1 | 0.12 | 0.06 | ||
| C. glabrata | 6 (2.8) | 6 | 2–32 | 16 | 0.25–16 | 1 |
| C. parapsilosis | 3 (1.4) | 3 | 32 | 1 | ||
| C. krusei | 1 (0.5) | 1 | 0.5–4 | 0.06–0.12 | ||
| Mixeda | 2 (1) | |||||
| S. cerevisiae | 1 (0.5) | |||||
| Other Candida species | 10 (4.7) | |||||
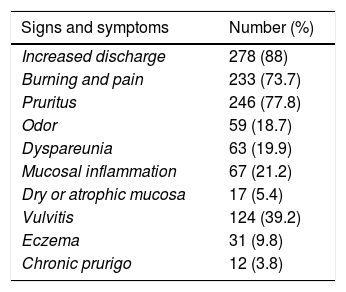
Table 4 summarizes patients’ data including clinical manifestations, time of evolution in the previous vulvovaginal episode, comorbidities and risk factors for Candida vaginitis. The most frequent clinical manifestations were increased discharge, itching, burning, vulvitis and dyspareunia. Almost 90% of the patients had previously received antifungal treatments.
Signs, symptoms and predisposing factors in 316 cases of vulvovaginitis.
| Signs and symptoms | Number (%) |
|---|---|
| Increased discharge | 278 (88) |
| Burning and pain | 233 (73.7) |
| Pruritus | 246 (77.8) |
| Odor | 59 (18.7) |
| Dyspareunia | 63 (19.9) |
| Mucosal inflammation | 67 (21.2) |
| Dry or atrophic mucosa | 17 (5.4) |
| Vulvitis | 124 (39.2) |
| Eczema | 31 (9.8) |
| Chronic prurigo | 12 (3.8) |
| Previous vulvovaginitis (length of the episode) | |
|---|---|
| ≤ 1 year | 133 (42.1) |
| >1 year (up to 20 years) | 183 (57.9) |
| Predisposing factors and comorbidities | |
|---|---|
| Antibiotic treatments | 46 (14.6) |
| Previous antifungal treatments | 278 (88) |
| Cystitis | 26 (8.2) |
| Chlamydia spp. or Ureaplasma spp. infections | 14 (4.4) |
| Viral infections (Herpes, HPV) | 7 (2.2) |
| Contraceptives use and estrogen or infertility treatments | 76 (24.1) |
| Pregnancy | 4 (1.3) |
| Intrauterine device | 6 (1.9) |
| Diabetes | 1 (0.3) |
| History of stress | 9 (2.8) |
| Rheumatoid arthritis | 1 (0.3) |
| Renal lithiasis | 1 (0.3) |
| Hysterectomy | 3 (0.9) |
| Anemia | 5 (1.6) |
| Chronic mucocutaneous candidiasis | 1 (0.3) |
| Corticosteroids use | 4 (1.3) |
Vulvovaginitis is a quite common infection suffered by a high percentage of women around the world. Acute vulvovaginal candidiasis affects about 75% of them at least once in a lifetime. About 5–8% of them suffer four or more episodes per year and in these cases the condition, which is difficult to manage, is called recurrent vulvovaginal candidiasis. In this study 89% of the included women had suffered from several previous episodes of vulvovaginitis that had been considered as vaginal candidiasis and, therefore, they had received local or oral antifungal treatments, with or without microbiological confirmation.
The prevalence of this disease varies according to the microbiological studies conducted. In our study it was found that cultures were positive only in the samples of 52.5% of the patients where the yeasts were observed microscopically. In 48 cases direct examination was negative but yeasts developed in the cultures, hence being difficult to distinguish between colonization and infection since the great majority of women had received previous antifungal treatment. The percentage of positive cultures was 66.8%, and ranges between 11.9% and 72.7% (in complicated and uncomplicated VVC) according to several studies.5,13,14,20–22,25,34,49,50 The presence of some risk factors could explain the differences among those data. For example, different studies suggest that the use of contraceptives,54 pregnancy17,30 and the use of antimicrobials that also increase vaginal yeast colonization22,28,36,43,48,53 may be involved in this pathology. The role of sexual partners seems to be important only in those cases of recurrent vulvovaginal candidiasis.6 Many risk factors are described in a large number of publications4,18,21,27,33,42,43, most of which were found in our study (Table 4).
