Oospores are the most persistent propagules of Phytophthora capsici. The viability of oospores is determined by staining methods such as the tetrazolium bromide (MTT) test.
ObjectivesThe aim was to assess the MTT test and a plasmolysis method for their utility in determining viability of oospores of P. capsici.
MethodsFor either procedure the reactions of non-treated (viable) and lethally heat-treated (non-viable) oospores were assessed and compared with different matings of isolates.
ResultsThe plasmolysis method provided lower estimates of oospore viability relative to MTT in almost all cases. Viability of autoclaved oospores determined by MTT ranged from 26 to 35%, while in the plasmolysis method, the false positive rate was null. Data of non-treated (viable) oospores determined by both techniques showed a significant positive correlation (r=0.998, P=0.0001). Viability assessments in the plasmolysis method of heated oospores in sterile water at 52°C (0-2-4-20hours) showed viability values of 81-4-0.7-0% while MTT values were 78-15.3-9-5.7% respectively. They both exhibited different values for the other tested mating (57.7-1-1-0% and 50-7.3-7.7-9% respectively). Data of autoclaved, heat-treated (52°C), and non-treated oospores determined by both techniques showed a significant positive correlation (r=0.946, P<0.0001). However, MTT always overestimated viability rates when values were near zero.
ConclusionsMTT staining was non-objective, unstable and with a high rate of false positives. In contrast, reliable results were obtained using the plasmolysis method. MTT should be used in combination with other techniques such as the plasmolysis method for determining viability of P. capsici oospores.
Las oosporas son los propágulos más persistentes de Phytophtora capsici. La viabilidad de las oosporas se puede determinar con métodos de tinción como el bromuro de tetrazolio (MTT).
ObjetivosEl MTT, y un método de plasmólisis, fueron comparados en su utilidad para determinar la viabilidad de las oosporas de P. capsici.
MétodosEn cada procedimiento se compararon las reacciones de oosporas no tratadas (viables) y tratadas con calor letal (no viables) en diferentes cruces de aislados.
ResultadosLa plasmólisis proporcionó viabilidades menores que el MTT en casi todos los casos. La viabilidad estimada con MTT en oosporas autoclavadas osciló entre 26 y 35%, siendo nula la falsa positividad con plasmólisis. Los resultados determinados con ambas técnicas se correlacionaron significativamente (r=0,998, P=0,0001) en oosporas no tratadas (viables). La viabilidad de oosporas tratadas con calor en agua estéril a 52°C (0-2-4-20 horas) en uno de los cruces ensayado mediante plasmólisis fue 81-4-0,7-0% y mediante MTT fue 78-15,3-9-5,7% respectivamente, mostrando diferentes resultados en el otro cruce ensayado (57,7-1-1-0 y 50-7,3-7,7-9% respectivamente). Los resultados determinados con ambas técnicas en el conjunto de oosporas (autoclavadas, calentadas 52°C y no tratadas) mostraron una correlación positiva significativa (r=0,946, P<0,0001), aunque el MTT sobreestimó la viabilidad cuando los valores se aproximaban a 0.
ConclusionesEl MTT resultó una prueba no objetiva, inestable y con alta proporción de falsos positivos al compararla con la plasmólisis en la que se obtuvieron resultados fiables. El MTT debería usarse junto a otras técnicas como la plasmólisis para determinar la viabilidad de las oosporas de P. capsici.
Phytophthora capsici Leon. is the causal organism of an important pepper (Capsicum annuum L.) crop disease worldwide. This major pathogen is able to attack all plant organs. Depending on the cropping system and the climate of each region, it can produce different syndromes of disease, including: collar rot, root rot, foliar blight, stem blight and fruit rot. The collar and root rot phase of the disease is ussually important in greenhouse cultivation with drip irrigation in the Mediterranean regions. The aerial blight phases are characteristic of outdoor cultivation during periods of heavy rainfall in the tropics. This oomycete is formed by vegetative propagules such as mycelium, sporangia and zoospores (rarely chlamydospores), and sexual resistant spores such as oospores. While vegetative propagules are the agents causing multicyclic development and reproduction of the disease during the cycle of the crop, oospores appear to be responsible for the disease persistence when the crop is not present. Oospores also contribute to the potential adaptability of the species to the environment due to the potential genetic variation resulting from meiosis7.
Consequently, oospore survival evaluation tools are of essential to phytopathological investigations. However, the germination as an absolute measure of oospore viability is complicated for most of the species of Phytophthora due to the dormancy of the oospores. This is particularly relevant in the case of heterothallic Phytophthora species. In the case of P. capsici (heterothallic), germination percentages for oospores are very low, ranging from 0 to 51%1,10,17,20,22.
In addition, bioassays are not the most suitable methods for viability evaluation because they are time-consuming and not provide quantitative measures. Therefore, it is recommended to use other techniques to determine the viability of oospores.
As of today, several staining procedures have been used. Staining with tetrazolic compounds has been a technique used by several authors to evaluate the viability of oospores. Tetrazolium bromide (MTT) has been used as a vital stain of oospores in different studies2,5,12,19,21,24.
In addition to staining techniques, plasmolysis methods have also been used with successful results8,12,18.
The main aim of our study was to assess the suitability of the MTT test for the determination of the viability of Phytophthora capsici oospores and its comparison to a plasmolysis method. For this purpose, the influence of factors inherent to the staining such as incubation time in MTT or elapsed interval time between microscopic mounting and observation were analysed. Previous conditions to the staining such as oospores permanence in different preservation media at several temperatures, as well as other variables (age or isolate) were also taken into account. Moreover, oospore viability was compared using the MTT test and a plasmolysis method with viable (non treated), and non viable (autoclaved or heat treated) oospores of different isolates.
