The frequency of Candida isolates as a cause of hospital infections has risen in recent years, leading to high rates of morbidity and mortality. The knowledge of the epidemiology of those hospital acquired fungal infections is essential to implement an adequate antifungal therapy.
AimsTo describe the epidemiology of Candida infections in Intensive Care Units (ICUs) from a surveillance network in Colombia.
MethodsInformation was collected from the microbiology laboratories of 20 tertiary healthcare institutions from 10 Colombian cities using the Whonet® software version 5.6. A general descriptive analysis of Candida species and susceptibility profiles focusing on fluconazole and voriconazole was completed between 2010 and 2013, including a sub-analysis of healthcare associated infections (HAIs) during the last year.
ResultsCandida isolates made up 94.5% of the 2680 fungal isolates considered, with similar proportions for Candida albicans and non-C. albicans Candida species (48.3% and 51.7%, respectively). Among the latter, Candida tropicalis (38.6%) and Candida parapsilosis (28.5%) were the most frequent species. Of note, among the blood isolates C. albicans was not the main species. Most of the species isolated were susceptible to fluconazole and voriconazole. From the HAIs reported, 25.5% were caused by Candida; central line-associated bloodstream infection was the most common HAI (58.8%). There were no statistically significant differences regarding length of hospital stay and device days among HAIs.
ConclusionsIn ICUs of Colombia, non-C. albicans Candida species are as frequent as C. albicans, except in blood samples where non-C. albicans Candida isolates predominate. Further studies are needed to evaluate Candida associated risk factors and to determine its clinical impact.
La frecuencia de aislamientos de Candida causantes de infecciones hospitalarias ha aumentado en los últimos años, lo que implica altas tasas de morbimortalidad. El conocimiento de la epidemiología de estas infecciones nosocomiales asociadas con hongos es indispensable para instaurar una terapia antifúngica adecuada.
ObjetivosDescribir la epidemiologia de las infecciones causadas por Candida en las unidades de cuidados intensivos (UCI) de una red de vigilancia de Colombia.
MétodosLa información se recogió en los laboratorios de microbiología de 20 instituciones de tercer nivel en 10 ciudades de Colombia a través de Whonet® versión 5.6. Se realizó un análisis descriptivo general de las especies de Candida más frecuentes y de su perfil de sensibilidad al fluconazol y al voriconazol desde 2010 hasta 2013, incluyendo un subanálisis de las infecciones asociadas con la atención de salud (IAAS) durante el último año.
ResultadosDe los 2.680 aislamientos de hongos, el 94,5% correspondió a especies de Candida, con proporciones similares entre Candida albicans y el resto de especies del género halladas (el 48,3 y el 51,7%, respectivamente). La mayor prevalencia entre estas últimas correspondió a Candida tropicalis (38,6%) y Candida parapsilosis (28,5%). En muestras de sangre, C. albicans no fue la especie más frecuente. La mayoría de especies fue sensible al fluconazol y al voriconazol. Candida causó el 25,5% de las IAAS reportadas, con la infección del torrente circulatorio asociada con catéter (58,8%) como la más frecuente de las patologías. No hubo diferencias estadísticamente significativas en el tiempo de estancia hospitalaria o en el de uso de cualquier eventual dispositivo entre las IAAS.
ConclusionesEn las UCI de Colombia, la prevalencia de C. albicans es muy similar al del resto de especies en conjunto. Únicamente en sangre fue evidente el predominio de otras especies del género diferentes de C. albicans. Otros estudios son necesarios para evaluar factores asociados con la infección por Candida y determinar su impacto en estos pacientes.
