Mycotic keratitis by moulds (MKM) is an important cause for corneal blindness and usually carries an unfavorable prognosis.
AimsThis study describes the risk factors and demographic and microbiological features of all MKM cases in Santa Lucía Ophthalmology Hospital during a period of 6 years.
MethodsA prospective study was performed for all MKM cases diagnosed between October 2007 and September 2013.
ResultsAmong 157 diagnosed cases, direct microscopic examination and culture were positive in 97 and 96% of the cases respectively. MKM represents 17% of all microbiologically confirmed corneal abscesses. No significant differences were detected in annual MKM frequencies across the study period, suggesting that MKM incidence remains constant over time. A male-to-female ratio was observed (2.8:1); the most affected age groups ranged from 31 to 40 years old (males) and 61–70 years old (females). The most frequent predisposing factor was trauma (40%) followed by the use of contact lenses (9%), herpetic abscesses (5%) and diabetes (4%). The predominant genera were Fusarium (66%), Aspergillus (10%), Curvularia (6%) and Alternaria (4%). The most frequent agent was Fusarium solani species complex (52%). More than two-thirds of the cases were produced by only 3 species or complexes. However, at least 29 different species were detected in the remaining cases. This is the first report of Pholiota sp. as causative agent of human MKM.
ConclusionsArgentina lacks extensive epidemiological and clinical data on MKM. This six-year study performed in Argentina is a first step leading to a better understanding of MKM epidemiology in our country.
La queratitis micótica causada por hongos miceliares (MKM) es una causa importante de ceguera corneal y presenta un pronóstico desfavorable.
ObjetivosDescribir los factores de riesgo y las características demográficas y microbiológicas de las MKM diagnosticadas en el Hospital Oftalmológico Santa Lucía durante 6 años.
MétodosSe llevó a cabo un estudio prospectivo de todos los casos de MKM diagnosticados entre octubre de 2007 y septiembre de 2013.
ResultadosSe diagnosticaron 157 casos de MKM: un 97% con examen directo positivo y un 96% con cultivo positivo. Las MKM representaron el 17% de todos los abscesos corneales microbiológicamente confirmados. No se encontraron diferencias significativas en esta proporción durante los seis años de estudio, lo que demuestra que la incidencia permaneció constante. La proporción varones:mujeres fue 2,8:1; las edades más afectadas fueron 31-40 años en los varones y 61-70 años en las mujeres. El factor predisponente más común fue el traumatismo (40%), seguido del uso de lentes de contacto (9%), abscesos herpéticos (5%) y diabetes (4%). Los géneros predominantes fueron Fusarium (66%), Aspergillus (10%), Curvularia (6%) y Alternaria (4%). Los agentes más frecuentes pertenecían al complejo de especies Fusarium solani (52%). Más de dos tercios de los casos fueron causados únicamente por 3 especies, o complejos de especies, pero en los restantes se detectaron al menos otras 28 especies. Este es el primer informe referente a Pholiota sp. como agente causal de MKM humana.
ConclusionesArgentina carece de datos epidemiológicos y clínicos consolidados sobre MKM. Este estudio contribuye a una mejor comprensión de la epidemiología de esta enfermedad en este país.
Mycotic infections of the eye continue to be an important cause of ocular morbidity. Aetiological factors and symptoms are useful for the diagnosis of fungal infections. The conservative estimate of the number of corneal ulcers occurring annually in the developing world is 1.5–2 million.35 Mycotic keratitis (MK), or keratomycosis, is an important ophthalmologic problem especially among outdoor workers in the tropics. It is an important cause of corneal blindness and usually carries an unfavorable prognosis due to its protracted course and requirement of specific therapy.15
Fungi are a large group of organisms commonly present in soil, air, water, and plant materials. Mycotic keratitis is an infection that begins mostly by trauma with plant material, insects or branches contaminated with conidia or in previously diseased eyes. Geographical location and climate are reported to influence the species diversity detected as the causative agents. Fungi exhibit the largest diversity of aetiological agents, represented by 144 species from 92 genera, of which filamentous fungi include 127 species from 87 genera.14 The most common aetiological agent belongs to the genus Fusarium, with Aspergillus in the second place, pigmented fungi, and others.14Fusarium solani ocular infections are reported to have the most severe prognosis because of their rapid progression, showing corneal perforation in only few weeks.31 In contrast, dematiaceous fungi such as Curvularia and Lasiodiplodia tend to result in persistent low-grade infection and may present with a slightly pigmented lesion.7
The main risk factors for MK include trauma, the use of contact lenses, prolonged topical medications, diabetes mellitus and chronic use of corticosteroids.28 Clinical manifestations begin 1–2 days after trauma, with symptoms similar to bacterial ulcers but usually painless. It may take weeks or months to identify the causative agent and the proper treatment.
Mycotic keratitis due to filamentous fungi is typified by a grey-white infiltrate with irregular feathery margins and raised borders, extending into adjacent stroma.14 The epithelium may appear intact over the lesion, which may be surrounded by a white halo caused by immune reaction. Sometimes, hypopyon and purulent suppuration may appear even though infiltrate seems minimal. Conjunctival and anterior chamber reaction may be intense.
