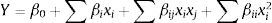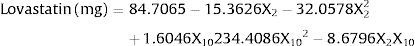Although lovastatin production has been reported for different microorganism species, there is limited information about lovastatin production by basidiomycetes.
AimsThe optimization of culture parameters that enhances lovastatin production by Omphalotus olearius OBCC 2002 was investigated, using statistically based experimental designs under solid state fermentation.
MethodsThe Plackett Burman design was used in the first step to test the relative importance of the variables affecting production of lovastatin. Amount and particle size of barley were identified as efficient variables. In the latter step, the interactive effects of selected efficient variables were studied with a full factorial design.
ResultsA maximum lovastatin yield of 139.47mg/g substrate was achieved by the fermentation of 5g of barley, 1–2mm particle diam., at 28°C.
ConclusionsThis study showed that O. olearius OBCC 2002 has a high capacity for lovastatin production which could be enhanced by using solid state fermentation with novel and cost-effective substrates, such as barley.
Aunque se ha descrito la producción de lovastatina por parte de diversas especies de microorganismos, existe poca información al respecto concerniente a los basidiomicetos.
ObjetivosSe ha investigado la optimización de aquellos parámetros que fomentan la síntesis de lovastatina en los cultivos de Omphalotus olearius OBCC 2002 mediante el uso de diseños experimentales estadísticos y condiciones de fermentación en estado sólido.
MétodosEn una primera etapa se utilizó el diseño Plackett Burman para estimar la importancia relativa de las variables que afectan a la producción de lovastatina. La cantidad de cebada y el tamaño de sus partículas se identificaron como variables de eficiencia. La última etapa consistió en el análisis de los efectos interactivos de las variables de eficiencia seleccionadas mediante un método factorial completo.
ResultadosEl rendimiento máximo de lovastatina, que resultó ser de 139,47mg por gramo de sustrato, se obtuvo realizando la fermentación a 28°C con 5g de cebada y un tamaño de partícula de 1-2mm.
ConclusionesEste estudio pone de manifiesto que O. olearius OBCC 2002 posee una gran capacidad para producir lovastatina, y que dicha capacidad se ve incrementada con condiciones de fermentación en estado sólido, y el uso de un sustrato novedoso y de bajo coste como es la cebada.
Statins, a widely used antihypercholesterolemic agent, competitively inhibits HMG-Co A reductase enzyme in the rate-limiting step of cholesterol biosynthesis in humans. Statins also have potential as therapeutic agents in the treatment of various cancer types.10 Commercial statins are derived from the natural product lovastatin, which is produced by various organisms as a secondary metabolite. Lovastatin, isolated originally from Aspergillus terreus,2 can be produced by actinomycetes19 and numerous fungi including strains of Penicillium citrinum8 and Monascus ruber.13 However, few reports exist describing the potential of basidiomycetes as a source of lovastatin.6,9 Recently, it was pointed out that inedible mushrooms also have the potential for producing beneficial metabolites.17 Studies have shown that species of the basidiomycetes Omphalotus – although poisonous – produce illudins that have antitumor and strong anticancer properties.18 In addition, it has been reported that the ethanol extract of Omphalotus olearius has good potential as a natural antioxidants.3 In our earlier studies, we demonstrated that O. olearius OBCC 2002 produces lovastatin.4 In this study, an attempt was made to enhance the cost-effective production of lovastatin by O. olearius OBCC 2002 under solid state fermentation (SSF) using statistically based experimental designs.
Materials and methodsO. olearius OBCC 2002, used for this study, belongs to the Basidiomycetes Culture Collection of Eskisehir Osmangazi University, Turkey. The pure culture was maintained on potato dextrose agar slants at 4°C until used. Seed media were inoculated with five mycelial discs and incubated on a rotary shaker at 100rpm and 28°C for 4 days. The harvested mycelia were washed with sterile distilled water (SDW) three times, and the total volume was adjusted to 100ml with SDW and homogenized to obtain mycelia suspension to be used as an inoculant (Heidolph, Silent Crusher M). The fermentation media were then inoculated with 4% (v/v) of the inoculant.