Fungal infections are one of the most common complications in the intensive care unit (ICU), leading to an enormous clinical impact. Classically these infections are characterized by their chronicity and difficulty to diagnose and treat, and have been associated with high rates of morbidity and mortality, as well as considerable healthcare costs. In the last few years, the emergence of fungal infections in ICU, with immunosuppressed and long-term hospitalization patients, has become a therapeutic challenge to clinicians due to diagnostic limitations and frequent reports of cases with fungal species resistant to azoles.18
The global prevalence of fungal infections, including healthcare associated infections (HAIs), is near 19%, being recognized as the third cause of infection in the ICU worldwide, only surpassed by Staphylococcus aureus (20.5%) and Pseudomonas (19.9%).50 Approximately, 10–15% of HAIs are caused by fungi; Candida and Aspergillus are the fungal genera most frequently implied. Invasive candidiasis (IC), especially candidemia, represents 70–90% of all fungal infections.16 Although the incidence of Candida is variable, there is a clear trend of increase in IC.5 Additionally, the incidence of candidemia is 5–10-fold higher in the ICU than in wards, which is explained by the risk factors (antibiotics, immunosuppression, long term hospitalization, and surgery, among others) inherent to critically ill patients; some authors have published an incidence ranging from 2 to 6.7 cases per 1000 admissions.16,33,47
In Latin America, the total incidence of candidemia in ICUs and wards varies between 1.18 and 2.49 cases per 1000 admissions.9,35 In contrast, Colombia has reported higher rates of candidemia in ICUs, with a prevalence of 1.4–5.2% and a rate of 2.3 cases per 1000 patient-days.10,11
The impact of IC is reflected in longer hospitalizations and worse outcomes. Almost one third of the cases evolve to sepsis or septic shock,10,11,16,35,54 and the attributable mortality ranges from 49% to 71%.16,21,24 Additionally, patients with IC incur higher healthcare costs. On average, the unadjusted costs range from $15,000 to $40,000 per case, surpassing the costs associated with bacterial bloodstream infections, which have been measured to range from $12,305 to $21,678.54
Recently, the epidemiology of IC has experienced a remarkable change. Although Candida albicans is still the leading cause of IC in the ICU according to most authors, up to 50% of cases may be caused by non-C. albicans Candida species such as Candida glabrata, Candida krusei, Candida parapsilosis and Candida tropicalis.11,16,17,36 Other species have also been reported in lower frequencies such as Candida guilliermondii, Candida lusitaniae and Candida rugosa.29 This trend, reported in several countries, including Colombia,10,11 is clinically relevant since Candida species present different resistant patterns, particularly to first-line antifungals, such as azole agents and echinocandins. The resistance to these antifungals has been reported to have led to an increase in mortality from 15% to 41% as a result of inadequate empirical therapy.6,25,34,48
Given that IC is a rising problem worldwide and that its therapeutic approach in critical care settings requires the knowledge of which Candida species are present, the aim of this study was to analyze the epidemiology and the susceptibility profile of the Candida clinical isolates from 20 tertiary healthcare institutions belonging to the National Bacterial Resistance and HAIs Surveillance Network in ten Colombian cities.
Methods and materialsParticipating institutionsData from the clinical isolates of Candida collected in ICUs of 20 tertiary healthcare institutions (that belong to the National Bacterial Resistance and HAIs Surveillance Network) between January 1st, 2010 and December 31st, 2013 were analyzed. Hospitals from the following Colombian cities were included: Barranquilla, Bogotá, Bucaramanga, Cali, Cúcuta, Ibagué, Medellín, Neiva, Pasto and Pereira. Most hospitals were private (n=14) and teaching hospitals (n=15) catalogued as medium size hospitals (200–500 beds, n=15). Three hospitals were considered small with less than 200 beds, and two hospitals were categorized as large with >500 beds. From 1040 ICU beds available, 59% corresponded to adults, 23% to neonatology and 18% to paediatrics.