Material and methodsProduction of oospores and fixation in nylon meshThree P. capsici isolates of the A1 compatibility type from Spain (98/T3-12-6 from Bizkaia greenhouse soil; 152/95 from Bizkaia pepper plant; 02/155 from Zaragoza pepper plant) were crossed with a P. capsici isolate of the A2 compatibility type from France (59 from the INRA collection). A1 and A2 isolates were paired in Petri dishes (90-mm diameter) containing 15ml of V8-juice broth (without agar). The plates were sealed with parafilm and incubated in darkness at 25°C until they were used (3, 4, 5, 6, 7, 10, 13 and 14 weeks old). Oospores were extracted by blending the V8 liquid culture in 100ml of deionized water. This suspension was filtered through a 100μm nylon mesh and washed with deionized water. Sporangia and mycelium propagules remained on the filter and oospores were recovered from the filtrate. Afterwards, the filtrated suspension was collected and refiltrated by vacuum through 25μm nylon mesh, cut into circles, and adjusted to a Buchner funnel. Consequently, oospores with a diameter size nearly the size of the mesh pores were embedded in the interstices, smaller ones passed through the mesh and larger ones were rinsed from the surface with deionized water13. The nylon mesh containing oospores was cut into squares of about one cm2 and were immediately used in the corresponding experiments. In each experiment, three mesh squares per treatment were used and 100 oospores were counted in each mesh square. The exposed data, expressed as a percentage, were the average of three counts of 100 oospores with their corresponding standard errors.
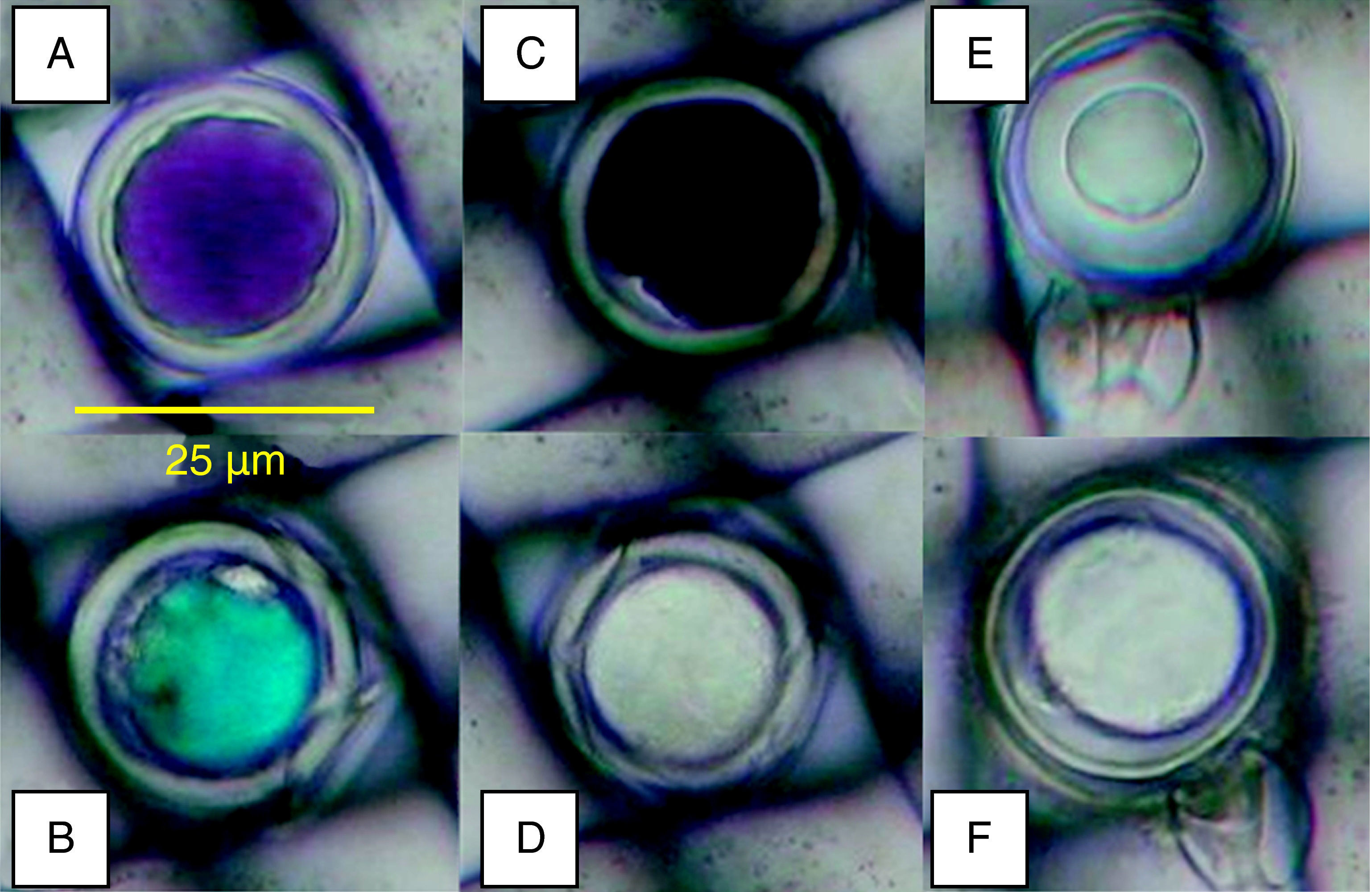
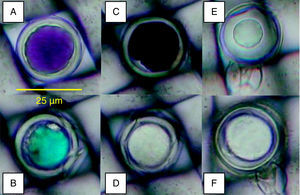
Oospores viability testsTetrazolium bromideTetrazolium compounds are reduced by the hydrogen enzymatic transport system to form an insoluble compound called formazan which stains living cells16. To evaluate the colours and viability of oospores by the MTT test, oospores embedded in mesh squares were introduced in 0.1% tetrazolium bromide (Sigma-Aldrich) in 1 m mol l−1 potassium phosphate buffer (pH=6.3) solution12 and were incubated for 24hours at 35°C. After the incubation period, the mesh squares were rinsed with deionized water and microscopically (Olympus CH40, x200) examined. Oospores were classified as red-rose, blue, black or unstained (fig. 1A-D). Oospores that stained red to rose were considered to be viable dormant, blue were viable activated (pre-germination phase), and black and unstained non-viable6.
