Nested PCR can be used to determine the status of Pneumocystis jirovecii infection in other lung diseases.
AimsThis study sought to detect a target DNA fragment (mitochondrial large subunit rRNA or mtL SUrRNA) of P. jirovecii in patients with lung disease who underwent bronchoscopy with collection of bronchoalveolar lavage (BAL).
MethodsThe results from toluidine blue staining were compared with those obtained using molecular methods that included an “in house” DNA extraction procedure, PCR and nested PCR.
ResultsFifty-five BAL samples from patients with atypical chest X-rays were screened for P. jirovecii. None of the samples was positive for P. jirovecii using toluidine blue staining. In contrast, P. jirovecii DNA was detected by nested PCR in BAL samples from 36 of 55 patients (65.5%). The lung diseases in the patients included cancer, pneumonia, tuberculosis, and chronic obstructive pulmonary disease (COPD). Other chronic problems in the patients included hypertension, diabetes, smoking, and alcoholism.
ConclusionsNested PCR showed high sensitivity for detecting P. jirovecii, especially when compared with toluidine blue staining. Using this method, P. jirovecii infection was detected in HIV-negative patients with lung disease.
El diagnóstico de laboratorio mediante la técnica de PCR anidada permite determinar estados de infección por Pneumocystis jirovecii en otras enfermedades pulmonares.
ObjetivosEl objetivo de este estudio fue detectar fragmentos de ADN mitocondrial (mtLSU rRNA) de P. jirovecii en muestras de lavado broncoalveolar (LBA) de pacientes con enfermedades pulmonares, sometidos a broncoscopia.
MétodosSe compara la técnica de coloración con azul de toluidina para la microscopia, con los métodos moleculares PCR y PCR anidada; se realizó una extracción in house de ADN para las reacciones moleculares.
ResultadosLa presencia de P. jirovecii fue estudiada en 55 muestras de LBA de pacientes que presentaron patrones radiográficos de tórax atípicos. Ninguna de las muestras fue positiva para P. jirovecii con la técnica de coloración con azul de toluidina. Por la técnica de PCR anidada se detectó el ADN de P. jirovecii en 36 de los 55 pacientes (65,5%). Las enfermedades pulmonares de los pacientes fueron cáncer, neumonía, tuberculosis y enfermedad pulmonar obstructiva crónica (EPOC). Las otras enfermedades crónicas presentadas por los pacientes fueron hipertensión, diabetes, alcoholismo y tabaquismo.
ConclusionesLa PCR anidada mostró ser altamente sensible en la detección de P. jirovecii en comparación con la coloración por azul de toluidina. Este método permite detectar infecciones por P. jirovecii en pacientes VIH negativos con enfermedades pulmonares.
Pneumocystis jirovecii, formerly known as Pneumocystis carinii f. sp. hominis, was until recently considered a protozoan. In 2002, a new taxonomic classification included P. carinii in the kingdom Fungi, phylum Ascomycota, subphylum Ascomycota. Currently, the Ascomycota is divided into three taxa based on sequence analyses of 18S rDNA and RPB2 genes.3,49 The genus Pneumocystis is included in the taxon Taphrinomycotina (Archiascomycota), class Pneumocystidomycetes, order Pneumocystidales and family Pneumocystidaceae.27,32
Pneumocystis species are obligatorily dwellers in normal mammalian lungs where they generally cause no ill effect, but can become pathogenic when host defenses are compromised. P. jirovecii specifically infects humans.12,57Pneumocystis pneumonia (PCP) continues to be common in patients with HIV infection. However, PCP also occurs in non-HIV immunosuppressed patients, particularly those receiving immunosuppressive agents in the settings of malignancy and organ transplantation.26,33,37,47
The laboratory detection of P. jirovecii can be done using various staining techniques, such as toluidine blue staining to detect P. jirovecii cysts. However, the specificity and sensitivity of these methods are dependent on several factors, such as the quality of the samples, the number of microorganisms present and the training and expertise of the professionals involved in the diagnosis.7,26