Lovastatin production in solid state fermentationThe effects of environmental and nutritional conditions on lovastatin production by O. olearius OBCC 2002 were examined. The tested trace element solution contained MgSO4·7H2O 500mg/l, ZnSO4·4H2O 3.4mg/l, NaCl 500mg/l, FeSO4·7H2O 5mg/l, CoCl2·6H2O 2mg/l, and MnSO4 1.6mg/l. The solid substrates were ground and sieved to obtain tested particle sizes. Substrates were then weighed in Petri plates (120mm×20mm), moistened to 70% with distilled water and autoclaved at 121°C for 40min.16,21 After cooling, the prepared media were inoculated and incubated at 28°C. After 6 days of incubation, the fungal extracts were assayed for lovastatin contents. All of the statistical experiments were performed in triplicate with the mean values taken for analysis.
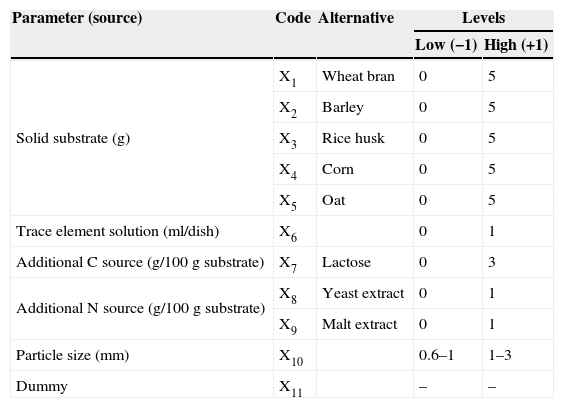
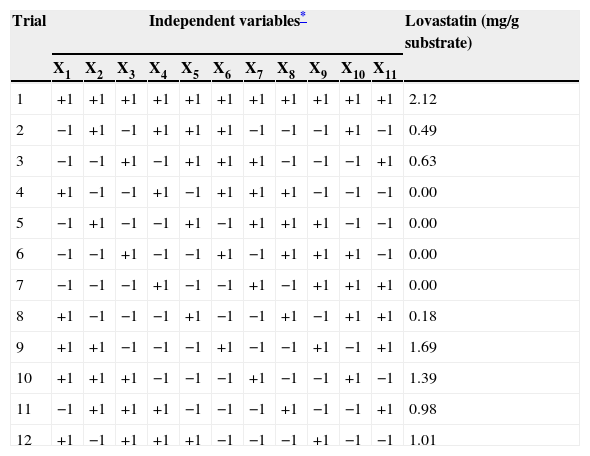
Selection of efficient variables: Plackett-Burman designThe effects of fermentation variables on lovastatin production of O. olearius were evaluated using the Plackett-Burman design (PBD). The PBD is an efficient technique that is used in selecting among a large number of variables those that influence increases in the production of microbial metabolites. It allows the investigation of N−1 variables with N experiments. Ten environmental or nutritional variables were evaluated. The dummy variable was only used to complete the design to 11 variables-12 runs standard PB form. The high (+1) and low (−1) levels of tested environmental/nutritional variables are presented in Table 1.
The independent variables and levels for Plackett-Burman design.