Data collectionMicrobiology laboratories sent monthly reports to Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), using Whonet database software version 5.6 (World Health Organization – WHO – Collaborating Centre for Surveillance of Antimicrobial Resistance). These reports included isolates that were identified by the microbiology laboratory in each hospital and entered into the Whonet database, using Backlink 2 for the transference of data from automated microbial identification systems such as VITEK 2® (bioMérieux, Lyon, France) and MicroScan® (Dade Behring, Sacramento, CA, USA). In the laboratories where manual identification was performed (e.g. morphological identification, carbohydrates assimilation and enzyme detection, in accordance with standard procedures), the data were entered directly into the software. For blood cultures, microbiology growth systems (either BACTEC™ and BacT/Alert®) were used. Each microbiology laboratory used internal and external quality controls during the study period to assure the validation of the reports. All ICU Candida isolates were included regardless of the age of the patient or any other clinical criteria. Minimum inhibitory concentration (MIC) to fluconazole and voriconazole for every isolate were achieved by means of E-test (AB Biodisk, Solna, Sweden) or automated VITEK 2® system according to the Clinical and Laboratory Standards Institute (CLSI) document M27-S4.13C. albicans, C. parapsilosis and C. tropicalis isolates with MIC breakpoints ≤2μg/ml to fluconazole were considered susceptible; those with MICs of 4μg/ml were considered susceptible dose-dependent (SDD), and those with MIC ≥8μg/ml were considered resistant; for C. glabrata, isolates with MIC≤32μg/ml were considered SDD, and those with MIC ≥64μg/ml were considered resistant. All C. krusei isolates were considered resistant regardless of the MIC value. For voriconazole, C. albicans, C. parapsilosis and C. tropicalis isolates with MIC≤0.12μg/ml were considered susceptible, those with MIC between 0.25 and 0.5μg/ml were considered SDD, and those with MIC≥1μg/ml were considered resistant; for C. krusei, isolates with MIC≤0.5μg/ml were considered susceptible, those with MIC 1μg/ml were considered SDD, and those with MIC≥2μg/ml were considered resistant. All isolates were classified according to their source of origin: blood samples derived from central or peripheral venous catheter; respiratory samples derived from tracheal secretion, bronchoalveolar lavage, and pleural fluid; urine samples from suprapubic aspirate or urinary catheter; and abdominal fluid samples were obtained during surgery procedures in deep wounds.
Between June 2012 and June 2013, data from HAIs caused by Candida isolates were prospectively collected using the software HAI solutions® version 1.151 in 11 institutions from the network. A general descriptive analysis of the most frequent Candida species was performed, including the report of their susceptibility profiles and a sub-analysis of the HAIs.
Device associated HAIs, which are infections associated with devices used in medical procedures such as central line-associated bloodstream infections (CLABSI), ventilator-associated pneumonias (VAP), and urinary catheter-associated urinary tract infections (CAUTI), were recorded in 6 cities (Bogotá, Cali, Medellín, Barranquilla, Neiva and Pasto). A tablet with the HAI Solutions® software was provided to the epidemiology surveillance committees of each institution, which recorded cases of HAIs based on 2012 Centres for Disease Control and Prevention (CDC) criteria,14 allowing collection of data in real time. Variables such as length of hospital stay (days), device days (total number of days per device), antibiotic consumption, and prolonged mechanical ventilation during hospitalization (defined as >7 days), were analyzed in these HAIs.
For each patient only the isolates from the first positive culture were included in the study.
Statistical analysisDescriptive analysis was performed to establish proportions for all categorical variables. Means or medians with their respective standard deviation or interquartile ranges (according to data distribution) were calculated for quantitative variables. Non-parametric tests were used to evaluate differences. Variables with p<0.05 were considered statistically significant. All statistical analyses were conducted using STATA version 9.0.
