Treatment with amphotericin B is highly effective in histoplasmosis. Caspofungin has shown good activity against Candida and Aspergillus spp. In vitro studies have demonstrated that Histoplasma capsulatum is inhibited by caspofungin.
ObjectivesThe purpose of this study was to evaluate the effectiveness of caspofungin in the treatment of histoplasmosis in an animal experimental model.
MethodsThree strains of Histoplasma capsulatum var. capsulatum were used. Treatment started one week post-inoculation and the animals were randomly assigned to six groups: amphotericin B 6mg/Kg/d, caspofungin 2mg/Kg/d, 4mg/Kg/d, 8mg/Kg/d and the other two groups received saline solution and dextrose solution. Blood samples for culture were obtained once a week, from day 7 to 35 post-inoculation. One week after the end of the treatment the animals were sacrificed and spleen cultures were performed.
ResultsBlood cultures were negative in all the hamsters which received amphotericin B (100%, P<0.001); those treated with caspofungin and the control animals presented 30 and 32% of positive cultures respectively (P=0.59). Spleen cultures were negative in the animals treated with amphotericin B, while the percentage of positive spleen cultures in the caspofungin groups varied from 25 to 100%, and in the control groups from 35 to 94.8% (P=0.07). The statistical analysis of the undiluted cultures showed the use of amphotericin B as the only independent predictor of negative culture (P<0.001).
ConclusionsThe efficacy of amphotericin B is well known for the treatment of histoplasmosis, though we could not demonstrate that caspofungin is better than control.
La anfotericina B es un fármaco efectivo para el tratamiento de la histoplasmosis. La caspofungina es activa contra especies de Candida y Aspergillus. Estudios in vitro han demostrado el efecto inhibitorio de este fármaco en Histoplasma capsulatum.
ObjetivoEvaluar la eficacia de la caspofungina comparada con la anfotericina B para el tratamiento de histoplasmosis en un modelo experimental en hámster.
MétodosSe utilizaron tres cepas de Histoplasma capsulatum var. capsulatum. El tratamiento comenzó una semana tras la inoculación y los animales fueron distribuidos aleatoriamente en 6 grupos, según los fármacos utilizados: anfotericina B 6mg/kg/día, caspofungina en dosis de 2, 4 y 8mg/kg/día, solución fisiológica y dextrosa al 5%. Se tomaron muestras semanales para hemocultivos a partir del séptimo día postinfección y a los 7 días de finalizado el tratamiento los animales fueron sacrificados, realizándose cultivos del bazo.
ResultadosLos resultados obtenidos de los hemocultivos realizados al finalizar el tratamiento fueron negativos en un 100% en el grupo tratado con anfotericina B, frente al 30% en el grupo tratado con caspofungina y el 32% en el grupo control (p=0,59). Los cultivos de bazo fueron negativos en el grupo de anfotericina B, mientras que en el grupo de caspofungina los valores fluctuaron entre el 25 y el 100%, y en el grupo control entre el 35 y el 94,8% (p=0,07). En el análisis estadístico del cultivo de la suspensión de bazo sin diluir el uso de anfotericina B fue el único predictor independiente de cultivos negativos (p=0,001).
ConclusionesLa anfotericina B es un fármaco efectivo para el tratamiento de la histoplasmosis. La administración de caspofungina no demostró mayor eficacia en comparación con el grupo control.
Histoplasmosis is an endemic systemic mycosis with high prevalence in Argentina. The development of progressive disseminated histoplasmosis depends on the host immunity, especially immunosuppressed patients who are unable to develop effective cell-mediated immunity. This includes patients with AIDS, transplant recipients, patients with hematological malignancies and those treated with corticosteroids.6 In Argentina, it is the third fungal disease in AIDS patients. Amphotericin B and itraconazole are highly effective, with response rates of 88%.15 However, amphotericin B is toxic and itraconazole has a variety of drug interactions and an erratic bioavailability. Therefore, it is important to explore new classes of drugs against histoplasmosis.
Caspofungin is a semi-synthetic derivate of pneumocandin B, a product of Glarea lozoyensis. This drug targets the cell wall, blocking the synthesis of β(1,3)-D-glucan by non-competitive inhibition of the enzyme β(1,3)-D-glucan synthase.8,13 Caspofungin has shown good activity against Candida and Aspergillus spp. In vitro studies have demonstrated that Histoplasma capsulatum is inhibited by caspofungin. This drug offers an alternative to the azoles and avoids amphotericin B-associated toxicity.4,8,13
The purpose of this study was to evaluate the effectiveness of caspofungin in the treatment of histoplasmosis in an animal experimental model.