| Parameter (source) | Code | Alternative | Levels | |
|---|---|---|---|---|
| Low (−1) | High (+1) | |||
| Solid substrate (g) | X1 | Wheat bran | 0 | 5 |
| X2 | Barley | 0 | 5 | |
| X3 | Rice husk | 0 | 5 | |
| X4 | Corn | 0 | 5 | |
| X5 | Oat | 0 | 5 | |
| Trace element solution (ml/dish) | X6 | 0 | 1 | |
| Additional C source (g/100g substrate) | X7 | Lactose | 0 | 3 |
| Additional N source (g/100g substrate) | X8 | Yeast extract | 0 | 1 |
| X9 | Malt extract | 0 | 1 | |
| Particle size (mm) | X10 | 0.6–1 | 1–3 | |
| Dummy | X11 | – | – | |
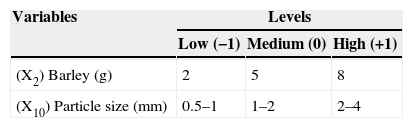
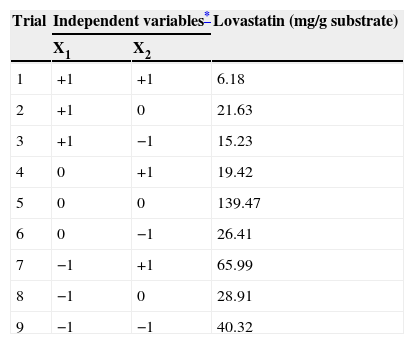
A 23 full factorial design (FFD) was used to determine the optimal levels of the two most efficient variables (barley and particle size). The effects and interactions of these variables on lovastatin production by O. olearius OBCC 2002 were studied using FFD. The levels of each variable are presented in Table 2. In this design, all possible combinations of variables were used to determine the effects on lovastatin production. The behavior of the system is explained by the following quadratic equation:
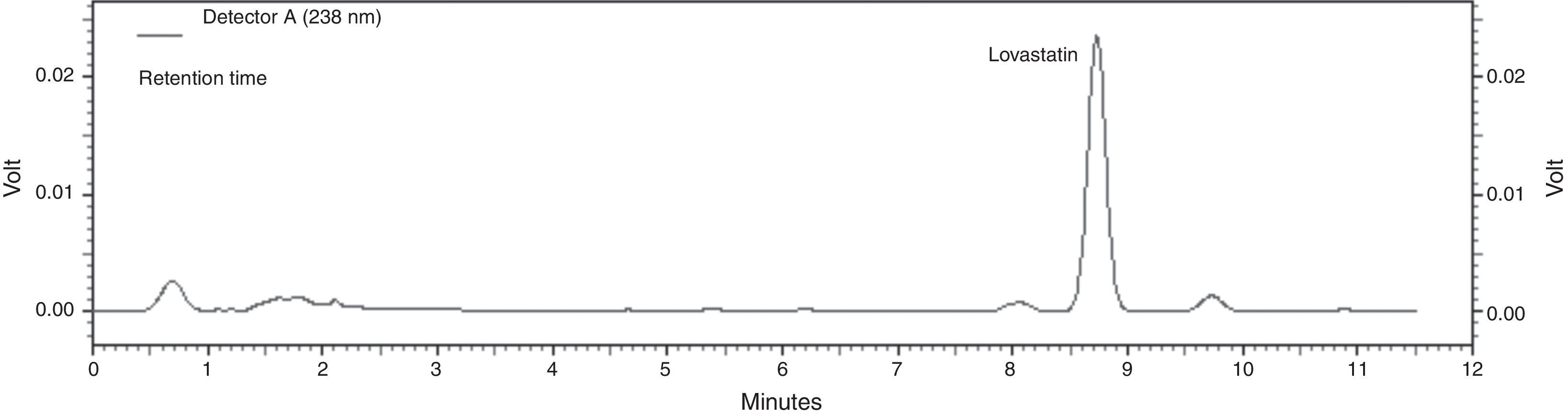
where yield (Y) is the predicted response, β0 is the intercept term, βi the linear effect, βii the squared effect and βij the interaction effect, and xi and xj are the uncoded independent variables. All experiments were carried out three times. The regression and graphical analysis of the experimental data were performed using Statistica 8.0.Analytical methodsThe fermented material (1g) was suspended in 5ml of ethyl acetate, and the suspension was incubated in a shaker incubator at 180rpm and 70°C for 1.5h. After incubation, the mixture was centrifuged at 3000×g for 8min. The supernatant was then collected and evaporated at 80°C, and then dissolved to a total volume of 1ml with acetonitrile. The extracted lovastatin was then determined by HPLC analysis (SHIMADZU, Japan). This analysis was carried out using a reversed-phase ID Intersil® 100 C18 column (250mm×4.6mm with 5μm particle size) at 45°C and a flow rate of 1.5ml/min with a mobile phase mixture of acetonitrile and 0.1% ortho-phosphoric acid (65:35, v/v). Measurements were made by using a UV detector (SPD10A VP) at a 238nm wavelength.11 Standard lovastatin (Sigma) solutions and extracts of fermentation samples were filtered through a 0.45μm filter, and analyzed in HPLC. The retention time of lovastatin in its β-hydroxy acid form was 8.76min (Fig. 1.).