Since the 1990s, there have been significant advances in the detection of P. jirovecii with the introduction of molecular methods such as the polymerase chain reaction (PCR).53,54 More than 17 coding and noncoding regions of Pneumocystis DNA are currently used for diagnosis, genotyping, assessment of biodiversity and identification of polymorphisms.6,30
P. jirovecii can be detected in clinical samples by using a combination of various target genes and PCR-related techniques. These genes include dihydropteroate synthase (DHPS), dihydrofolate reductase (DHFR), thymidylate synthase (TS), β-tubulin (β-tub), cell division cycle (cdc) gene family, internal transcribed spacer regions of rRNA (ITS), 5S rRNA, 18S rRNA, major surface glycoprotein (MSG), mitochondrial large subunit rRNA (mtLSUrRNA) and mitochondrial small subunit rRNA (mtSSUrRNA).15,16,45,52
In 1989, Wakefield used the mitochondrial mtLSU rRNA gene locus, a highly conserved region, to develop a simple PCR with the primers pAZ102-H and pAZ102-E. These primers generated fragments of 346bp.54 In addition to PCR, nested PCR has been used to generate fragments of 260bp.51,53 Nested PCR is a highly sensitive and reproducible means of detecting Pneumocystis DNA in biological samples from the respiratory tract, biopsies and environmental samples. In addition to its application in diagnosis, mtLSU rRNA is widely used for genotyping and assessing transmission, as well as for identifying latent infections, subclinical carriers and the reactivation of infection.53,54
PCR and nested PCR have also been used to detect P. jirovecii in subjects with other pathologies, especially lung diseases such as tuberculosis, asthma, cystic fibrosis, chronic obstructive pulmonary disease (COPD) and other types of bacterial pneumonia.16,24,31,47 In addition, P. jirovecii infection has been diagnosed in patients with chronic diseases such as systolic arterial hypertension (SAH), diabetes mellitus (DM), neoplasms, alcoholism and smoking.24,31,47
The detection of P. jirovecii is generally based on samples obtained from the respiratory tract, including induced sputum, bronchoalveolar lavage (BAL) and nasopharyngeal swabs.29 BAL is considered one of the best sources for P. jirovecii detection because it is taken straight from the lung epithelium where the low bacterial population facilitates the finding of P. jirovecii, which often occurs at a low population density.4
The aim of this study was to use BAL samples from bronchoscopy to examine the relationship between lung diseases of various etiologies and the presence of P. jirovecii based on the detection of a target DNA fragment (mtLSU rRNA) by nested PCR.
Materials and methodsClinical specimens and patientsThe specimens used in this study were collected from January to September, 2013. BAL samples were collected from patients >18 years old who underwent bronchoscopy at the university teaching hospital of the State University of Campinas (HC/UNICAMP). The BAL samples were stored in sterile vials at 2–8°C until used for the detection of the target fragment of P. jirovecii by nested PCR and for staining with toluidine blue, a standard technique used in our laboratory.
Sample preparation and staining with toluidine blueBAL samples were treated with 0.1% dithiothreitol, centrifuged and the pellet was used for slide preparation. The staining method was a modification of the toluidine blue procedure described by Gosey19 and Aderaye.2
DNA extractionA 200μl aliquot of the centrifuged material was subjected to genomic DNA extraction using an ‘in house’ technique based on proteinase K digestion and DNA purification with phenol-chloroform, as described by Coutlee.11 The extracted and purified DNA was quantified spectrophotometrically in a Nanodrop™ (Uniscience) spectrophotometer and diluted to 50ng/μl for use in PCR. The quality of the extracted DNA was assessed by using primers (5′-CAA CTT CAT CCA CGT TAC ACC-3′ and 5′-GAA GAG CCA AGG ACA GGT AC-3′) to amplify a 268bp fragment of the human β-globin.
Determination of primer specificityPrimer specificity was detected using primers flanking a conserved region of the P. jirovecii mitochondrial large-subunit (mtLSU) rRNA gene.