Oospores were embedded in 25-μm-pore nylon mesh. Staining colours (A: red to rose; B: blue; C: black; D: unstained) obtained with the tetrazolium bromide (MTT) test (24h, 35°C, 0.1% MTT solution) and viable (E: plasmolized) or unviable (F: non-plasmolized) oospores with the plasmolysis method (45minutes, 4mol l−1 sodium chloride solution).
Mesh squares were introduced in 4mol l−1 sodium chloride solution for about 45min and microscopically (x200) observed. The cytoplasm of the viable oospores was contracted to form a central ball-like structure and non viable ones did not plasmolize as they had lost the differential permeability of their cellular membrane12. Oospores were classified as viable (plasmolized) and unviable (non-plasmolized) (fig. 1E-F).
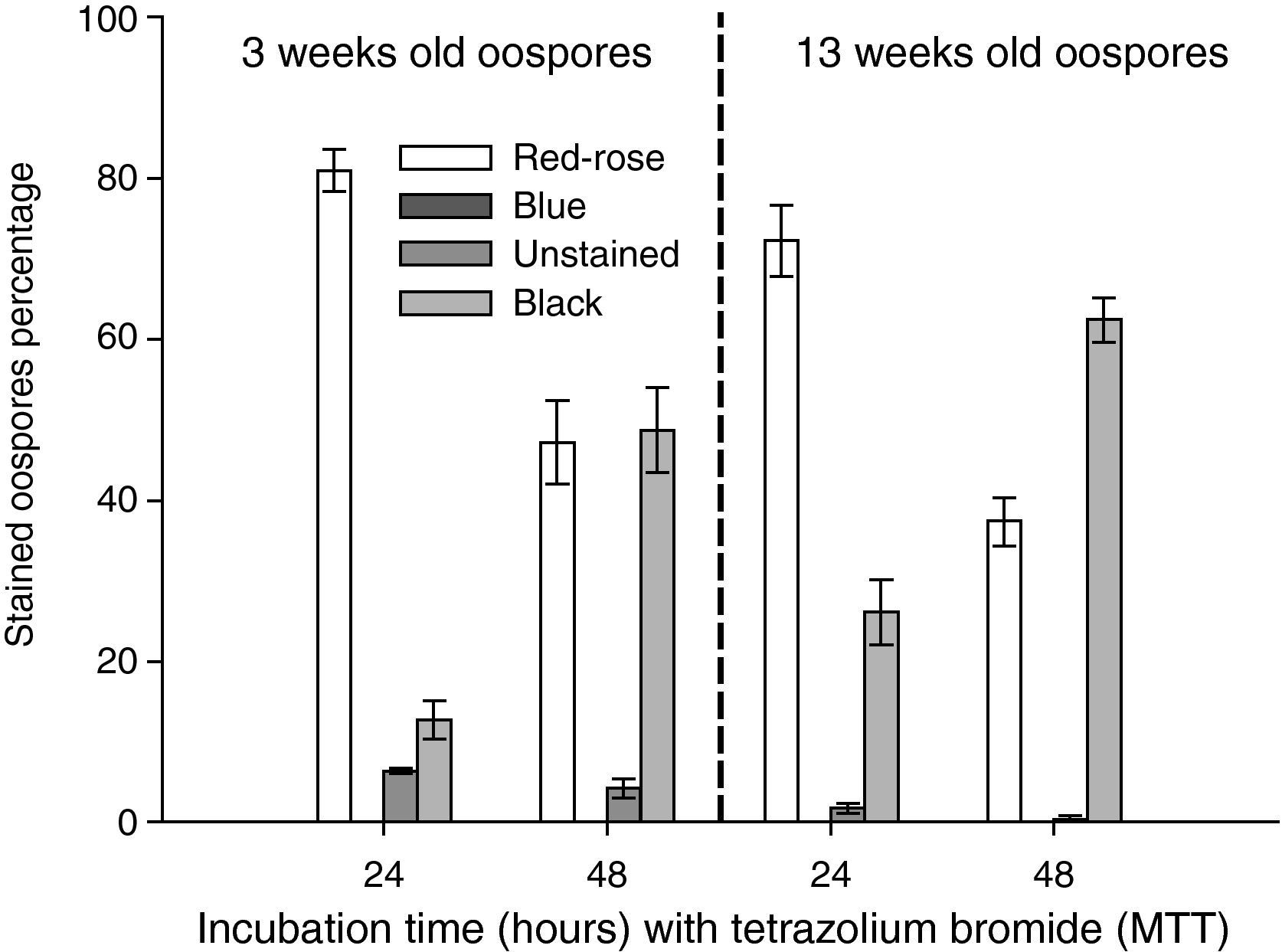
Instability of MTT testThe instability of the MTT staining technique was evaluated in different experiments that analyzed the effects on oospores staining of some factors combinations, such as: a) incubation time in MTT solution (24 and 48hours) and oospores age (3 and 13 weeks old) (fig. 2); b) elapsed time interval between microscopic mounting and observation (0, 24 and 144hours) and oospores age (3 and 13 weeks old) (fig. 3); c) preservation media (sterile moistened soil [22.5% v/v] or sterile water) and exposure time (6, 12 and 24hours) (fig. 4); d) temperature (25 and 85°C) and oospores age (4, 7 and 14 weeks old) (fig. 5).
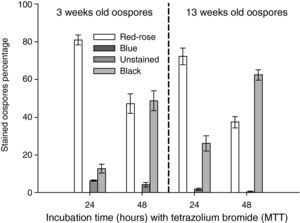
Effect of incubation time (24 or 48hours) at 35°C with 0.1% tetrazolium bromide (MTT) solution on the staining of oospores of different ages (3 or 13 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red–rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.