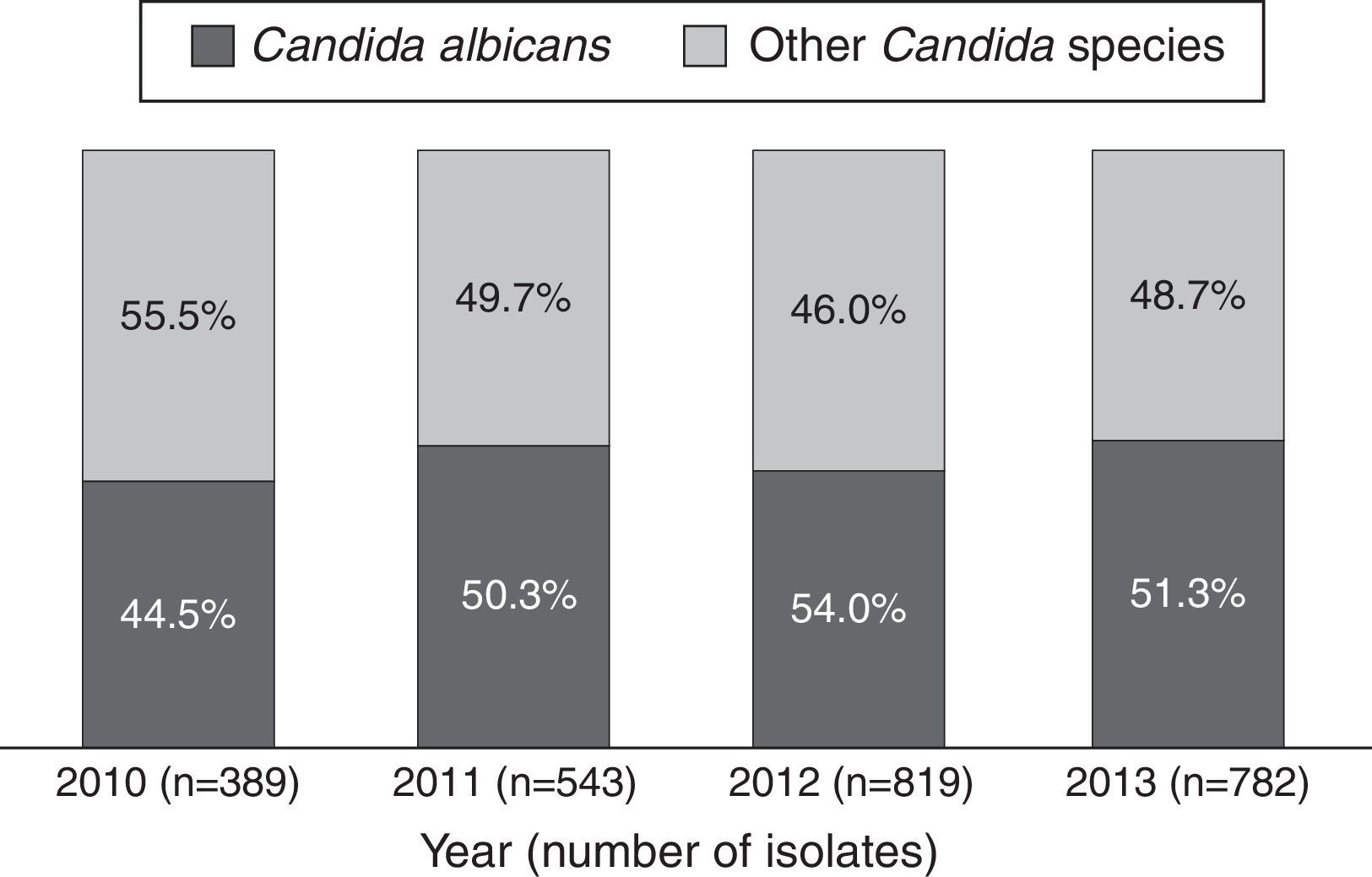
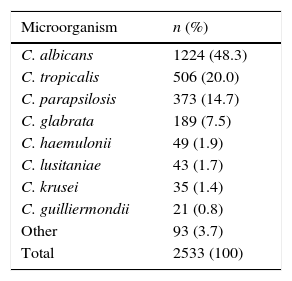
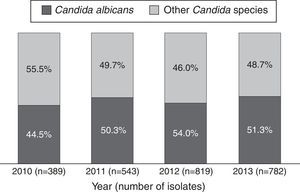
ResultsBetween 2010 and 2013, 44,438 microbiological isolates, including bacteria and fungi, were reported; 2680 (6%) corresponded to fungi. Out of these, 2533 (94.5%) belonged to Candida genus (Table 1). During the study period C. albicans and non-C. albicans Candida species had similar proportions (48.3% and 51.7% respectively) and did not present significant differences over time (p=0.24/Wilcoxon Mann–Whitney test) (Fig. 1). The most prevalent species among the non-C. albicans Candida species were C. tropicalis (38.6%) and C. parapsilosis (28.5%). Other isolates (7.1%) corresponded to C. famata, C. dubliniensis, or C. lipolytica, among others.
