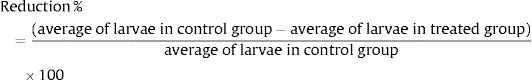Ancylostoma species have demanded attention due to their zoonotic potential. The use of anthelmintics is the usual method to prevent environmental contamination by Ancylostoma eggs and larvae. Nematophagous fungi have been widely used in their biological control due to the fungus ability to capture and digest free nematode forms.
AimsThe aim of this study was to evaluate the effect of four different fungal extracts of Paecilomyces lilacinus (n=2), Trichoderma harzianum (n=1) and Trichoderma virens (n=1) isolates on the hatchability of Ancylostoma eggs.
MethodsFungal extracts consisted of fungal broth culture supernatant without filtration (crude extract) and filtered broth (filtered extract), macerated mycelium (crude macerate), and macerated mycelium submitted to filtration (filtered macerate). The Ancylostoma eggs were obtained from the feces of naturally infected dogs. In vitro assays were performed in five replicates and consisted of four treatments and one control group.
ResultsThe activity of the fungal extracts of each evaluated fungus differed (p<0.05) from those of the control group, showing significant ovicidal activity. The hatching of the eggs suffered reduction percentages of 68.43% and 47.05% with P. lilacinus, and 56.43% with T. harzianum, when crude macerate extract was used. The reduction with the macerate extract of T. virens was slightly lower (52.25%) than that for the filtered macerate (53.64%).
ConclusionsThe results showed that all extracts were effective in reducing the hatchability of Ancylostoma eggs. The ovicidal effect observed is likely to have been caused by the action of hydrolytic enzymes secreted by the fungi.
Las especies del género Ancylostoma son de gran importancia debido a su potencial zoonótico. El uso de antihelmínticos es el método habitual en la prevención de la contaminación ambiental por huevos y larvas del género Ancylostoma. Los hongos nematófagos se utilizan ampliamente en el control biológico de aquellos, debido a su capacidad de capturar y digerir nematodos libres.
ObjetivoEl objetivo del estudio fue evaluar el efecto de cuatro extractos diferentes de hongos (Paecilomyces lilacinus [n=2], Trichoderma harzianum [n=1] y Trichoderma virens [n=1]) en la eclosionabilidad de huevos de especies de Ancylostoma.
MétodosLos extractos de hongos constaban del sobrenadante del cultivo en caldo fúngico sin filtración (extracto crudo) y caldo filtrado (extracto filtrado), micelio macerado (macerado crudo) y micelio macerado sometido a filtración (macerado filtrado). Los huevos de Ancylostoma se obtuvieron a partir de heces de perros infectados de manera natural. Se realizaron cinco repeticiones de los ensayos in vitro con cuatro tratamientos y un grupo control.
ResultadosLa actividad de los extractos fúngicos de cada hongo evaluado difiere (p<0,05) de la de aquellos del grupo control, lo que demuestra una actividad ovicida significativa. Con el extracto crudo macerado, la reducción de la eclosión mostró porcentajes del 68,43 y el 47,05% en el caso de P. lilacinus y del 56,43% para el caso de T. harzianum. El porcentaje de reducción en el uso del macerado crudo en T. virens fue del 52,25%, algo inferior respecto al macerado filtrado (53,64%).
ConclusionesLos resultados mostraron que todos los extractos fueron eficaces en la reducción de la eclosionabilidad de huevos de Ancylostoma. Es probable que el efecto ovicida observado haya sido causado por la acción de enzimas hidrolíticas secretadas por los hongos.