A rapid presumptive diagnosis of MK should be made by observing clinical signs and symptoms and by direct microscopic examination of corneal scrapings. Material collected by scraping is used to prepare smears or mounts for direct microscopic examination and to inoculate culture Plates.31 Growth in culture is deemed significant if the same growth is obtained on more than one occasion or on one occasion with direct microscopy of corneal material revealing the concordant presence of fungal elements. In some cases, corneal biopsy may be required.30
Mycotic keratitis due to moulds (MKM) continues to be difficult to treat despite the use of topical and systemic antifungal agents. Available antifungals are not very effective because they have limited penetration into corneal tissue and are irritating or toxic to the eye surface. In Argentina, natamycin, amphotericin B, fluconazole, miconazole, ketoconazole, voriconazole, clotrimazole, flucytosine and silver sulfadiazine are the available antifungal drugs.25 However, only natamycin and voriconazol are commonly used for ophthalmic treatments.
In some cases, debridement of the ulcer is recommended to remove microorganisms, necrotic stroma and other inflammatory necrotic debris, and may also promote the penetration of the antifungal agent. When there is no response to the medical treatment, performing a lamellar keratectomy with a conjunctival flap may be necessary. This kind of surgery was the most frequent and important treatment before the appearance of antifungal therapy.18
The aim of this study was to describe the risk factors and demographic and microbiological features of all MKM cases in the Santa Lucía Ophthalmology Hospital between October 2007 and September 2013.
MethodsA prospective study was performed for all patients with laboratory-proven MKM diagnosed in the mycology laboratory of the Santa Lucía Ophthalmology Hospital between October 2007 and September 2013. All patients’ data included age, gender, predisposing factors (antibiotics or steroid treatment, use of cosmetic or therapeutic contact lenses), history of infectious evolution (trauma, inoculation, previous surgeries, etc.) and other systemic diseases (HIV, diabetes, etc.). Chi square P-values were used for categorical data comparison.
Corneal scrapings were collected from all patients for microscopy and culture. Multiple scrapings were used for direct microscopic examination of Gram- and Giemsa-stained smears. The scrapings were aseptically inoculated on Sabouraud dextrose agar. Cultures were incubated at 25°C and observed daily for up to 4 weeks.
Fungal keratitis was defined as a corneal epithelial defect with underlying stromal infiltrate with presence of fungal elements by direct examination and/or isolation (positive culture) from a corneal scraping or biopsy. The criteria to determine the significance of a culture included (i) the presence of the organism detected by direct microscopic examination and by direct growth in culture media; and/or (ii) growth of the same isolate in all tubes inoculated; and/or (iii) repeated isolation of the organism from different samples. In all cases, when there was discordance between direct examination and culture results (positive and negative, respectively, or vice versa), sample collection was repeated to confirm the diagnosis.
Morphologic identification of all the strains was performed in the Mycology Laboratory of the Santa Lucía Ophthalmology Hospital, and later confirmed in the National Reference Mycology Laboratory, Mycology Department, National Institute of Infectious Diseases (INEI) “Dr. Carlos G. Malbrán” by standard laboratory techniques such as rate of growth, colony morphology and microscopic features of fungal hyphae and conidia in lactophenol cotton blue mount and slide culture.4,17,29,32 Particularly, Pholiota sp. identification was complemented by ITS sequencing, described as follows.34 Conidial suspension (106–108conidia/ml) was seeded in small Petri dishes (6cm diameter) containing MEYA broth (1% malt extract, 0.4% yeast extract, 0.4% dextrose; 4ml/plate) and incubated at 28°C until abundant development. Mycelium was collected with a pipette tip and dried completely on sterile Whatmann filter paper No. 2. Dried mycelium was transferred to a 50ml tube, where 4mm glass beads were added. Mycelium was ground by placing it in liquid nitrogen for 1min and vortexing at maximum speed for 30s. The mycelium powder was resuspended in 800μl of lysis buffer (200mM Tris–HCl, 500mM NaCl, 10mM EDTA, 1% SDS) and DNA was extracted with phenol–chloroform–isoamyl alcohol (25:24:1), precipitated with isopropanol and washed with 70% ethanol. Dried DNA pellet was resuspended in sterile distilled water.
The internal transcribed spacer region was amplified in a 50μl polymerase chain reaction assay. Briefly, amplification was carried out using 1.5mM MgCl2, 250μM dNTPs, Tris–HCl 20mM (pH 8.4), KCl 50mM, 2.5U Taq DNA polymerase (Invitrogen, Life Technologies, CA), 0.2μM of each primer, ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and 10ng DNA template. Thermal cycling was performed using the MasterCycler EppGradient (Eppendorf, Hamburg, Germany) under the following conditions: an initial denaturating step at 94°C for 10min, followed by 35 cycles of denaturation at 94°C for 1min, annealing at 55°C for 1min and extension at 72°C for 1min, and a final extension step at 72°C for 10min. Polymerase chain reaction products were sequenced on both strands using a Big Dye Terminator kit and the 3500 ABI genetic analyzer (Applied Biosystems). The sequences obtained were edited using Bioedit Sequence Alignment Editor v7.0.4.1 and sequence similarities were searched and retrieved from the NCBI GenBank database using the BLAST algorithm with automatically adjusted parameters.