Polymerase chain reaction (PCR)Polymerase chain reactions were done for all samples using the external primers pAZ102-H (5′-GATGGCTGTTTCCAAGCCCA-3′) and pAZ102-E (5′-GTGTACGTTGCAAAGTACTC-3′) for the first stage of amplification, resulting in a 346bp fragment, and the internal primers pAZ 102-X (5′-GTGAAATACAAATCGGACTAGG-3′) and pAZ 102-Y (5′-TCACTTAATATTAATTGGGGAGC-3′) for the second stage of amplification, resulting in a 260bp fragment.3,54 Gene amplification by PCR was done essentially as described by Wakefield (1990),53 with some modifications. The PCR mixture (25μl) contained 2μl of DNA (50ng/μl), 50mM KCl, 10mM Tris, 1.5mM MgCl2, 0.20mM dNTPs, 0.4μM of both primers and 1.8U of Taq polymerase. The positive control samples were from patients with HIV/AIDS who developed P. jirovecii-mediated pneumonia, whereas samples from individuals not infected with P. jirovecii and purified water were used as negative controls.
Two microliters of the first PCR product were used for nested PCR. The conditions for the first and second round amplifications were the same and consisted of 40 cycles of denaturation at 94°C for 2min, annealing at 58°C for 1min and extension at 72°C for 7min. After the final amplification, 5μl of each nested PCR product was subjected to electrophoresis on a 1.5% agarose gel containing ethidium bromide and visualized under ultraviolet light.
To avoid cross-contamination, different rooms and areas were used for DNA extraction, PCR amplification and viewing of the agarose gel. Filter tips were used for all pipetting steps. The distilled water used in each PCR mixture was included as a negative control in each series of reactions. The assays were repeated for all samples, under the same conditions, in order to validate the results.
Statistical analysisData analysis and statistical comparisons were done using SAS (Statistical Analysis System) software.48 Possible associations between pulmonary and other diseases were analyzed using the Chi-square test. Fisher's exact test was used for categorical observations. A value of p<0.05 indicated significance.10,17