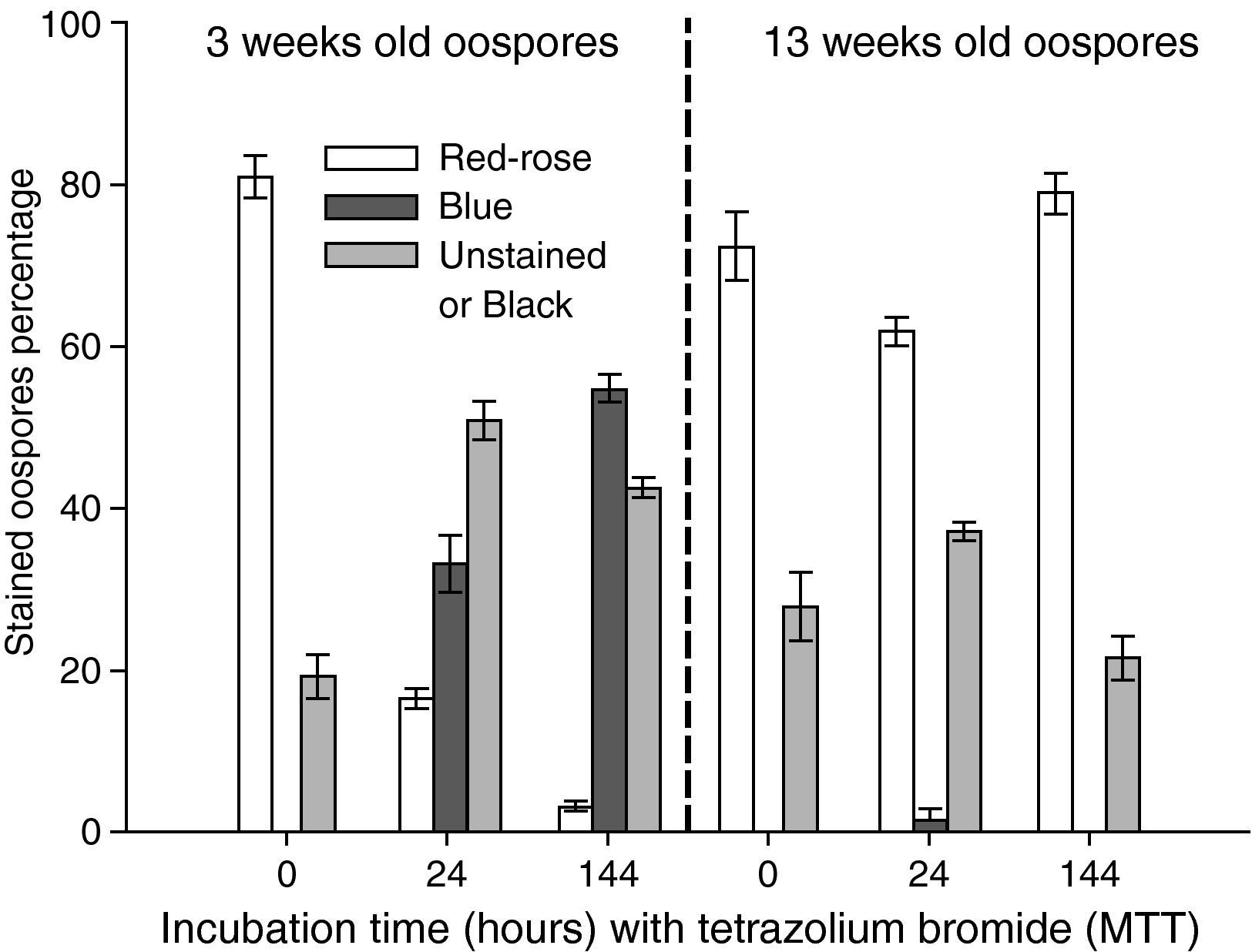
Effect of elapsed time interval between microscopic mounting and observation (0, 24 or 144hours) on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (3 or 13 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.
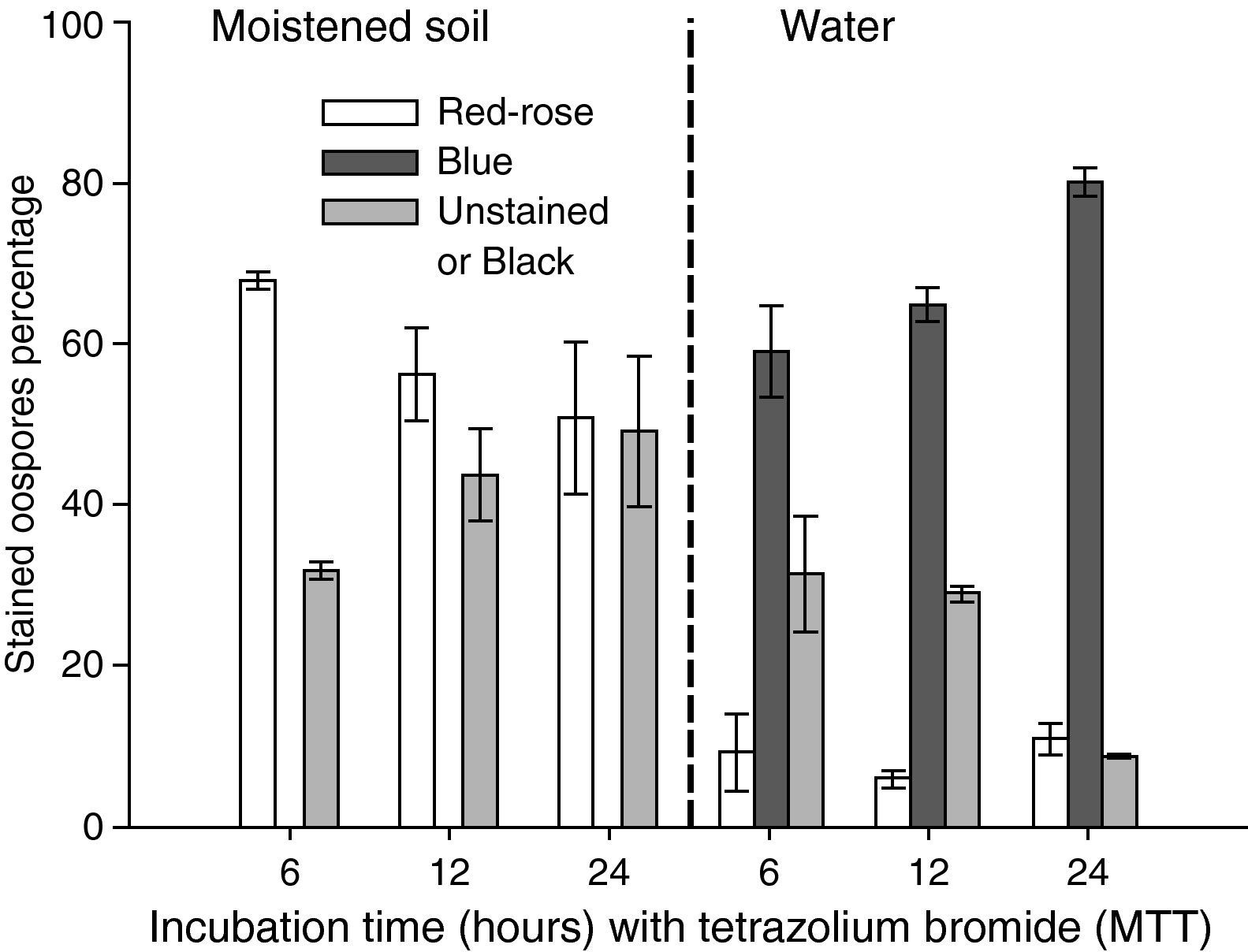
Effect of preservation media (sterile moistened soil and water) during 6, 12 and 24hours on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of 3 weeks old aged oospores. Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.