ResultsIn this study, ten environmental/nutritional variables were examined for their influence on lovastatin production by O. olearius in solid state fermentation using the Plackett-Burman statistical design. A total of 12 trials were performed following the PBD model. The rows and columns in Tables 3 and 4 represent the different trials and variables, respectively. The highest lovastatin production was found in experimental trials 1 and 5, with PPD and FFD under SSF, respectively.
Plackett–Burman design for lovastatin production by Omphalotus olearius and measured response.
| Trial | Independent variables* | Lovastatin (mg/g substrate) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | ||
| 1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | 2.12 |
| 2 | −1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | 0.49 |
| 3 | −1 | −1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 0.63 |
| 4 | +1 | −1 | −1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 0.00 |
| 5 | −1 | +1 | −1 | −1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | 0.00 |
| 6 | −1 | −1 | +1 | −1 | −1 | +1 | −1 | +1 | +1 | +1 | −1 | 0.00 |
| 7 | −1 | −1 | −1 | +1 | −1 | −1 | +1 | −1 | +1 | +1 | +1 | 0.00 |
| 8 | +1 | −1 | −1 | −1 | +1 | −1 | −1 | +1 | −1 | +1 | +1 | 0.18 |
| 9 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | −1 | +1 | −1 | +1 | 1.69 |
| 10 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | −1 | +1 | −1 | 1.39 |
| 11 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | −1 | +1 | 0.98 |
| 12 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | −1 | 1.01 |
* For high (+1) and low (−1) levels of tested variables see Table 1.
The full factorial design for lovastatin production by Omphalotus olearius and measured response.
| Trial | Independent variables* | Lovastatin (mg/g substrate) | |
|---|---|---|---|
| X1 | X2 | ||
| 1 | +1 | +1 | 6.18 |
| 2 | +1 | 0 | 21.63 |
| 3 | +1 | −1 | 15.23 |
| 4 | 0 | +1 | 19.42 |
| 5 | 0 | 0 | 139.47 |
| 6 | 0 | −1 | 26.41 |
| 7 | −1 | +1 | 65.99 |
| 8 | −1 | 0 | 28.91 |
| 9 | −1 | −1 | 40.32 |
* For high (+1), medium (0) and low (−1) levels of tested variables see Table 2.
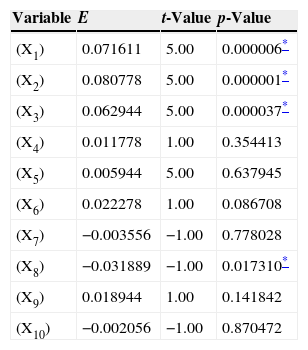
The main effects of the fermentation variables, regression coefficient, t values and P values are illustrated in Table 5. A P-value (>0.05) indicates that the model terms are significant. A coefficient of determination (R2) of 0.8407 indicates that the model can explain the obtained data in 84.07% of the cases, and this also supports the reliability of the model. When the concentration effect value (E) of the tested variable was positive, the influence of the variable was greater at the higher concentration tested, and when it was negative, the influence of the variable was greater at the lower concentration.