Nematophagous fungi comprise different types of fungi and are the major nematode natural enemies, so, they have been used in their biological control due to the fungus ability to capture and digest free nematode forms.10,11
Ancylostoma caninum and Ancylostoma braziliense have demanded considerable attention due to their zoonotic potential, which is directly related to soil contamination with the feces of infected animals.7 Although the use of anthelmintics is the usual method to prevent environmental contamination by Ancylostoma eggs and larvae, the development and implementation of alternative measures for control of geohelminths are crucial to reduce environmental contamination by the infective forms of this parasite.7 Furthermore, the increase in the number of reports of nematodes’ resistance to the different drugs available and the growing trend toward using products that do not harm the environment stimulate the search for alternative methods.10 In this context, nematophagous fungi can be used in combination when the environment is already contaminated.7
Ovicidal or opportunistic fungi such as Paecilomyces lilacinus and Pochonia chlamydosporia have been used successfully for the in vitro control of gastrointestinal helminth eggs from animals.1,3,7 Studies have shown that the mechanism of infection of these fungi can be mechanical, enzymatic, or a combination of both.2 However, in the last decade, the identification of numerous extracellular enzymes has confirmed their involvement as important virulence factors associated with the infection process.11 A significant enzymatic activity has been reported when filtered cultures of Paecilomyces lilacinus and Trichoderma were used on phytonematodes,2,12,16 or when the crude enzymatic extract of Pochonia chlamydosporia and Duddingtonia flagrans were used on eggs and larvae of animal nematodes.4–6
However, enzymatic extracts of Paecilomyces lilacinus and Trichoderma have not yet been tested on geohelminths eggs, such as Ancylostoma, which hatch for a short period of time in the environment. The aim of this paper was to evaluate the in vitro action of four different Paecilomyces lilacinus, Trichoderma harzianum and Trichoderma virens fungal extracts on Ancylostoma eggs.
Material and methodsFungal culturesFour fungal isolates were used – CG193 Paecilomyces lilacinus and CG502 Trichoderma harzianum provided by Cenargen (Embrapa Genetic Resources and Biotechnology), MICLAB009 Paecilomyces lilacinus and MICLAB008 Trichoderma virens obtained from the collection of fungi of the Mycology Laboratory, Biology Institute, Federal University of Pelotas, Brazil properly identified by DNA sequencing. The cultures kept in test tubes containing potato agar (PDA) at 4°C were subcultured on Petri dishes with PDA and incubated at 25°C for 10 days. Then 4mm fungal culture disks of each isolate were transferred to Erlenmeyer flasks containing 150ml minimal medium broth [glucose (1.8g/l); NH4NO3 (0.4g/l); MgSO4 7 H2O (0.12g/l); Na2HPO4 7 H2O (3.18g/l), KH2PO4 (0.26g/l), yeast extract (0.3g/l) and gelatin for bacteriological use (2g/l)]. The flasks were incubated at 28°C on a rotary shaker at 120rpm for five days.6
Preparation of fungal extractsFour different extracts were obtained from the cultures in minimum medium broth: crude extract (CE), consisting of supernatant broth; filtered extract (FE) obtained by filtering the supernatant broth on filter paper (Whatman N°1); crude macerate (CM), obtained by macerating mycelium in three liquid nitrogen baths until a powdery consistency was obtained, subsequently resuspended in the supernatant broth; and filtered macerate (FM), obtained in the same manner as crude macerate, but subjected to filtration through filter paper (Whatman N°1). All extracts were prepared and used on the same day.
Fecal samplesA 500g fresh feces pool from naturally infected dogs of the Pelotas City Kennel was collected every day during the experiment. Initially, the feces were diluted and macerated in warm water and then filtered through 1mm, 105μm, 55μm and 25μm sieves. The residue of the last sieve was washed in distilled water and the suspension centrifuged at 3000rpm for five minutes, the supernatant was then discarded, and the pellet was suspended in supersaturated saline and centrifuged again under the same conditions. Following, the supernatant was filtered through a 25μm sieve and the eggs collected by distilled water wash, counted in a Neubauer chamber and used on the same day.
Experimental assaysThe in vitro assays consisted of four treatments and a control group. Four ml of CE, FE, CM and FM fungal extracts were poured into 60mm×15mm Petri dishes. Then 1ml of a suspension containing 103Ancylostoma eggs was added. The control group dishes were poured a suspension containing 103Ancylostoma eggs in 4ml minimum medium broth. All dishes were incubated at 25°C for 24h. Each treatment consisted of five replicates. After 24h, the reading was performed by a stereoscope and the total number of Ancylostoma larvae present in the treated and control groups was estimated.