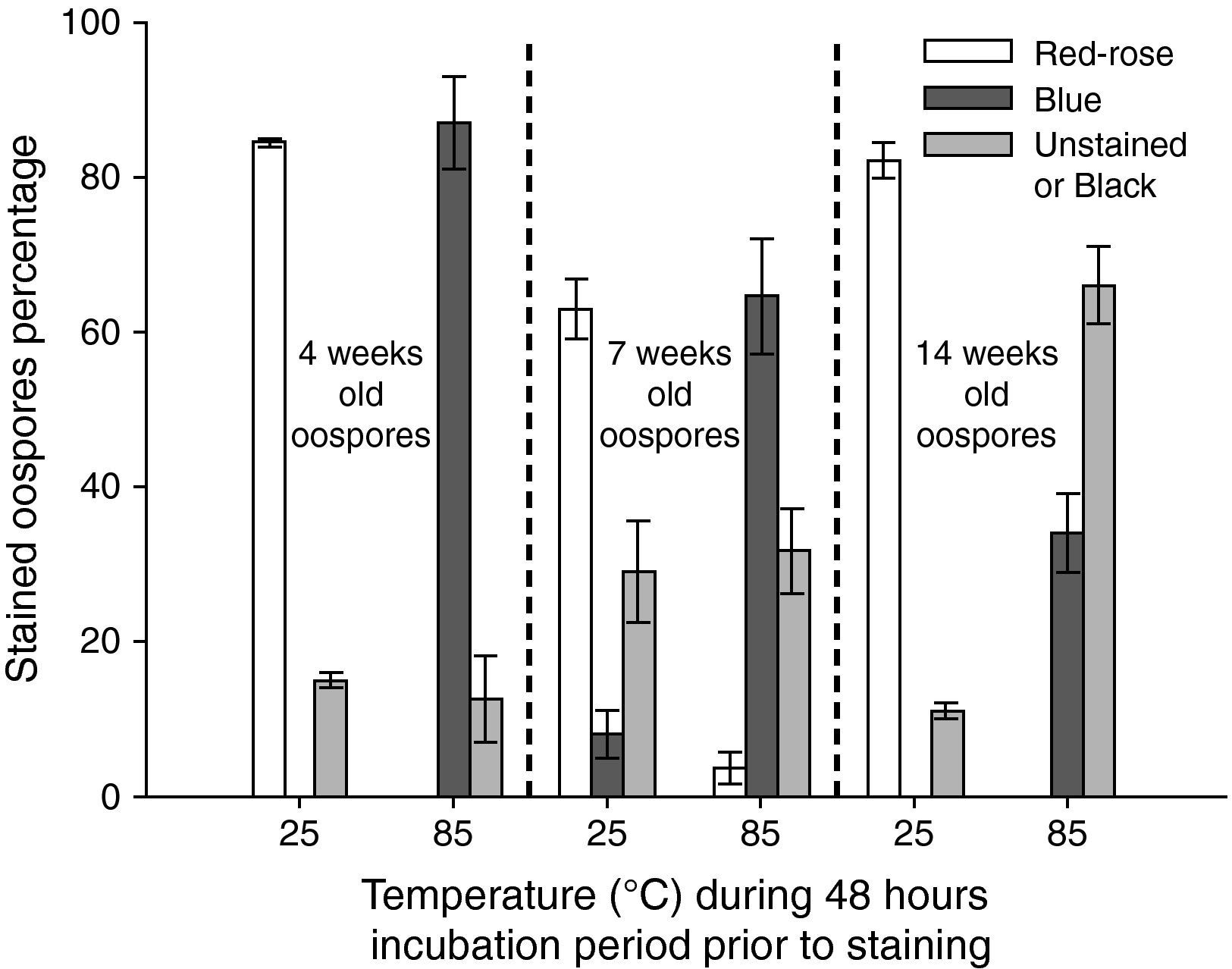
Effect of temperature (25 and 85°C) during 48hours in sterile water on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (4, 7 and 14 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.
Viability tests (MTT and plasmolysis) were applied to heat-treated and non-treated oospores and the results were compared in order to verify their congruence. An autoclave (Raypa Sterilmatic AE-7) and a heater (Selecta 573) were utilized. The following combinations of temperature and exposure times were used: 121°C during 15minutes, 52°C during 2, 4 and 20hours and 85°C during 48hours. Relationships between the MTT test and the plasmolysis method were statistically determined by Pearson's correlation analysis using the CORR procedure of SAS (version 8 for windows) software.
ResultsInstability of MTT testAfter 48hours of incubation, there were more black coloured oospores and less red to rose coloured ones in comparison to the 24 hour incubation period, independent of the oospore age. The percentage of black stained oospores increased with age when comparing the incubation time periods (fig. 2).