The analysis of variance of the calculated model of process parameters for lovastatin production.
| Variable | E | t-Value | p-Value |
|---|---|---|---|
| (X1) | 0.071611 | 5.00 | 0.000006* |
| (X2) | 0.080778 | 5.00 | 0.000001* |
| (X3) | 0.062944 | 5.00 | 0.000037* |
| (X4) | 0.011778 | 1.00 | 0.354413 |
| (X5) | 0.005944 | 5.00 | 0.637945 |
| (X6) | 0.022278 | 1.00 | 0.086708 |
| (X7) | −0.003556 | −1.00 | 0.778028 |
| (X8) | −0.031889 | −1.00 | 0.017310* |
| (X9) | 0.018944 | 1.00 | 0.141842 |
| (X10) | −0.002056 | −1.00 | 0.870472 |
E, effects of variables on lovastatin production; R-sqr (coefficient of determination)=0.84077; Adj (adjusted coefficient of determination)=0.7678; standard error=0.055999.
By looking at the data in Table 3, it can be easily inferred that X2 (barley) have highly significant effect among the tested solid substrates (X1–X5) on lovastatin production. As an additional carbon source, lactose (X7) has no significance. Among additional nitrogen sources (X8–X9), X8 (yeast extract) showed negative effect on lovastatin production although it has significance.
The solid substrate must have a certain particle size. Therefore, although the particle size of the solid substrates did not have a statistically significant effect on lovastatin production, this variable was selected for a further step. As a result, barley (X2) and particle size (X10) were selected for further study to understand the effect of their interaction on lovastatin production under SSF. Therefore, a full factorial design was employed to study the interactions among barley and particle size and also their optimal levels. The ANOVA of quadratic regression model demonstrates that the model is significant, as is evident from the F-test with a very low probability value (Pmodel>F=0.001). P-values less than 0.05 indicate that model terms are significant. The coefficients of regression were calculated, and the following second order polynomial equation was obtained:
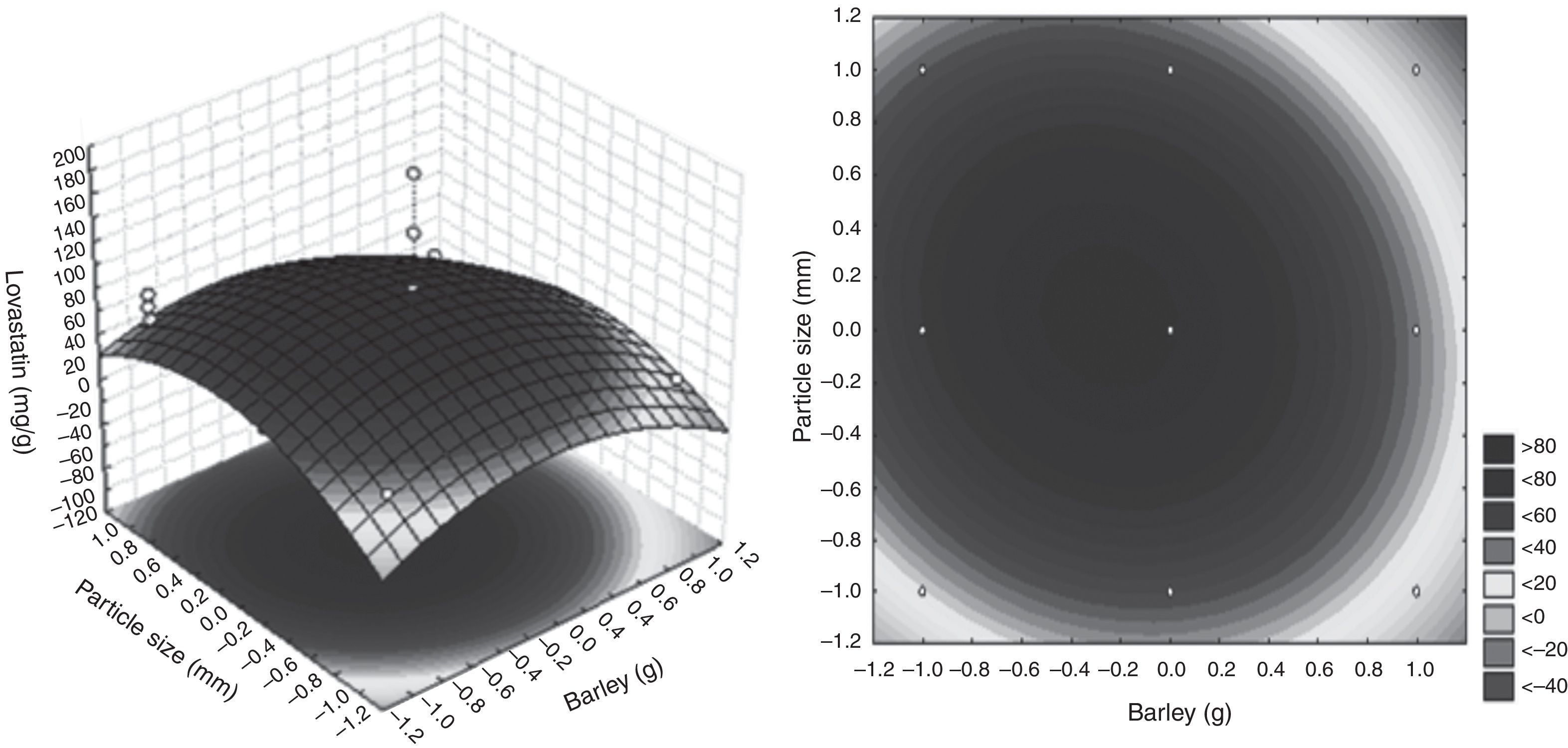
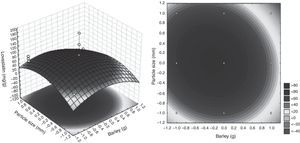
where lovastatin yield is the predicted response, and X2 and X10 are the coded values of barley and particle size, respectively.The lovastatin production at the optimum levels of the variables was predicted by using the equation. The model equation illustrates the interaction between these two factors. According to the equation barley interacts positively with particle size with respect to lovastatin production. Response surface and contour were also obtained from this equation (Fig. 2.). In this figure, predicted lovastatin