Isolates of Candida spp. from ICUs of 20 tertiary healthcare institutions in Colombia (2010–2013).
| Microorganism | n (%) |
|---|---|
| C. albicans | 1224 (48.3) |
| C. tropicalis | 506 (20.0) |
| C. parapsilosis | 373 (14.7) |
| C. glabrata | 189 (7.5) |
| C. haemulonii | 49 (1.9) |
| C. lusitaniae | 43 (1.7) |
| C. krusei | 35 (1.4) |
| C. guilliermondii | 21 (0.8) |
| Other | 93 (3.7) |
| Total | 2533 (100) |
Among the 2533 Candida isolates, 32% were isolated from blood, 30.1% from urine, 9.7% from respiratory secretion and 6.8% from abdominal fluid. In blood, non-C. albicans Candida species (66.2%) predominated over C. albicans (33.8%); the most frequent non-C. albicans Candida species were C. parapsilosis (45.6%), C. tropicalis (22.3%), C. haemulonii (8.7%) and C. glabrata (7.8%). In urine, a superior proportion of C. albicans over non-C. albicans Candida species (54.9% and 45.1%) was observed; among the latter, C. tropicalis (53.4%), C. glabrata (24.4%) and C. parapsilosis (13.6%) were the most common. In respiratory secretion, the proportion of C. albicans was higher when compared to non-C. albicans Candida species (65.8% and 34.2%); C. tropicalis (63.1%) predominated among the non-C. albicans Candida species, followed by C. parapsilosis (13.1%). In abdominal fluid, the proportion of C. albicans was greater when compared to non-C. albicans Candida species (54.3% and 45.7%); C. tropicalis (45.5%), C. parapsilosis (20.2%) and C. glabrata (15.1%) were the most frequent among the latter.
With regard to fluconazole susceptibility profiles, 1.4% of C. albicans and 2.8% of C. tropicalis isolates were resistant; this percentage was higher for C. glabrata (5%) and C. parapsilosis (15.3%); SDD was observed in 1.6% of C. albicans isolates, 1% of C. tropicalis, 13% of C. parapsilosis and 95% of C. glabrata. Susceptibility to voriconazole ranged between 94% and 97% in C. albicans, C. tropicalis, and C. krusei isolates; in C. parapsilosis, 85% of the isolates were susceptible and 12.3% were SDD.
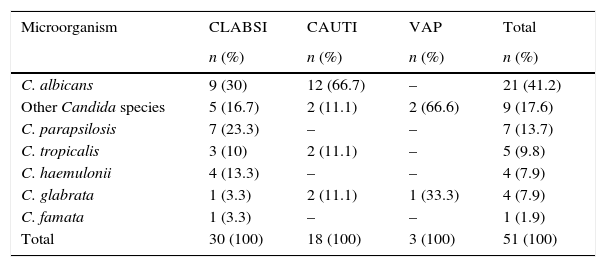
HAIs analysisDuring 2013, among the 200 HAIs with microbiological identification reported, 25.5% (n=51) had positive cultures for Candida (Table 2). Regarding the type of location, 27 (52.9%) HAIs were reported in adult ICUs, 21 (41.1%) in paediatric ICUs and 3 (6%) in neonatal ICUs. In adult ICUs, CLABSI was the most frequent (n=13, 48%), followed by CAUTI (n=11, 40%), and VAP (n=3, 12%); in paediatric ICU, 14 (66.7%) cases corresponded to CLABSI and 7 (33.3%) to CAUTI; finally, in neonatal ICUs all cases corresponded to CLABSI (n=3).