In the 3 weeks old oospores, as the interval between microscopic mounting and observation increased, so did the percentage of blue stained oospore (fig. 3). If oospores were examined immediately, no blue oospores were observed, but if the same oospores were counted 24hours later, 33% of the oospores were blue coloured, and 144hours later, 54% of the oospores were stained blue. Furthermore, the percentage of unstained or black oospores was 19% (if oospores were examined immediately), and increased to 51% and 43% (if examined at 24 and 144hours respectively). However, 13 week old oospores retained the stain much better, as only some blue stained oospores (1.33%) appeared 24hours after the microscopic mounting was carried out, even though the amount of red to rose coloured oospores fluctuated from 62 to 80% (fig. 3).
No blue oospores were counted in 3 week old oospores that were buried in moistened soil and maintained at 25°C during 6, 12 and 24hours, but oospores of the same age immersed in water and in the same conditions stained mostly blue (59-80%) (fig. 4).
In contrast, older oospores (4, 7 and 14 weeks) maintained in water at 25°C during 48hours did not stain blue or stained in a very low percentage (8% for those corresponding to 7 weeks); but if oospores of the same age were exposed to 85°C during 48hours, an amount of 87, 65 and 34% of blue stained oospores were observed respectively (fig. 5).
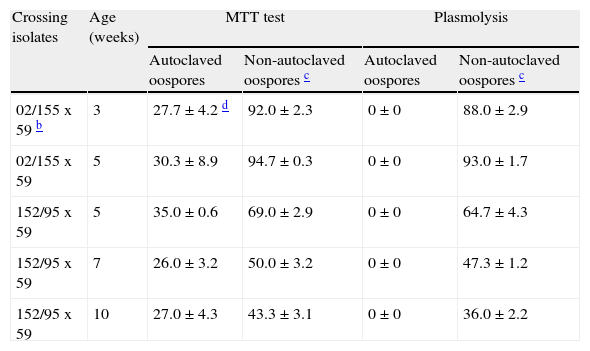
MTT test versus plasmolysis methodIn the MTT test, red to rose and blue stained oospores were considered viable, and black or unstained ones, non-viable. Thus, oospores of different crosses and ages immersed in water and autoclaved (121°C, 15minutes) gave false positives (ranging from 26 to 35%) with the MTT test, but not with the plasmolysis method (Table 1). However, the viability data of non-autoclaved oospores determined by both techniques (MTT and plasmolysis) showed a significant positive correlation (r=0.998, P=0.0001).
Viability assessments of autoclaved (121°C, 15 min) and non-autoclaved oospores of P. capsici determined by using either MTT testa or plasmolysis in 4M NaCl solution.
| Crossing isolates | Age (weeks) | MTT test | Plasmolysis | ||
| Autoclaved oospores | Non-autoclaved oospores c | Autoclaved oospores | Non-autoclaved oospores c | ||
| 02/155 x 59 b | 3 | 27.7±4.2 d | 92.0±2.3 | 0±0 | 88.0±2.9 |
| 02/155 x 59 | 5 | 30.3±8.9 | 94.7±0.3 | 0±0 | 93.0±1.7 |
| 152/95 x 59 | 5 | 35.0±0.6 | 69.0±2.9 | 0±0 | 64.7±4.3 |
| 152/95 x 59 | 7 | 26.0±3.2 | 50.0±3.2 | 0±0 | 47.3±1.2 |
| 152/95 x 59 | 10 | 27.0±4.3 | 43.3±3.1 | 0±0 | 36.0±2.2 |
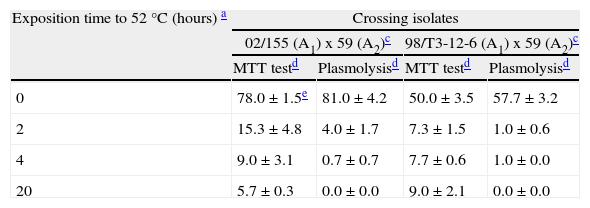
Six week old oospores kept in sterile water at a temperature of 52°C during different exposure times (2, 4 and 20hours) presented viability reductions as assessed by both techniques, even though the MTT test overestimated the viability of oospores when compared with the plasmolysis method (Table 2). When heated and non-heated oospores data were joined, a positive correlation was observed between viability data obtained with the MTT test and the plasmolysis method (r=0.993, P<0.0001).
| Exposition time to 52°C (hours) a | Crossing isolates | |||
| 02/155 (A1) x 59 (A2)c | 98/T3-12-6 (A1) x 59 (A2)c | |||
| MTT testd | Plasmolysisd | MTT testd | Plasmolysisd | |
| 0 | 78.0±1.5e | 81.0±4.2 | 50.0±3.5 | 57.7±3.2 |
| 2 | 15.3±4.8 | 4.0±1.7 | 7.3±1.5 | 1.0±0.6 |
| 4 | 9.0±3.1 | 0.7±0.7 | 7.7±0.6 | 1.0±0.0 |
| 20 | 5.7±0.3 | 0.0±0.0 | 9.0±2.1 | 0.0±0.0 |
When autoclaved, heat treated in sterile water at 52°C, and non-treated oospores data were pooled, even though the two viability tests correlated well (r=0.946, P<0.0001), the MTT test always showed a trend towards overestimation of viability rates when values were near zero.
DiscussionThe survival of oospores of P. capsici that have been exposed to various environmental conditions can be assessed with the use of germination as a measure of spore viability. However, a drawback of this method is the dormancy of oospores, as reported in an early study by Ribeiro et al19, who used MTT as a vital stain of oospores. Williams et al25questioned the use of triphenyl tetrazolium chloride (TTC) as a vital stain with oospores of Sclerospora graminicola in relation to environmental factors that led to non-reproductive results. Sutherland and Cohen24 maintained that the use of MTT as a vital stain was a useful technique for the study of the physiology of pithyaceous fungi, but they suggested that MTT alone could not be used for determining viability of oospores without more detailed studies. Jiang and Erwin12 obtained reliable results with the MTT test as they compared the morphology, the plasmolysis and the staining with MTT as methods for determining viability of oospores of Phytophthora (P. cactorum, P. megasperma f. sp. medicaginis and P. citricola) and obtained a positive correlation between the morphology and the MTT test, between morphology and germination, between morphology and plasmolysis, and between plasmolysis and germination. Bowers et al3 used the MTT test to evaluate the viability of oospores of P. capsici buried in soil. They reported that the reaction indicating that an oospore is respiring is the red staining of that oospore. However, they concluded that the stained oospores do not allow for determining the capacity of oospores's germination and consequently, it can not indicate if the oospores are able to cause disease.