production values according to barley amounts and particle sizes can be seen. The optimal levels of each variable for maximum lovastatin production were determined by means of the three dimensional response surface plots, which were constructed by plotting response (lovastatin production) on the Z axis against any two independent variables. The density of the contours indicated whether the interactions between the factors were significant or not. It was observed that high lovastatin productivity is achieved at median levels of barley with a particle size of 1–2mm.
The lovastatin yield under non-optimal conditions was 2.23mg/g substrate. Under optimal conditions, the predicted response for lovastatin production was 84.70mg/g substrate, and the observed validated experimental value was 139.47mg/g substrate. The maximum production of lovastatin (139.47mg/g substrate) by O. olearius was obtained by SSF, which was established using barley 5g of barley in particle sizes of 1–2mm, and a relative humidity of 70% at 28°C after 6 days.
DiscussionLovastatin is a multi-billion dollar drug that is produced in a polyketide pathway by different species cultivated under controlled nutritional and environmental conditions. Recently, several molecular studies have been performed to compare lovastatin production of A. terreus in solid state and submerged fermentation. The results show that lovastatin gene expression was ten times higher on SSF than in submerged fermentation, indicating that the physiological features related to SSF offers an advantage to high lovastatin production.5 Therefore, we focused on enhancing the production of lovastatin of O. olearius with SSF.
Different substrates were tested for lovastatin production by the O. olearius strain OBCC 2002. Generally, wheat bran is considered the most suitable substrate for secondary metabolite production by other fungi species in SSF. In several studies comparing the effects of different solid substrates on lovastatin production, wheat bran was found as the most effective, and chickpea and bagasse were found to the least effective substrates.11,21 In contrast, we found barley to be the best substrate, followed by wheat bran and rice husks. Also, Subhagar et al.20 reported that lovastatin production by Monascus purpureus MTCC 369 was 193.7mg/g in SSF with barley. The particle size of the substrate is one of the most important variables in SSF because biosynthesis of lovastatin depends on the oxygen supply. Our results show that barley particles have to be in 1–2mm for maximum lovastatin production by O. olearius.