Distribution of the main Candida species by type of healthcare associated infection (HAI) in ICUs from 11 tertiary healthcare institutions in Colombia (2013).
| Microorganism | CLABSI | CAUTI | VAP | Total |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| C. albicans | 9 (30) | 12 (66.7) | – | 21 (41.2) |
| Other Candida species | 5 (16.7) | 2 (11.1) | 2 (66.6) | 9 (17.6) |
| C. parapsilosis | 7 (23.3) | – | – | 7 (13.7) |
| C. tropicalis | 3 (10) | 2 (11.1) | – | 5 (9.8) |
| C. haemulonii | 4 (13.3) | – | – | 4 (7.9) |
| C. glabrata | 1 (3.3) | 2 (11.1) | 1 (33.3) | 4 (7.9) |
| C. famata | 1 (3.3) | – | – | 1 (1.9) |
| Total | 30 (100) | 18 (100) | 3 (100) | 51 (100) |
CLABSI: central line-associated bloodstream infections; CAUTI: catheter-associated urinary tract infection; VAP: ventilator-associated pneumonia
The median hospital stay before HAI diagnosis was 20 days (range 2–109 days), with a mean of 27 days (standard deviation±25 days). The median number of devices days before the HAI diagnosis was 13 days (range 2–56 days), with a mean of 14 days (standard deviation±12 days). With regard to the type of HAIs, non-statistically significant differences were found among length of hospital stay or device days (p=0.36 and p=0.71/Kruskal–Wallis test). Antimicrobial therapy two weeks before admission was reported in 80% of the patients, and prolonged mechanical ventilation in 17.8%.
DiscussionFungal infections are challenging healthcare infections in critically-ill patients due to the difficulty in diagnosis and empirical management; these infections are usually associated with increased rates of morbidity and mortality. Candida species are the third most frequent cause of bloodstream infections in ICU50 and the main clinical presentation is invasive candidiasis (IC). Prolonged length of stay, presence of invasive devices, major burn injuries, hemodialysis, neutropenia, bone-marrow or solid organ transplant, parenteral nutrition, recent chemotherapy or radiotherapy, high doses of corticosteroids, and prolonged exposure to broad-spectrum antimicrobial therapy are some conditions associated with Candida infections.37 These conditions highlight the severity of co-morbidities, and the high-exposure to healthcare services common to patients with IC.
This study demonstrates that during the study period, non-C. albicans Candida species were as frequent as C. albicans in Colombian ICUs. Additionally, these findings are similar to those of other studies from other countries, which describe a shift towards non-C. albicans Candida species as the main cause of candidemia.31 Worldwide, the frequency of C. albicans is decreasing while the prevalence of C. parapsilosis and C. tropicalis is rising.26 In our study, one third of Candida isolates were collected from blood samples, where non-C. albicans Candida species predominated, with C. parapsilosis, C. tropicalis and C. haemulonii being the most common. In contrast, in the United States, C. glabratra is the first species causing bloodstream infections.7
The findings of our study are similar to those of another Latin American candidemia study; from 672 isolates included, 109 isolates were collected from 4 hospitals in two Colombian cities; C. parapsilosis was the most prevalent species followed by C. albicans, C. tropicalis and C. glabratra.35 In contrast, Cortes et al. describe C. albicans as the most common fungal species causing bloodstream infections, followed by C. tropicalis and C. parapsilosis in two Colombian studies; the first study collected isolates from ICUs and wards of 7 tertiary healthcare institutions in Bogotá (n=131), and the second study from 6 hospitals in Bogotá and 1 in Ibagué (n=382).10,12
Our results may differ from other studies due to the large number of hospitals included from different geographical areas in Colombia, as a result of possible variations in risk factors and empirical treatments, as well as by the fact that all Candida isolates were obtained from patients hospitalized in the ICU. The fact that isolates came only from ICU may increase the possibility that patients have had previous exposure to azoles, and may indicate a higher clinical severity, which has been associated with a higher proportion of non-C. albicans Candida species. However, because this is a descriptive study, it is not possible to evaluate these differences.