VVC is more frequent among fertile women, and fertility is one of the main factors influencing the development of VVC according to several reports. In a study carried out in Greece where 1217 women from 10256 (11.9%) suffered from vulvovaginal candidiasis, prevalence for women over 50 years was 12.4%, while this value was 37.5% for younger women.25 In another study the frequency of vulvovaginal candidiasis ranged between 44% (women of 21–30 years) and 18% (41–50 years old).21 However, in some investigations an increase in VVC was observed in postmenopausal women who received hormone replacement or were diabetic, and infection by C. glabrata was more frequent.46 In our study, vaginal candidiasis in women over 45 years represents only 12.3% of the 211 cases. C. glabrata was isolated from patients between 25 and 42 years and from one 50-year-old woman (one case).
In our research, C. albicans was the etiological agent in 88.6% of the cases, and a mixed infection with two Candida species was found in only two patients. C. glabrata was isolated in 2.8% of the cases. This is consistent with the findings of many publications, although several studies have found that the proportion of non-C. albicansCandida infections are higher. There are even studies where C. glabrata was the most frequently isolated species, especially in certain regions such as Lebanon, Egypt or Pakistan, where non-C. albicansCandida vulvovaginitis may exceed that caused by C. albicans.1,17,21,23,24,31,54
Resistance to oral antifungals, mainly fluconazole, which is the most widely used antifungal drug in this pathology, has been increasing over the years, particularly in recurrent VVC.2,7,10,11,13,15,16,23,38,44,47 Numerous resistance mechanisms have been described, the main ones being the over-expression of the CDR1 gene, which would be more common in non-C. albicans Candida species,7 and also the over-expression of CDR2 and MDR1, related to efflux pumps.29,55 Mutations in the ERG3 (encoding Δ5,6 desaturase) or ERG11 (encoding 14-α demethylase) genes, involved in the ergosterol synthesis, have been observed in fluconazole resistant isolates.29 The increase of fluconazole-resistant Candida strains may be due to an inadequate use (low dosage and/or short-term antifungal treatment).26 In recurrent cases resistance appears more frequently. In our study we found an exceedingly high level of resistance to both fluconazole (80.1%) and itraconazole (58.8%) in C. albicans isolates. As previously mentioned, about 90% of the patients had already received treatments with these triazoles at least once.
ConclusionsThis study reinforces the importance of carrying out the microbiological study in each episode of vulvovaginitis since similar signs and symptoms are observed in uncomplicated or complicated vulvovaginal candidiasis, bacterial vaginosis, vaginal dysbacteriosis or mixed infections. Each of them must be treated accordingly to avoid therapeutic failures and increased resistance to the antifungal agents useful in this pathology. In all cases where yeasts are isolated, the species must be identified since some of them may be less susceptible or intrinsically resistant to some antifungals. Susceptibility tests should also be performed due to the great number of resistant isolates in previously treated patients.7
Our study revealed that C. albicans was the most frequently isolated species (88.6%) in both young and postmenopausal women, being C. glabrata the second species in frequency (2.8%). These results are also evidenced in numerous publications. Finally, we would like to highlight the high proportion of cases in which Candida vaginitis was associated with abnormal bacterial biota, the significant percentage of isolates resistant to fluconazole and itraconazole, and the large number of women who received antifungal treatment without microbiological confirmation. These results emphasize the necessity of an adequate diagnosis to reach successful clinical management of any patient and prevent future antifungal resistance.
Conflict of interestThe authors declare no conflict of interest.