Carbon and nitrogen sources can be important limiting factors in the production of lovastatin. Lactose is known as a good carbon source for lovastatin production by a lot of fungus species16,21, but it had no effect on lovastatin production by O. olearius in this study. In addition, nitrogen limiting conditions are favorable for lovastatin production and we found that the yeast extract decreases lovastatin production. These results show that barley alone is sufficient for maximizing lovastatin production by O. olearius. We theorize that this is because barley is high in fermentable sugars and low in protein contents.
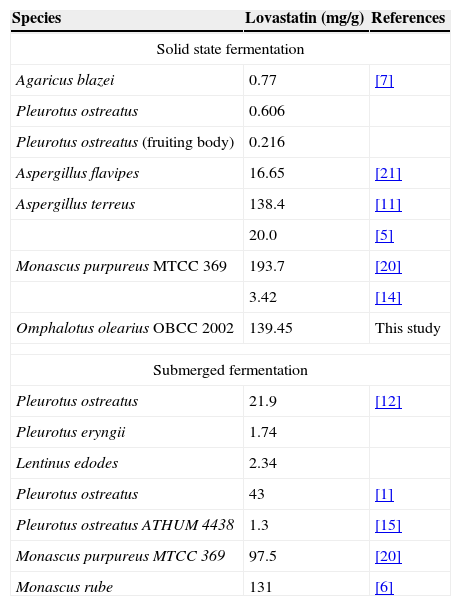
In conclusion, the optimum conditions established for the maximum production of lovastatin (139.47mg/g substrate) were 5g of barley with 1–2mm particle size, and a 70% relative humidity at 28°C. The yield of lovastatin was 62.5 times higher than that produced under non-optimized conditions of SSF. To the best of our knowledge, the literature pertaining to lovastatin production by macrofungi is limited, and this is the first report to show higher lovastatin production by O. olearius. According to other reports (Table 6), we can argue that O. olearius OBCC 2002 has a high capacity for lovastatin production, which could be enhanced by using SSF with novel and cost-effective substrates, such as barley.
Lovastatin production of different fungus species in submerged and solid state fermentation.
| Species | Lovastatin (mg/g) | References |
|---|---|---|
| Solid state fermentation | ||
| Agaricus blazei | 0.77 | [7] |
| Pleurotus ostreatus | 0.606 | |
| Pleurotus ostreatus (fruiting body) | 0.216 | |
| Aspergillus flavipes | 16.65 | [21] |
| Aspergillus terreus | 138.4 | [11] |
| 20.0 | [5] | |
| Monascus purpureus MTCC 369 | 193.7 | [20] |
| 3.42 | [14] | |
| Omphalotus olearius OBCC 2002 | 139.45 | This study |
| Submerged fermentation | ||
| Pleurotus ostreatus | 21.9 | [12] |
| Pleurotus eryngii | 1.74 | |
| Lentinus edodes | 2.34 | |
| Pleurotus ostreatus | 43 | [1] |
| Pleurotus ostreatus ATHUM 4438 | 1.3 | [15] |
| Monascus purpureus MTCC 369 | 97.5 | [20] |
| Monascus rube | 131 | [6] |
The authors declare that there are no potential conflicts of interest in relation to this article, including no financial, consulting, personal or other relationships with other people or organizations.
The authors are thankful to Fatih Buruksu and Talat Ozan Doğan for their technical support and Musa Şölener for HPLC analysis. The first author (BA) thanks Fungiculture laboratory staff for help in laboratory experiments. The authors would like to thank Laurie Gengenbach of the Ag Communications team at North Carolina A&T State University, Greensboro, NC, USA and the anonymous reviewer for his/her comments that helped to improve the manuscript.