C. parapsilosis is considered an important cause of infection in hospitalized patients and is associated with failures in hand-hygiene and inadequate usage of catheters and vascular devices. In addition, C. parapsilosis has been reported to exhibit less in vitro susceptibility to echinocandins than other Candida species,22,39 although this has not been demonstrated in some clinical trials.8,53 While fluconazole is considered the first option for the empirical treatment of IC due to C. parapsilosis,1,30,38 in the present study up to 15% of the isolates were resistant to fluconazole, with a similar proportion to voriconazole. These findings support the need to have antifungal treatment guidelines based on local epidemiology in order to choose the therapeutic options with the best fungal coverage and least selective pressure, ideally in the context of an antifungal stewardship programme.2
Remarkably, C. haemulonii complex ranked third in non-C. albicans Candida species isolated in blood, and fifth in CLABSI. This complex is associated with antifungal multiresistance, therefore these clinical isolates should be followed strictly.42C. haemulonii rarely causes human infections32; however, some reports of patients with bacteremia emphasize its clinical relevance because this species may have resistance to amphotericin B and fluconazole.44 Complementary studies using molecular identification are necessary to corroborate this finding, in order to differentiate species, and to evaluate the real impact of C. haemulonii in candidemia.
C. lusitaniae is a rare cause of candidemia. In this study it corresponded to 1.7% of all isolates and represented 1.8% (n=15) of the bloodstream isolates. C. lusitaniae is frequently associated with resistance to amphotericin B and immunosuppressive conditions.4 Its acquisition has been related to colonization and exogenous sources; however, the main mechanism remains unclear.45
One of the major challenges is distinguishing between Candida infection and colonization; while the presence of Candida isolates in blood or sterile tissues is an infection criterion, the clinical significance of respiratory, urinary and abdominal isolates is still controversial. However, infection may be considered when microorganisms are isolated in clinical situations highly suspicious of infection such as intra-operative samples or abdominal collections from patients with strong risk factors for candidemia.49
Respiratory isolates showed a trend similar to those reported by other authors, with C. albicans as the most common fungal species, followed by C. tropicalis and C. parapsilosis. C. albicans colonization is more common in patients receiving mechanical ventilation for more than 2 days; colonization is also associated with higher risk of Pseudomonas aeruginosa ventilator-associated pneumonia and worse clinical outcomes.3 It is not clear if this association is causal or if C. albicans colonization is a marker of disease severity and mortality in those patients.
Regarding the urine isolates, C. albicans and non-C. albicans Candida species were similar (54% vs. 45%), as described also in other reports.23C. glabrata ranked third, after C. albicans and C. tropicalis, which is relevant because of its resistance to azoles. Candiduria is not a strong risk factor of candidemia or invasive candidiasis19; however, candiduria has been associated with high mortality rates in patients with multiple co-morbidities, even when the attributable mortality to candiduria is low.
In abdominal isolates, C. albicans was the most common species, as previously described.15 Systemic dissemination risk is close to 20%, with gastrointestinal surgical perforations and surgical-treated pancreatic perforations as the main risk factors to develop intra-abdominal candidiasis.15,16
Susceptibilities of C. albicans, C. tropicalis and C. parapsilosis to fluconazole and voriconazole were 95%, 96% and 71%, respectively; among the 189 isolates of C. glabrata, 95% showed dose-dependent susceptibility to fluconazole. Unfortunately, susceptibility to echinocandins was not screened in all laboratories, so it is not reported here. In Latin America, the resistance rate of C. glabrata to fluconazole is 7.1%, and C. krusei is considered intrinsically resistant.35
In contrast to other Latin American studies where broth microdilution method was employed, the institutions included in our study used manual and automated methods. However, these methods have been shown to have good clinical agreement (CA) with the reference broth microdilution method: for E- test, CA values of 94.6% and 98.1% have been reported for fluconazole and voriconazole, respectively,40 and similar percentages are described for VITEK 2 ® with CA values of 96.8% and 96.5%.41 It is important to note that changes in susceptibility profiles should be followed strictly over time in every hospital, and faster diagnostic tools for Candida should be incorporated in critically ill patients in order to generate empiric antifungal therapy guidelines based on local fungal epidemiology, which is the first step in any antifungal stewardship programme.