Statistical analysisThe experimental design was completely randomized with five treatments and five replicates. As the response variable showed no normality, the data were subjected to the nonparametric Kruskal–Wallis test; when differences between treatments were found, the means were compared by the Bonferroni test. The analyses were performed with the aid of SAS statistical software, assuming a 5% probability. The mean reduction percentage of larvae was calculated through the following equation4,5
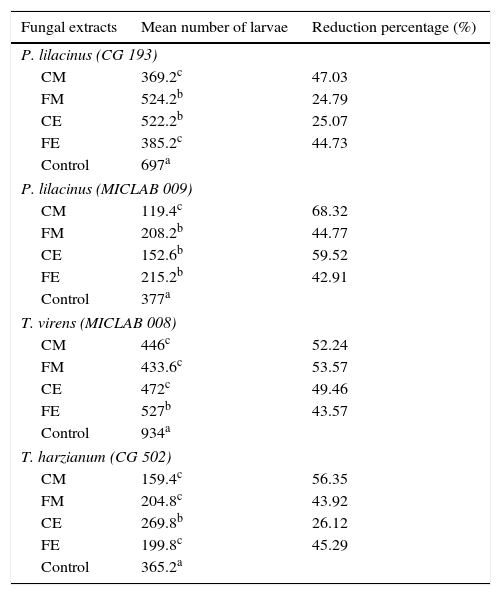
ResultsAfter a 24-h-interaction period, the fungal extracts (CE, FE, CM and FM) evaluated were observed to reduce the Ancylostoma hatchability to some extent, as compared to control (Table 1). Statistical analysis showed differences (p<0.05) in the number of larvae between the fungal extracts of each fungus and the control group. Moreover, it showed that CE, FE, CM and FM did not present the same pattern for each fungus tested (Table 1). However, when the hatchability reduction percentage of Ancylostoma eggs was analyzed, it was evidenced that the greatest hatchability reduction occurred when CM was used, and 68.43% MICLAB009 P. lilacinus, 47.05% CG193 P. lilacinus and 56.43% CG502 T. harzianum reduction percentages were observed. The percentage CM reduction (52.25%) was slightly lower than that of FM (53.64%) only for the T. virens isolate.
Mean number of larvae and hatching reduction percentages of Ancylostoma eggs subjected to treatment with different fungal extracts of P. lilacinus (n=2), T. harzianum (n=1) and T. virens (n=1) in a 24-h-period.
| Fungal extracts | Mean number of larvae | Reduction percentage (%) |
|---|---|---|
| P. lilacinus (CG 193) | ||
| CM | 369.2c | 47.03 |
| FM | 524.2b | 24.79 |
| CE | 522.2b | 25.07 |
| FE | 385.2c | 44.73 |
| Control | 697a | |
| P. lilacinus (MICLAB 009) | ||
| CM | 119.4c | 68.32 |
| FM | 208.2b | 44.77 |
| CE | 152.6b | 59.52 |
| FE | 215.2b | 42.91 |
| Control | 377a | |
| T. virens (MICLAB 008) | ||
| CM | 446c | 52.24 |
| FM | 433.6c | 53.57 |
| CE | 472c | 49.46 |
| FE | 527b | 43.57 |
| Control | 934a | |
| T. harzianum (CG 502) | ||
| CM | 159.4c | 56.35 |
| FM | 204.8c | 43.92 |
| CE | 269.8b | 26.12 |
| FE | 199.8c | 45.29 |
| Control | 365.2a | |
Means followed by different letters in the column differ statistically (p<0.05).
CM, crude macerate; FM, filtered macerate; CE, crude extract; FE, filtered extract.
Nematophagous fungi have been widely used for biological control because of their ability to capture and infect nematodes through enzymatic action.11,12
The results of this study show the ovicidal activity of the evaluated fungi on Ancylostoma eggs and suggest that the activity may be due to the action of hydrolytic enzymes. Furthermore, the use of enzymatic extracts of the fungi significantly reduced Ancylostoma eggs hatching after a 24-h-exposure period. This ovicidal activity could be an advantage, since it would allow the employment of fungus enzymatic extracts on geo-helminth eggs that hatch in a short period of time in the environment, as Ancylostoma, that hatch in approximately 5 days.17
Although the pathogenic mechanisms of nematophagous fungi are not fully understood, evidence shows that extracellular hydrolytic enzymes, including proteases, collagenases and chitinases, may be involved in the digestion and penetration of the cuticle of nematodes.11,14,20 Kahn et al.12 upon evaluating the effect of P. lilacinus on Meloidogyne javanica eggs, showed that the disintegration of the vitelline, lipid and chitinous layers of the eggs was caused solely by enzymatic degradation of proteases and chitinases. P. lilacinus serine protease was responsible for the damage to the shell and vacuolization of Meloidogyne hapla eggs, playing a key role in the fungus pathogenicity.2 In addition to serine protease, chitinase activity has also been observed in P. lilacinus culture supernatant.12
In this study, the two P. lilacinus isolates evaluated were able to reduce the hatching of Ancylostoma eggs, showing significant ovicidal activity. Previous studies have demonstrated the ovicidal effect of this fungal species on Toxocara canis1 and Taenia saginata.3
Studies have shown the potential of Trichoderma in the biological control of different Meloidogyne species.8,16 In 2013, Filho et al.,9 upon evaluating the ovicidal ability of fungi isolated from Brazilian soil on Toxocara canis eggs, identified a Trichoderma isolate with promising ovicidal activity.