ResultsFifty-five patients (35M, 20F, 21–83 years old; mean age: 53 years) were enrolled in this study and at least two samples (one for routine test and one for our investigation) were collected from each patient.
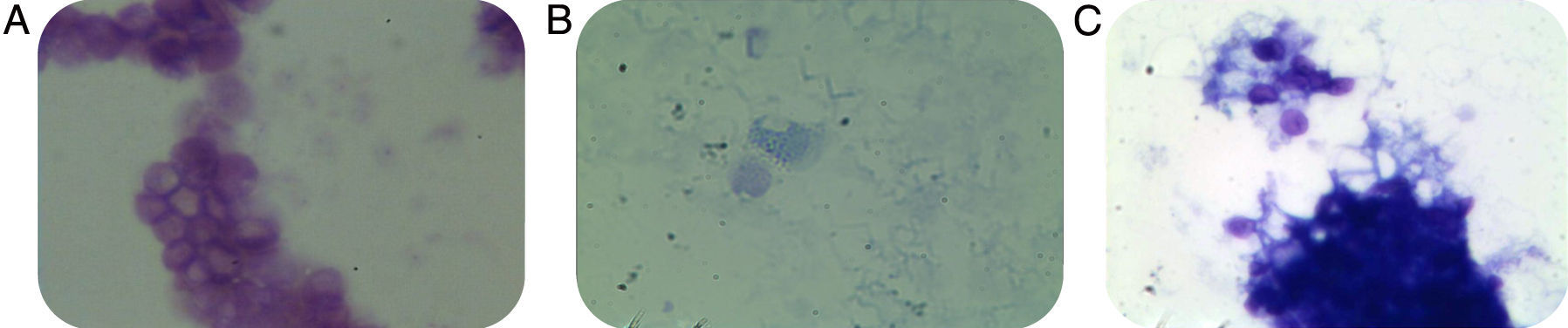
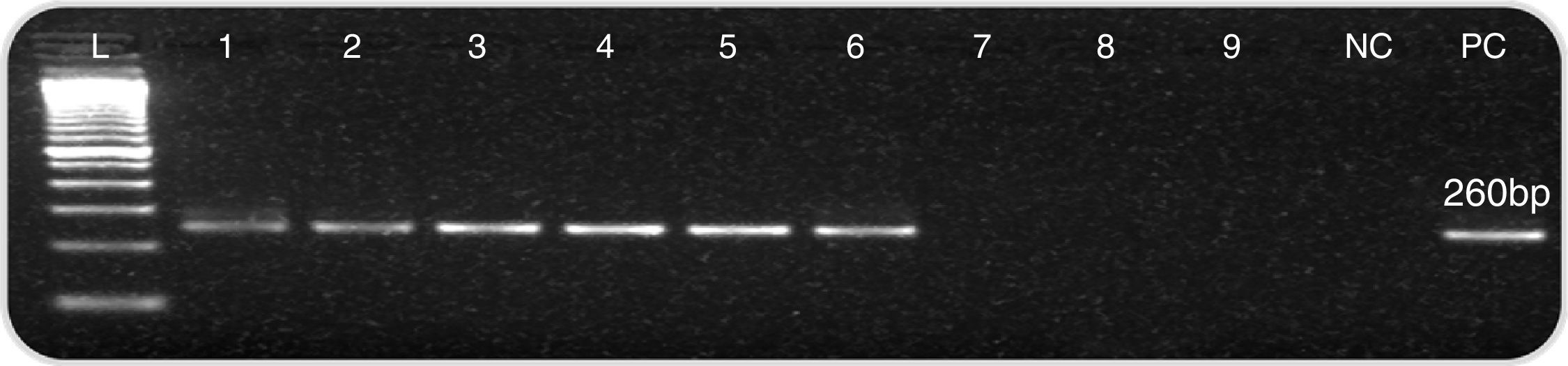
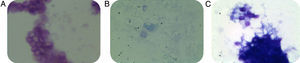
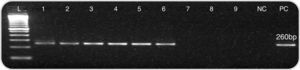
None of the slides stained with toluidine blue was positive for P. jirovecii cysts. All controls showed good performance (C. albicans as a stain control and P. jirovecii cysts as a positive control) (Fig. 1). Two additional specialized mycologists evaluated all slides suspected of containing P. jirovecii cysts. Nested PCR for the mitochondrial DNA fragment showed that 36 of the 55 samples (65.5%) were positive for P. jirovecii; 19 samples (34.5%) were negative (Fig. 2).
Nested PCR screening of BAL samples forP. jirovecii. Electrophoretic analysis of fragments generated by nested PCR resulted in a characteristic 260-bp fragment for positive samples. The samples were run in 1.5% agarose gels stained with ethidium bromide. L: molecular markers (100bp), lanes 1–6: 260bp fragments (samples positive for P. jirovecii), lanes 7–9: no amplification; NC, negative control; and PC, positive control.
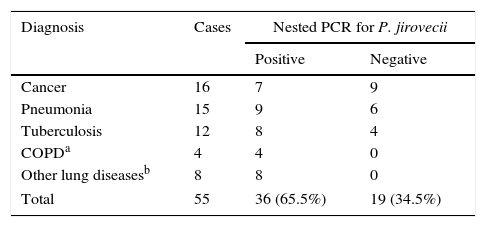
All patients were diagnosed with one or more pulmonary diseases or other chronic disease. The most common pulmonary diseases were lung cancers (n=16), pneumonia (n=15), tuberculosis (TB; n=12) and COPD (n=4). Eight patients had non-pulmonary disease and/or lung damage, such as lung abscess, respiratory failure, Wegener's granulomatosis, pulmonary emphysema, bronchiectasis (two cases), asthma and pleural effusion.
P. jirovecii was detected by nested PCR in four out of four (4/4) samples from COPD patients, in 8/12 patients with tuberculosis, in 9/15 patients with pneumonia and in 7/16 patients with cancer (p>0.05) (Table 1). The cases of lung cancer consisted of non-small cell lung cancer, with 14 cases of adenocarcinoma and two cases of squamous cell carcinoma.