Finally, in the HAIs analysis period, 25.5% of cases were associated with Candida isolates; non-C. albicans Candida species predominated in CLABSI (especiallyC. parapsilosis), and C. albicans in CAUTI. This description is similar to the reported by Hidron et al. in United States.28,46 Non-statistically significant differences were found between HAI type and length of hospital stay or device days. This is the first Colombian study that aims to describe fungal healthcare-associated infections and the microbiology profile of fungal isolates in the ICUs. It is important to mention that these infections appeared late and were related to ICU device usage and delayed clinical diagnosis. Due to the fact that this is a descriptive study, mortality analysis was not performed.
LimitationsThe main limitations of this study include its retrospective design, the inability to distinguish between colonization and infection in respiratory, urine and abdominal samples reported by Whonet, the inability to calculate population-based rates, and the potential to underestimate the proportion of fungemia due to the low sensitivity of fungal blood cultures. The sensitivity of the identification systems used in this study is variable and requires careful analysis when interpreting any result.20,27,43 Additionally, as this study is based on laboratory isolation of Candida, it is difficult to establish an association with infection and thus to draw conclusions about the clinical impact of these isolates.
Another possible limitation is the use of automated methods for susceptibility testing; although these methods have a good correlation with the broth microdilution method, they are not the gold standard to evaluate susceptibility profiles.52 Additionally, HAIs were described only in 11 of the 20 participating healthcare institutions during the last year of the study, so this information may be not representative of the whole country, and its analysis may be difficult due to the number of settings.
In conclusion, this study describes the epidemiology of Candida isolates in patients hospitalized in Colombian ICUs, demonstrating that non-C. albicans Candida species are as frequent as C. albicans, particularly in blood samples. Prospective studies, including analytical studies, with better fungal diagnostic tools are necessary in order to improve empirical and culture-based treatment of fungal infections.
FundingThis study was partially supported by research grants from Merck Sharp & Dohme (FC-000687-2012) and Pfizer S.A. (FC-000583-2012). Merck Sharp & Dohme., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) and Pfizer S.A. had no influence on designing and writing the protocol, the collection of the data, performing the analysis of the results, or the writing of the manuscript.
Conflict of interestDr. Christian Pallares is a speaker for Pfizer SA, Merck Colombia S.A. and Merck Sharp & Dohme. Dr. José Oñate is a speaker for Pfizer S.A. and Merck Colombia S.A. Cristhian Hernandez is speaker for Merck Sharp & Dohme. Dr. Villegas is a consultant or speaker for Merck Sharp & Dohme, Merck Colombia S.A. and Pfizer S.A., and received research grants for the conformation of the network from Merck Sharp & Dohme, Merck Colombia S.A. and Pfizer S.A.
The other authors declare no conflicts of interest relevant to this article.
We thank the people from the participant institutions and hospitals (2010–2013): Bogotá (Guillermo Prada, Stella Vanegas, Adriana Merchan, Henry Mendoza, Francisco Ortiz, Marta Patricia Meléndez, Carlos Álvarez, Sandra Valderrama, Katherine Gómez, Carlos Pérez, Julián Escobar, Luz Ángela Pescador, Sandra Gualtero, Gerson Arias), Cali (Leonor Dicué, Sandra Ossa, Martín Muñoz, Fernando Rosso, Marly Orrego, Lorena Matta, Socorro Trujillo), Pereira (Carmen Elisa Llanos, Berenice Isaza), Pasto (Marco Solarte, Rocío Ortega, Andrea Cerón, Mónica Guerrero), Neiva (Johanna Osorio, Jorge Ramos), Ibagué (Elsy Amparo Ovalle), Cúcuta (Luz Marina Osorio), Medellín (Ligia María Zapata), Bucaramanga (Luis Guillermo Uribe, Beatriz López) and Barranquilla (Ruben Camargo, Guiselle Olivares). We also thank Dr. Elkin Lemos Luengas and Brandon Berger for their valuable help in the final review of this manuscript.