Materials and methodsPathogenThree strains of H. capsulatum var. capsulatum were used: they were named “hamster”, “non-AIDS” and “AIDS”, belonging to the Mycology Center Collection. The “hamster” strain is currently used as a standard strain for in vivo sensitivity with antifungal agents.3 The other strains of recent isolation were derived from patients with disseminated histoplasmosis. We inoculated the strains in animals to establish the persistence of pathogenicity. The yeast obtained in this procedure was used for the inoculation.
Animals and inoculation techniqueA total of 360 hamsters (Mesocricetus auratus) of both sexes, weighting approximately 100g, were used. They were anesthetized and inoculated intracardially with 0.1ml of a suspension containing 1×107 living cells/ml of the yeast phase, in isotonic saline solution. The viability of yeast phase cells was controlled by culturing the inocula in dextrose agar broth (Biokar Diagnostics, Francia) at 37°C. The animals were maintained according to the Guide for the Care and Use of Laboratory Animals of the National Research Council.5
Drug preparationAmphotericin B (Sigma-Aldrich) was dissolved in dextrose solution at a final concentration of 2.5mg/ml. Caspofungin (Merck Sharp & Dohme) was prepared in isotonic saline solution at a concentration of 4mg/ml, and then diluted to be used in three different doses: 2, 4 and 8mg/kg of body weight. Caspofungin doses were based on toxicity and pharmacokinetic data from mice of previous reports on caspofungin.7
Treatment scheduleThe antifungal treatment started 7 days post-inoculation and was carried out for 3 weeks by intraperitoneal route once a day. The drugs used were amphotericin B 6mg/kg/d at a volume of 0.25ml, caspofungin 2, 4 and 8mg/kg/d at a volume of 0.2ml. Control animals received drug solvents at a volume of 0.2ml (saline and dextrose solution).
Animals were randomly assigned to three groups of 120 hamsters each. The “hamster” strain was used in the first group, the “non-AIDS” strain in the second and the “AIDS” strain in the third. Each group was randomly assigned to six subgroups of 20 animals each, according to the different doses and drugs used.
Treatment evaluationThe experimental mycosis outcome was controlled once a week by blood culture. Blood samples for culture were obtained at days 7-14-21-28 and 35 post-infection. A volume of 0.2ml of blood was cultured in Sabouraud dextrose agar and incubated at 28°C for 30 days, according to previous experiments.2