In most published reports, oospores that stained red to rose were considered to be viable oospores in dormancy, blue staining oospores were activated and ready to germinate, and those unstained or stained black were considered non-viable3,6-9,12,18,24. However, other authors2,4,11,15,23considered as viable the oospores that stained rose with a mature thick wall, and as non-viable, the aborted oospores that stained rose, blue oospores, black oospores and unstained ones. According to McCarren et al14, oospores of P. cinnamomi were assessed alive (rose) or dead (black or either unstained without a visible ooplast). They classified as having indeterminate viability the oospores that did not stain, but had a visible ooplast. In a recently published report, 4 species of Phytophthora were studied: P. ramorum, P. cambivora and P. cryptogea (heterothallic species) and P. cactorum (homothallic species). With the use of MTT the four types of coloured oospores (rose, blue, black and unstained) were observed in all four species2. Oospores from 60 day old cultures showed that most of them (> 70%) stained rose whereas the proportions of blue, black or unstained oospores were generally low for the four species. With older cultures (110 days old), there were fewer oospores with the rose staining and more in the blue and black classes for P. ramorum and P. cambivora.
We have proved problems in the experiments performed with the MTT test. First, we consider that this technique depends partially on the subjectivity of the observer. For instance, when reading the staining coloration results, tonalities from red to rose may swing significantly: occasionally oospores stained with a very light rose were difficult to classify either as viable (rose) or as non-viable (unstained).
Second, interpretation of viability was hampered by the fact that oospores were very dark coloured with a red or black tonality. As incubation time in MTT was increased from 24 to 48hours, red coloured oospores got darker, most probably due to the excessive amount of stain accumulated, and it also seemed to be black under microscopic observation (fig. 2). Several authors have used a 48 hour incubation period at 35°C for the staining of oospores with MTT4,8,12, as opposed to others3 who incubated oospores of P. capsici for 24hours at 35°C. According to Sutherland and Cohen24, oospores of Phytophthora megasperma f. sp. glycinea acquired a satisfactory coloration following a 24 hour incubation period. The oospores showed a trend towards a decrease in red-rose stained oospores and an increase in the number of oospores that stained black when they were kept in MTT several weeks at 23 or 35°C. Thus, these authors speculated that overstaining of spores caused them to appear black.
Third, another obstacle encountered was the instability of the staining. The colour of oospores did not maintain stability in young oospores (3 weeks old): as elapsed time interval between microscopic mounting and observation was increased, the proportion of red-rose stained oospores decreased and the percentage of blue coloured oospores increased (fig. 3). However, older oospores (13 weeks old) preserved the colour more uniformly (fig. 3). Of note, young oospores (3 weeks old) immersed in a watery preservation medium during 6, 12 and 24hours prior to the staining procedure were mostly coloured blue while those buried in soil on the same conditions were red to rose (fig. 4). All these facts could be explained through the influence of certain exogenous factors in the permeability of the oospore wall. Williams et al25 reported several issues in the stability of staining oospores of Sclerospora graminicola with TTC, as they could not understand why oospores rapidly lost the stain in some instances, reporting that triphenyl formazan was stable and nondiffusible. Autoclaved sporangium of Synchitrium endobioticum that were incubated in a TTC solution that contained the fungus Aspergillus fumigatus were 56% coloured red-rose16, which was explained by the fact that A. fumigatus may produce an extracellular, non-enzymatic agent that reduces the tetrazolic compound inside the inactivated sporangium.
Jiang and Erwin12 indicated that active oospores ready to germinate only stained blue when they were incubated with a phosphate buffer. In the absence of that buffer, active oospores, ready to germinate, stained black in some cases, while dead oospores were sometimes blue coloured. The use of a phosphate buffer was critical for the stability of the staining12, but other authors have reported that incubation in water or with a buffer did not affect the staining of oospores24,25. In our study, with the use of a phosphate buffer, young (3 week old) non-treated (viable) oospores maintained in water at 25°C for 6, 12 and 24hours prior to the staining, were mostly (60 to 80%) blue coloured (fig. 4). Yet, oospores (4, 7 and 14 week old) that were exposed to lethal treatments (48hours at 85°C) were also stained blue in a higher proportion in the case of younger oospores (87 and 65%) as opposed to the older oospores (14 week old) which only stained in a low percentage (34%) (fig. 5). We do not understand the interpretation of the contradictory blue staining of the oospores since both viable oospores and non-viable ones were blue coloured. However, according to Boutet et al2, the blue coloration in Phytophthora ramorum was considered to be the result of residual activity of aborted gametangia.
A striking and poorly understood fact in our experiment was that viable untreated oospores (3 week old) preserved in sterile water prior to staining with MTT showed a low percentage of rose staining (fig. 4) while older ages (ranging from 4 to 14 weeks) showed a significantly higher percentage of rose staining for untreated oospores, but it also showed a low proportion of rose staining for lethally heated oospores (fig. 5). It would appear that tetrazolium bromide does not ascertain viability of P. capsici oospores as proven by the low percentage that stained rose whether they were exposed to lethal stresses or untreated. Our results are in line with those reported by Dyer and Windels5. They reported that newly formed oospores of Aphanomyces cochlioides (harvested 3-4 days after inoculation of hypocotils) showed that 82% of the oospores were stained with MTT, but by 8-9 days only<10% were stained. This was apparently due to the decline in permeability of the spore wall to MTT as oospores matured. Nevertheless, the above mentioned authors observed a small, but consistent number of oospores that stained rose to lavender irrespective of age. This suggested an artifact or the possibility that oospores remained in an active physiological condition after breaking dormancy.