Relationship between lung disease of various etiologies and nested PCR positivity for P. jirovecii in BAL samples.
The 15 cases of pneumonia were caused by wide different pathogens such as Escherichia coli, Klebsiella pneumoniae, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae and Aspergillus niger. There were eight indefinite cases of pneumonia agents, none of which included P. jirovecii.
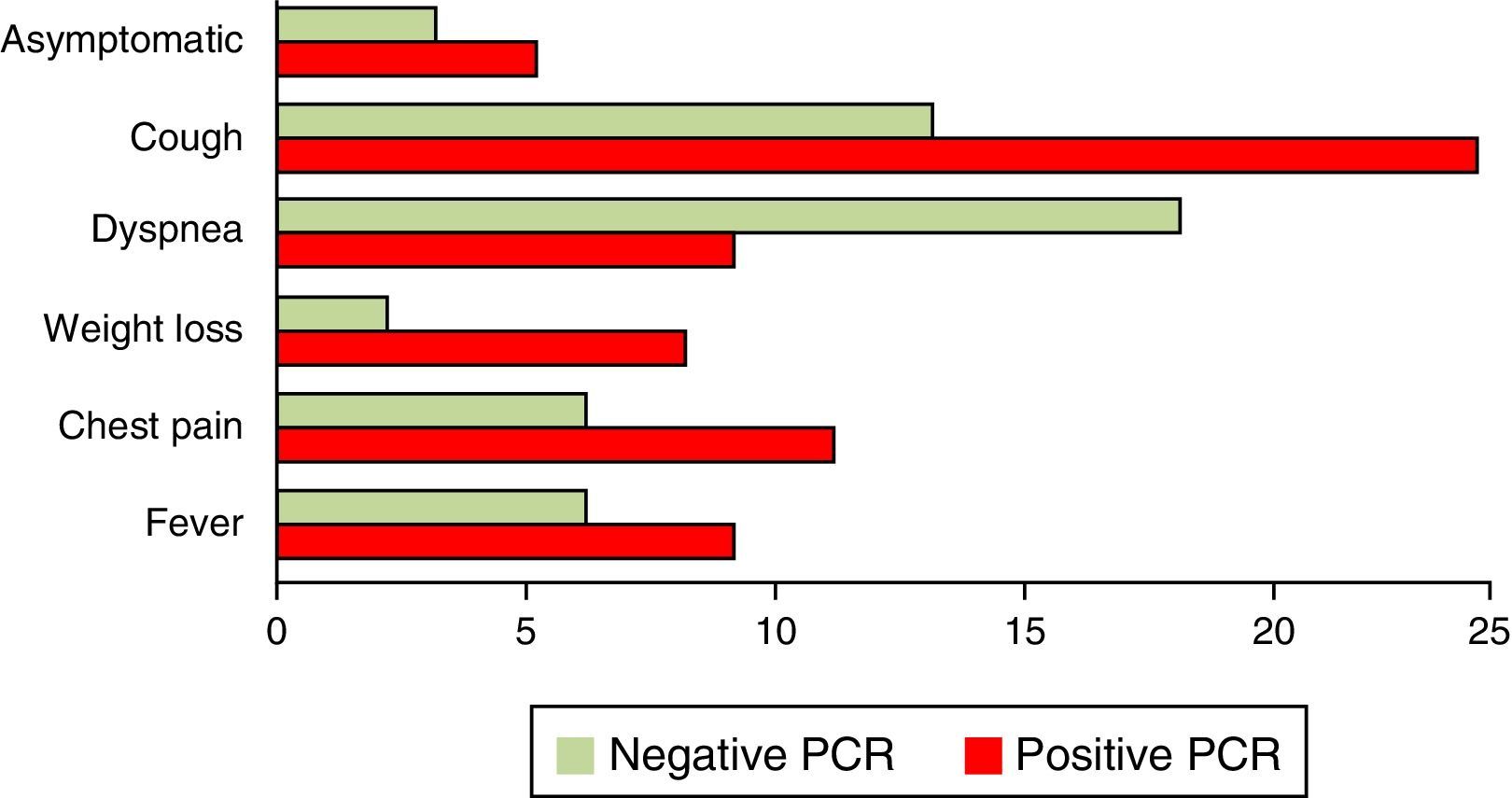
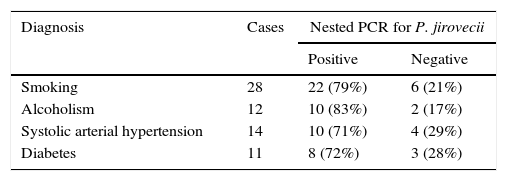
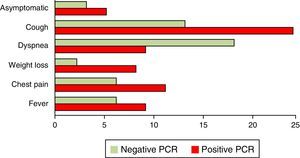
Other chronic diseases present in our series of patients included hypertension, alcoholism and smoking. Except for smoking, which was significantly associated with the occurrence of P. jirovecii (p<0.05), there were no other significant correlations with the presence of this microorganism (p>0.05) (Table 2). The patients had one or more symptoms or no symptoms at all. The most common symptoms were cough, dyspnea, fever, chest pain and weight loss. In 51 patients, the two most common symptoms were dyspnea and coughing (Fig. 3).
Relationship between other chronic diseases and nested PCR positivity for P. jirovecii in BAL samples.
| Diagnosis | Cases | Nested PCR for P. jirovecii | |
|---|---|---|---|
| Positive | Negative | ||
| Smoking | 28 | 22 (79%) | 6 (21%) |
| Alcoholism | 12 | 10 (83%) | 2 (17%) |
| Systolic arterial hypertension | 14 | 10 (71%) | 4 (29%) |
| Diabetes | 11 | 8 (72%) | 3 (28%) |
All patients had image data (radiological or CT scans) with minor changes, the most frequent of these being the presence of pulmonary nodules and opacities. Even without laboratory diagnosis based on toluidine blue staining, six of the 55 patients were diagnosed as having pneumocytosis with clinical signs such as cough, dyspnea, oxygen saturation <92% and X-ray images of interstitial infiltrates; these patients were treated with trimethoprim/sulfamethoxazole (15–20mg/kg/day). In these six patients clinically diagnosed with pneumocystosis the DNA target fragment of P. jirovecii was detected by nested PCR of the corresponding BAL.
DiscussionThe BAL samples from our patients showed a high rate of positivity (65%) for the P. jirovecii DNA mitochondrial fragment. At least three factors influenced this high rate: the quality of the BAL sample, the characteristics of the patients studied and the sensitivity of the technique.20,21,23,29
Although the sampling procedure for BAL is invasive because the tissue is obtained directly from lung epithelium, the resulting sample has a low microbial flora, a characteristic that increases the quality of the sample for staining and molecular tests.25,40,56 Consequently, immediate use of the BAL sample increased the sensitivity of the assay.