One week after the end of treatment (35 days), the animals were sacrificed and autopsy was performed. Response to therapy was determined by quantifying the viable yeasts. Homogeneous suspensions of the spleen in distilled water were prepared in sterile mortars, at a concentration of 100mg/ml. For CFU determination, 0.1ml of 1/10 and 1/100 dilutions of this original suspension were cultured in Petri dishes containing Sabouraud dextrose agar. The plates were incubated at 28°C for four weeks and growth was controlled weekly.
Undiluted suspension of the spleen was performed in the same culture medium and temperature.
Statistical analysisBlood cultures were analyzed as contingency tables by means of the χ2 test. ANOVA was performed for CFU comparison.9 Since the variable for the amphotericin B group was always zero, this group was excluded. Additionally, a log transformation was used to reach the normality of the variable in each group assumption. For statistical analysis of the undiluted cultures, a stepwise backwards logistic regression was implemented with a breakpoint of 0.05 as the probability to exist. The presence or absence of microorganisms in massive spleen cultures was used as the predicted variable. Dummies were created, indicating the use of each of the six treatment variants (amphotericin B, caspofungin at three different concentrations, isotonic saline solution and dextrose solution), and the three inoculated strains (“hamster”, “non-AIDS” and “AIDS”) as the predictor variable. The “R” 2.5.1 software Free Software Foundation, GNU General Public License was used for statistical analysis.16
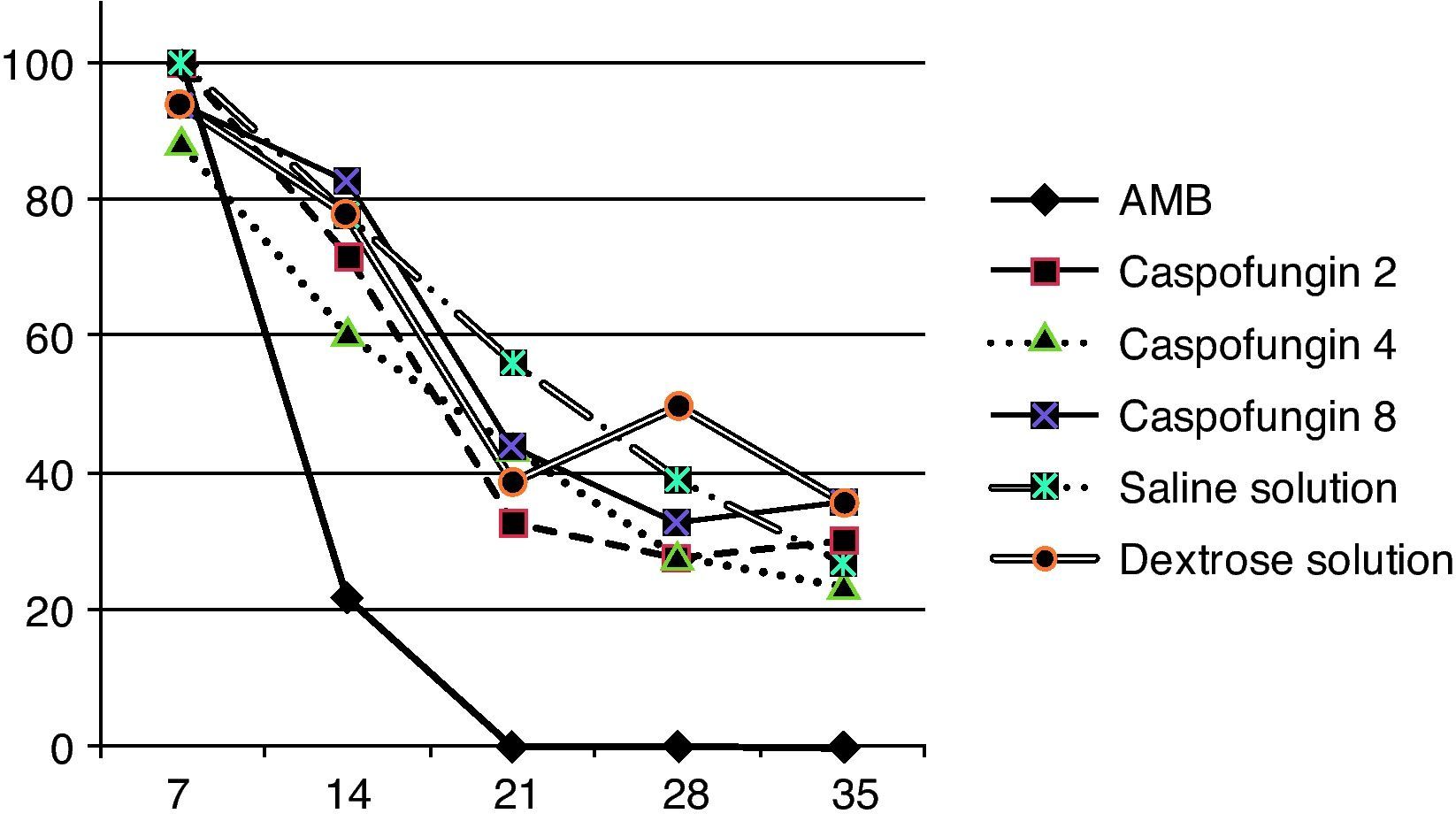
ResultsAll the animals of the amphotericin B group presented negative blood culture and exhibited an associated P value <0.001 compared with caspofungin group. A persistent fungemia was detected at day 35 in the other groups: 30% of the caspofungin group (29% caspofungin 2, 24% caspofungin 4, and 36% caspofungin 8) and 32% of the control group (36% dextrose solution and 27% isotonic saline solution), without significant differences among these results (P=0.59) (fig. 1).
Percentage of positive blood cultures during treatment of the three strains together because they showed a similar curve pattern. Seven days post-inoculation, 90-100% of the cultures were positive. The AMB group (amphotericin B) showed a significant reduction in positive cultures after 2 weeks of treatment (P<0.001). In the other groups, at day 35 between 24.1 and 36.2% were still positive.
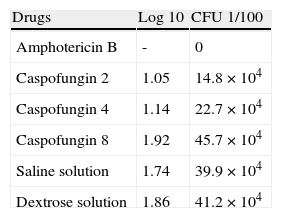
Variables indicating CFU counts were explored. In the amphotericin B group, all the spleen cultures were negative. Table 1 shows the means of log and CFU for each group. Between caspofungin and control groups we found an associated P=0.071.