We have observed false positive staining with the MTT test with autoclaved (non-viable) oospores, with viability ranging from 26 to 35% for different crosses of isolates and oospores age (Table 1). Several early works have also indicated this problem. Sutherland and Cohen24 obtained a 5% rate of false positives (rose stained) with non-viable autoclaved oospores (121°C, 15minutes) of P. megasperma f. sp. glycinea and Pittis and Shattock18 reported false positive results ranging from 0 to 49% with oospores of 20 different crosses of P. infestans. Dyer and Windels5 reported a rate of 8% of false positives with lethally treated (boiling water, 20minutes) oospores of Aphanomyces cochlioides with the use of tetrazolium bromide and a 9% rate of false positives with plasmolysis method, while no false positivity was found with direct microscopic examination.
Delcan and Brasier4 considered non-viable the oospores that stained red-rose but were aborted, which could explain the presence of false positives. In line with this observation, Boutet et al2 maintain that abnormal rose oospores (without ooplast) have been observed in P. ramorum oospores, leading to false positive staining with MTT. In contrast, oospores with normal morphological features were never seen in the other colour classes (blue, black and unstained).
Dyer and Windels5 assessed and compared plasmolysis, MTT staining and microscopic appearance for their usefulness in determining viability of oospores of Aphanomyces cochlioides. Only 16% of the oospores stained rose to lavender with MTT and were assessed as viable, even though plasmolysis and microscopic appearance indicated that 85% were viable.
In our study comparing plasmolysis and the MTT test, the former was devoid of false positive results with autoclaved oospores and provided high ranges of viability percentages in untreated oospores from crosses of several isolates, comparable to those obtained with the MTT test (Table 1). Several authors8,18have reported lower viability rates with the plasmolysis method when compared with the MTT test, perhaps due to the use of low concentration of NaCl solution (2mol l−1). However, incubation in NaCl 4mol l−1 for 45min could explain our more satisfactory results.
Pittis and Shattock18 suggested that the underestimation of viability provided by the plasmolysis method might be explained because exclusively oospores activated to germinate (and no longer dormant) are able to plasmolyse when immersed in a solution with high osmotic potential, as opposed to dormant oospores with thick impermeable walls which would fail to plasmolyse.
A possible pitfall of both methods (plasmolysis and MTT) is that false negative results cannot be estimated whereas false positive results are easily evaluated with the use of lethally treated oospores.
In order to overcome the difficulty of false negative evaluations, Medina and Platt15 have proposed the use of P. infestans oospores with MTT because this method provides a lower number of false negative results when compared with the plasmolysis method. With the aim of decreasing the false positive results, they correct them with the use of autoclaved (dead) oospores that stain as viable with the MTT.
Flier et al8 treated the oospore–mycelium suspension of P. infestans with certain enzymes (NovoZym 234) to eliminate mycelial fragments and sporangia and to promote oospore activation and germination. Both the plasmolysis and the MTT method (the latter applied before and after the treatment with enzymes) and with the use of a plating technique were employed in parallel to monitor the oospores viability. The treatment with enzymes induced a shift of the oospores from a dormant to an activated stage in all matings between isolates of P. infestans leading to viable oospores. The viability results of the active oospores based on MTT staining without enzymatic treatment were similar and correlated significantly with the values obtained with plasmolysis. Thus, they support the findings of Pittis and Shattock18 about the underestimation of the plasmolysis method.
In conclusion, despite the fact that the MTT test has been widely used to determine Phytophthora oospores viability, the subjectivity in the reading of colour, the instability, the incongruity reported widely in the literature about the staining colour of viable oospores and the existence of false positives, suggest that this technique is not the most suitable. The plasmolysis method seems to be a reliable technique to determine viability of oospores of P. capsici. MTT should be used in combination with other techniques such as the plasmolysis method for determining viability of P. capsici oospores.
To the best of our knowledge, this paper is the first to assess the different methodologies and their utility in determining the viability of the oospores of P. capsici, a worldwide major pathogen of pepper crops and other vegetables.
Author's disclosureThe authors have nothing to declare. Authors have no conflict of interests.
Aitzol Etxeberria was the recipient of a grant from the Basque Government. This research was supported by the National Institute for Agricultural and Food Research and Technology (INIA) of the Spanish Ministry of Agriculture, Fisheries and Food (project RTA-2008-00058-C03; Plan Nacional de Investigacion Científica, Desarrollo e Innovación Tecnológica) and by the Department of Agriculture, Fisheries and Food of the Basque Government (project BIOFUMI).






![Effect of elapsed time interval between microscopic mounting and observation (0, 24 or 144hours) on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (3 or 13 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors. Effect of elapsed time interval between microscopic mounting and observation (0, 24 or 144hours) on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (3 or 13 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.](https://static.elsevier.es/multimedia/11301406/0000002800000001/v1_201305061352/S1130140610001129/v1_201305061352/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Effect of preservation media (sterile moistened soil and water) during 6, 12 and 24hours on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of 3 weeks old aged oospores. Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors. Effect of preservation media (sterile moistened soil and water) during 6, 12 and 24hours on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of 3 weeks old aged oospores. Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.](https://static.elsevier.es/multimedia/11301406/0000002800000001/v1_201305061352/S1130140610001129/v1_201305061352/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Effect of temperature (25 and 85°C) during 48hours in sterile water on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (4, 7 and 14 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors. Effect of temperature (25 and 85°C) during 48hours in sterile water on the staining (24hours, 35°C, 0.1% tetrazolium bromide [MTT] solution) of oospores of different ages (4, 7 and 14 weeks old). Crossing P. capsici isolates: 152/95 (mating type A1) x 59 (mating type A2). Red-rose: viable dormant; Blue: viable activated; Black or Unstained: non viable oospores. The exposed data are the average of three counts of 100 oospores with their corresponding standard errors.](https://static.elsevier.es/multimedia/11301406/0000002800000001/v1_201305061352/S1130140610001129/v1_201305061352/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