Most patients in this study had comorbidities such as smoking, cancer, pneumonia and COPD, with a P. jirovecii infection. These patients are at risk of having an active infection with P. jirovecii, and this could have influenced the high rate of positive samples.6,24,31,39,41,47
There was a significant correlation between smoking and a positive nested PCR for P. jirovecii. This finding agreed with other studies showing that HIV-positive smokers have an increased risk of Pneumocystis-mediated pneumonia.8,35,38,43 For non-HIV populations, the correlation between tobacco use and P. jirovecii infection can be up to 30%.6,9,23,37 This correlation can be explained by the fact that many smokers get lung impairments that can lead to productive cough.13,55 Such individuals can therefore serve as both reservoirs and transmitters of opportunistic pathogens such as P. jirovecii.1
The six patients who were clinically and molecularly diagnosed with PCP were symptomatic for P. jirovecii. However, two patients who were not clinically diagnosed with PCP had symptoms and a nested PCR positive for P. jirovecii. These two cases and others that were not clinically considered to have P. jirovecii pneumonia, even with a positive PCR, had other lung diseases. Because of this, they were not considered to have PCP. For cases in which the diagnosis of pneumonia cannot be completed or confirmed because the symptoms of PCP are extremely similar to other diseases, e.g., tuberculosis, differential diagnoses are needed to distinguish between these diseases.50,58
The tropical climate of Brazil is particularly conducive to a higher incidence of infection by P. jirovecii5,28. A study in Chile, which has a subtropical climate, revealed a high incidence of Pneumocystis, with 65% of cases being positive; this incidence is similar to the 65.5% reported here and suggests a possible relationship to the regional incidence of this fungus.50,44
Since the main form of dissemination for P. jirovecii is via the airways, nosocomial transmission may have been an important factor in the spread of this pathogen among our patients and could have contributed to the high rate of positivity seen here.12,18,31 Some reports have shown that active PCP patients can transmit the pathogen more than a meter from their bed, leading to a significant increase in transmission in hospital settings. This observation suggests that individuals who attend hospital or remain close to patients with PCP can be infected with P. jirovecii.31,37,57
The high incidence of P. jirovecii can also be explained by the method of detection used. Nested PCR is a highly sensitive and specific procedure that allows the diagnosis of patients with a large number of microorganisms, as well as individuals who possess a small number of P. jirovecii without PCP, as in a latent infection.20,22,34,37 Even with a latent infection, individuals colonized by P. jirovecii and who develop some form of immunosuppression or comorbidities are at high risk of P. jirovecii proliferation. Thus, the nested PCR result can assist in prescribing prophylactic treatment for such patients.15,34,37
Patients in our study who were not diagnosed with PCP pneumonia, but had a nested PCR positive for P. jirovecii, can be classified as colonized.37 A study using nested PCR to screen BAL fluid samples from immunocompetent individuals showed that 19% of these individuals had P. jirovecii but with no apparent lung damage.20 In this case, P. jirovecii may reside in the lung epithelium without causing pneumonia or other damage, possibly as a colonizing agent in its latent form.14,15,36,37
A negative PCR result for P. jirovecii in the case of pulmonary infection may be related to prophylactic treatment with sulfamidic acid. These drugs are administered in primary prophylaxis against P. jirovecii.21 Once a patient has been treated with sulfamidic acid the nested PCR is likely to be negative because P. jirovecii is sensitive to this drug.37
A negative result for staining with toluidine blue might be related to the technique used. Staining with toluidine blue is specific for the cystic forms of P. jirovecii, meaning that trophozoite forms cannot be visualized by this procedure; this implies that other forms of the fungus that cannot be seen with toluidine blue may also be present on negative slides.2,19 Thus, since the toluidine blue technique is restricted to the identification of the cystic form of the fungus, the successful diagnosis of PCP requires the evaluation of the patient's clinical signs and symptoms, radiological and laboratory tests and the results of toluidine blue staining.2,19,46
The lack of reports in which PCR has been used to diagnose P. jirovecii infections in Campinas (southeastern Brazil) means that there is no local reference to compare with the present findings. However, studies from southern Brazilian subjects without HIV infection but with lung disease, such as cystic fibrosis, indicate that P. jirovecii is present in 38.2% of these individuals.41 A recent study with HIV-positive patients reported colonization by P. jirovecii in 49% of individuals, suggesting that the HIV-infected population is a major reservoir and source of P. jirovecii infection.42
As already demonstrated in other studies, nested PCR is an important tool for the detection of P. jirovecii infection. The results of our study indicated the presence of P. jirovecii-associated primary risk factors and comorbidities in the patients that we examined. When there is a positive result for nested PCR in the diagnosis of pneumocystosis, we recommend this result be interpreted in conjunction with the patient's clinical status.
Conflict of interestThe authors declare no conflict of interest.
The authors thank Diego Muraca, Stephen Hyslop and Jacqueline Campalans Barnier for editing the English and Spanish of the manuscript, and Paulo Fanti de Oliveira of the Statistical Support section of the Faculty of Medical Sciences/UNICAMP, for help with the data analysis. The authors also thank the reviewers and editor for their constructive comments that helped to improve the quality of the manuscript.