Quantitative culture results from hamsters’ spleen at day 35.
| Drugs | Log 10 | CFU 1/100 |
| Amphotericin B | - | 0 |
| Caspofungin 2 | 1.05 | 14.8×104 |
| Caspofungin 4 | 1.14 | 22.7×104 |
| Caspofungin 8 | 1.92 | 45.7×104 |
| Saline solution | 1.74 | 39.9×104 |
| Dextrose solution | 1.86 | 41.2×104 |
Values were expressed in CFU and their log transformation. In the amphotericin B group, all the cultures were negative.
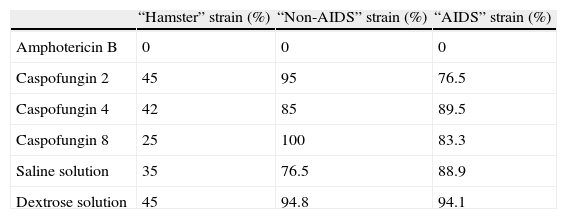
In the statistical analysis of the undiluted cultures, the use of amphotericin B and the inoculation with the “hamster” strain was found to be the only independent predictors of negative culture. This model was validated by means of the difference between the deviance obtained for the model with the variables (280.23) and the deviance of the model with the constant alone (463.86). The P value of the difference was < 0.001. The estimated odds ratios were 0.01 (95% CI: 0.00-0.03) for the amphotericin group and 0.08 (95% CI: 0.04-0.15) for the “hamster” strain. The results obtained from each strain are shown in Table 2.
Cultures of homogeneous suspension of spleen. Percentage of positive cultures from different strains and drugs doses used and control.
| “Hamster” strain (%) | “Non-AIDS” strain (%) | “AIDS” strain (%) | |
| Amphotericin B | 0 | 0 | 0 |
| Caspofungin 2 | 45 | 95 | 76.5 |
| Caspofungin 4 | 42 | 85 | 89.5 |
| Caspofungin 8 | 25 | 100 | 83.3 |
| Saline solution | 35 | 76.5 | 88.9 |
| Dextrose solution | 45 | 94.8 | 94.1 |
This study was created to establish an animal model which resembles human progressive disseminated histoplasmosis. Our previous experimental studies demonstrated that the hamster is a very suceptible host as well as an excellent animal model for the evaluation of antifungal drugs in experimental histoplasmosis.2,3
In the amphotericin B group, blood cultures at day 14 after treatment and spleen cultures at the end of the experiment were negative, showing a significant statistical difference associated to the use of amphotericin B. All blood cultures in the amphotericin B group turned negative and this result correlates well with the other parameters taken into account for therapeutic evaluation, as observed elsewhere.2 We could not find any significant differences between caspofungin and control groups regarding the positive blood cultures. Steve Kohler et al.7 compared caspofungin vs. amphotericin B for the treatment of pulmonary histoplasmosis in a murine model showing a limited efficacy of caspofungin, since the group treated with this drug did not prolong survival and lowered the quantitative colony counts only 0.5 log with no significant differences. Contradictory results were found by John Graybill et al.4 in an experimental model using immunocompetent and immunodeficient BALB/c mice, comparing caspofungin vs. control (water), and showed that the caspofungin group had a prolonged survival and a significant reduction of CFU of liver and spleen. These findings are in agreement with the results described by Gabriela Rodriguez Arellanes et al.,12 showing an important fungal clearance when using caspofungin monotherapy vs. amphotericin B.
The analysis of the CFU (log) showed that the values in the caspofungin 2 and 4mg/kg was lower than the one in the caspofungin 8mg/kg and control groups, with no significant differences. David van Duin et al. demonstrated that melanized cells of H. capsulatum were significantly less susceptible to caspofungin than non-melanized cells.14 The same authors found significant differences in rates of survival at concentration of 2 and 4μg/ml of caspofungin for the two strains tested. In addition, the results of the study conducted by Joshua Nosanchuk et al. indicate that H. capsulatum yeast cells synthesize melanin in vitro and during mammalian infection.10
Comparing the results obtained from the three strains, we observed that the percentage of spleen cultures in the “hamster” strain was lower than in the other groups, showing a significant difference. In the “AIDS” and “non-AIDS” strains, the percentage of the cultures was significantly high. One possible explanation is that it may be associated with different virulence factors, as demonstrated elsewhere.1,11
FinancingSupported by a research grant from the U.S. Investigator-Initiated Studies Program of Merck & Co., Inc. to D.P.K.
Conflict of interestJorge Finquelievich was awarded with a grant provided by Merck & Co. and has received speaker fees.
The authors express their gratitude to Eliana Cabali for her work as a laboratory animal technician, Santiago Pola as a laboratory technician of the Mycology Center and Francisco Fernández for statistical analysis. In addition, we thank Prof. Ricardo Negroni for his assistance in reviewing this manuscript.