The use of different enzymatic extracts of T. harzianum and T. virens in this study demonstrates the ovicidal potential of these fungal species on Ancylostoma eggs. Morton et al.14 and Romão-Dumaresq et al.15 argue that the chitinolytic activity of these fungi is probably the most relevant effect to the egg sheath injury.
Although the authors of this study have not identified and purified the enzymes present in the fungi extracts evaluated, it is believed that the ovicidal effect observed resulted from the enzymatic degradation of proteases and chitinases. Since the eggs of parasites of the Phylum Nematoda may have one to three layers, an inner lipoproteic layer, an intermediate chitinous one and an outer vitelline one, these layers are likely to be susceptible to these enzymes. Previous studies demonstrated the lytic effect of purified proteases and chitinases on Haemonchus contortus eggs.13 However, the development of future studies aimed at the identification and characterization of enzymes and their activities on animal pathogen helminth eggs is essential for the continuity and use of these fungi in the biological control of parasites.
Even though most studies have assessed filtered cultures of fungi on eggs of phytonematodes and gastrointestinal nematodes of domestic animals,2,4–6,12,16 we opted to test different fungal extracts involving filtered and macerated cultures. It was observed that, regardless of the fungal extract, there always was some level of ovicidal activity. However, the highest hatchability reduction percentage was observed with crude macerate extract, particularly that from MICLAB009 P. lilacinus isolate. The authors suggest that such activity could result from the presence of intracellular enzymes released during the maceration process which, together with the action of extracellular enzymes, would increase the fungus efficiency. However, this can only be confirmed with the development of studies that evaluate the isolated and combined action of the enzymes involved. On the other hand, some studies have evaluated the enzymatic activity of filtered cultures or purified enzymes of nematophagous fungi on larvae and eggs of gastrointestinal helminths of animals. When the results of these studies were evaluated, it was observed that, upon testing the enzymatic activity of a serine protease isolated from Monacrosporium thaumasium, the reduction in the number of Angiostrongylos vasorum larvae was only 23.9%.18 Nevertheless, when D. flagrans crude enzymatic extract was used on larvae of the same nematode, the reduction percentage reached 71.3%,6 suggesting that the combination of hydrolytic enzymes increases the ovicidal activity. Similarly, it was found in other studies using crude enzyme extracts of Pochonia chlamydosporia on Cyathostominae4 and Ancylostoma eggs6 an egg hatchability reduction of 72.8% and 76.8%, respectively, which is similar to that reported by us in the present study. In a previous research, Huang et al.11 found that the synergism of proteases and chitinases of P. lilacinus was able to significantly reduce the development and hatching of M. javanica eggs. Likewise, Tikhonov et al.19 reported that the combined action of proteases and chitinases destroys the lipid layers of the egg, causes hydrolysis of chitin and alters the vitelline layer.
ConclusionThe use of enzymatic extracts of the fungi P. lilacinus, T. harzianum and T. virens significantly reduces Ancylostoma eggs hatching after a 24-h-exposure period. Thus, these fungi are, together with other known nematophagous fungi, promising biocontrol agents of geohelminths in the environment. Yet, additional studies are needed so that the molecules responsible for the observed effects can be identified and characterized.
FundingThis study was supported by internal funding. CNPq (National Council for Scientific and Technological Development) of Brazil and CAPES (Coordination for the Improvement of Higher Education Personnel) of Brazil provided scholarship and research grants to the authors.
Conflict of interestThe authors declare that there is no conflict of interest.