Invasive aspergillosis (IA) is a highly lethal disease that mostly affects immunocompromised patients.6 Galactomannan (GM), a component of the genus Aspergillus cell wall, can be detected in clinical samples early in the course of the disease by the Platelia™ Aspergillus assay (Bio-Rad, Marnes-la-Coquette, France). One of the main reported disadvantages of this test is the false-positive results in patients receiving some beta-lactam antibiotics, especially piperacillin/tazobactam (TZP).1 According to recent reports, newer TZP formulations have been free of this effect.5,9 Nevertheless, this false-positive reaction may remain a problem with the use of some generic formulations.2
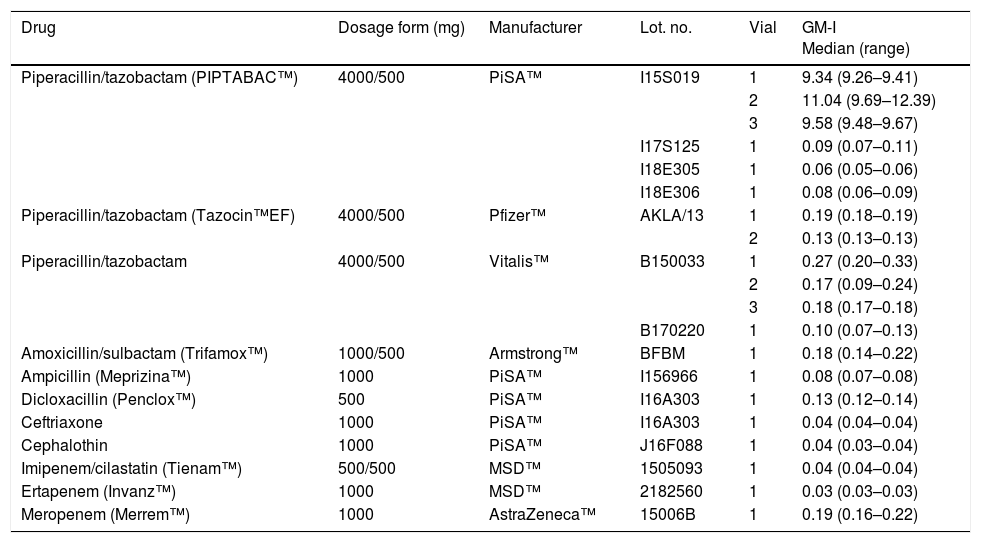
Twelve TZP vials from three different manufacturers available in Mexico (PiSA™, Mexico; Vitalis™, Colombia; and Pfizer™, USA) and other beta-lactam antibiotics were analyzed for the GM antigen by the Platelia™ Aspergillus assay. The antibiotics were diluted with saline 0.9% at the maximum concentration recommended for their intravenous administration. We used as negative controls saline 0.9% and the solution included in the kit. The results were presented as the GM index (GM-I). All tests were performed in duplicate by trained personnel blinded to the samples. Among the beta-lactam antibiotics tested, three TZP vials from one lot manufactured by PiSA (PIPTABAC™) yielded the highest GM-I (median [range]: 9.34 [9.26–9.41], 11.04 [9.69–12.39], and 9.58 [9.48–9.67]), Table 1. The other beta-lactam antibiotics examined, including the TZP manufactured by Vitalis™, Pfizer™, and the remainder lots from PiSA™, as well as the negative controls, consistently showed negative GM-I.
In vitro analysis of beta-lactams available in Mexico with the Platelia™ Aspergillus enzyme-linked immunosorbent assay.
| Drug | Dosage form (mg) | Manufacturer | Lot. no. | Vial | GM-I Median (range) |
|---|---|---|---|---|---|
| Piperacillin/tazobactam (PIPTABAC™) | 4000/500 | PiSA™ | I15S019 | 1 | 9.34 (9.26–9.41) |
| 2 | 11.04 (9.69–12.39) | ||||
| 3 | 9.58 (9.48–9.67) | ||||
| I17S125 | 1 | 0.09 (0.07–0.11) | |||
| I18E305 | 1 | 0.06 (0.05–0.06) | |||
| I18E306 | 1 | 0.08 (0.06–0.09) | |||
| Piperacillin/tazobactam (Tazocin™EF) | 4000/500 | Pfizer™ | AKLA/13 | 1 | 0.19 (0.18–0.19) |
| 2 | 0.13 (0.13–0.13) | ||||
| Piperacillin/tazobactam | 4000/500 | Vitalis™ | B150033 | 1 | 0.27 (0.20–0.33) |
| 2 | 0.17 (0.09–0.24) | ||||
| 3 | 0.18 (0.17–0.18) | ||||
| B170220 | 1 | 0.10 (0.07–0.13) | |||
| Amoxicillin/sulbactam (Trifamox™) | 1000/500 | Armstrong™ | BFBM | 1 | 0.18 (0.14–0.22) |
| Ampicillin (Meprizina™) | 1000 | PiSA™ | I156966 | 1 | 0.08 (0.07–0.08) |
| Dicloxacillin (Penclox™) | 500 | PiSA™ | I16A303 | 1 | 0.13 (0.12–0.14) |
| Ceftriaxone | 1000 | PiSA™ | I16A303 | 1 | 0.04 (0.04–0.04) |
| Cephalothin | 1000 | PiSA™ | J16F088 | 1 | 0.04 (0.03–0.04) |
| Imipenem/cilastatin (Tienam™) | 500/500 | MSD™ | 1505093 | 1 | 0.04 (0.04–0.04) |
| Ertapenem (Invanz™) | 1000 | MSD™ | 2182560 | 1 | 0.03 (0.03–0.03) |
| Meropenem (Merrem™) | 1000 | AstraZeneca™ | 15006B | 1 | 0.19 (0.16–0.22) |
GM-I: galactomannan optical density index.
The standard methods for diagnosing IA (i.e., histopathology and culture) have low sensitivity and a long turn-around time, leading to treatment delays and poor outcomes.6 In consequence, the indirect evidence of IA through diagnostic methods such as GM has a significant role in IA diagnosis.7 False positive reactions can lead to unnecessary morbid therapeutic and diagnostic procedures.8 Although there is evidence that some brand and generic TZP formulations available around the world are no longer associated with false positive results in GM detection (USA, Italy, Brazil and Republic of Korea), concern remains about some generic TZP formulations may still present a problem in some countries (France and Turkey).2-5,9,10 Here, we demonstrated the presence of high GM-I in one lot of the generic TZP formulation manufactured by PiSA (PIPTABAC™). Previous studies that have analyzed generic TZP formulations for GM contamination have also found variation even between batches of the same brands.10 Our results highlight the importance of maintaining an active surveillance for the possibility of GM contamination in generic TZP formulations.
In conclusion, a widely used generic TZP available in Mexico may still be cause of GM cross reactions by the Platelia™ Aspergillus assay. Prospectively testing for GM false-positive results in TZP formulations vials from new batches from PiSA and generic formulations from other manufacturers seems to be necessary in order to avoid IA over-diagnosis. Moreover, an inspection and improvement of the manufacturing process of PIPTABAC™ might be needed given that recent TZP products from other manufacturers did not show galactomannan false-positive reactions.