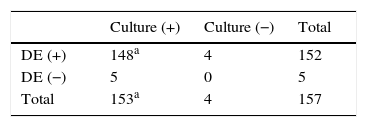
ResultsOne hundred fifty-seven cases of MKM were diagnosed in the mycology laboratory of Santa Lucía Ophthalmology Hospital between October 2007 and September 2013. Direct microscopic examination was positive in 152 cases (96.8%) and negative in 5 cases (3.2%), in which fungi could only be detected by culture (Table 1). There was a 94.3% agreement between direct examination and culture.
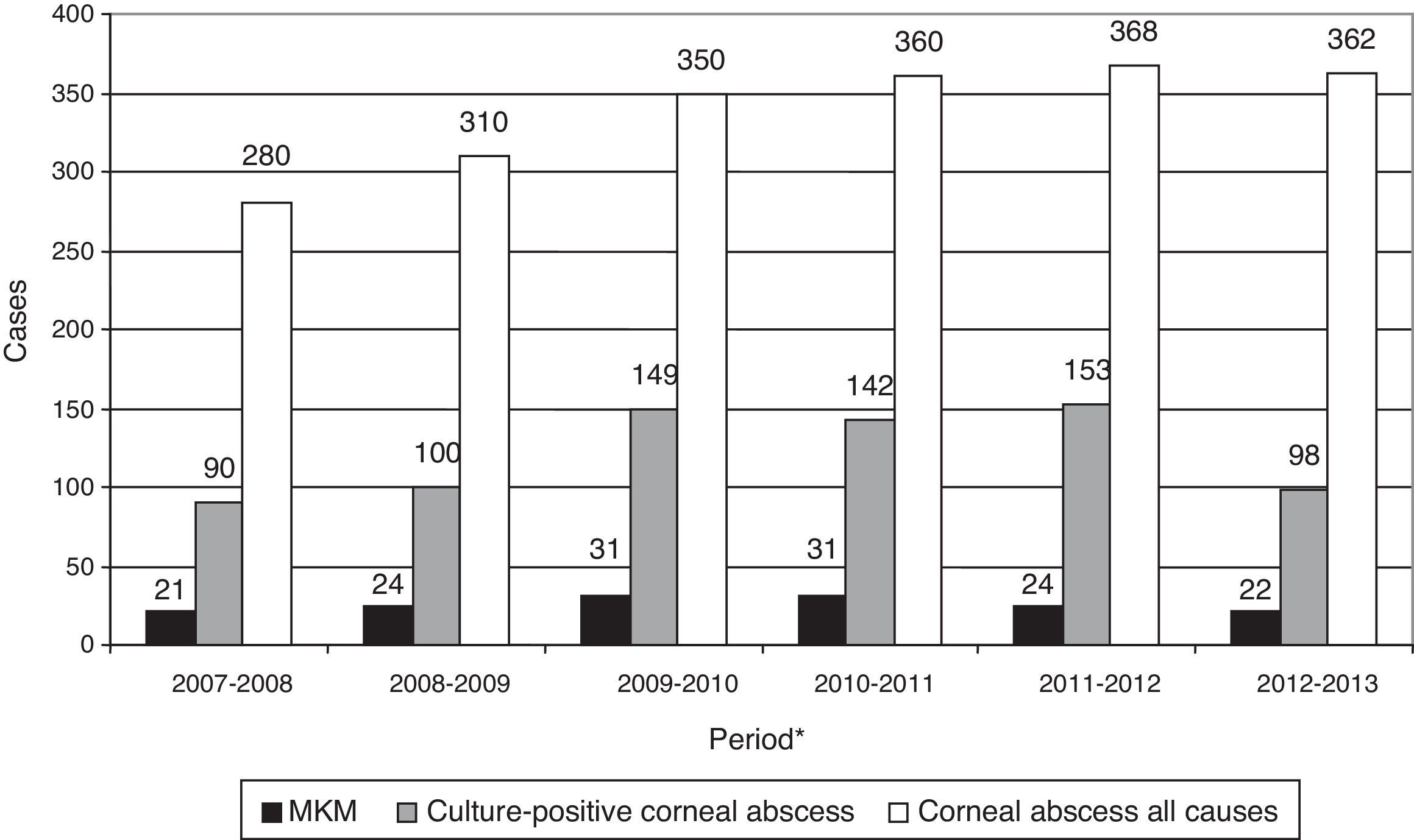
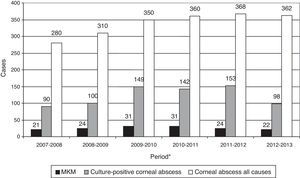
Considering all the causes of corneal lesion, a total of 2030 corneal abscesses were managed. Eight hundred and eighty five cultures out of 2030 were positive, and MKM represents 7.7% of the total corneal lesions and 17.3% of the microbiologically demonstrated corneal abscesses diagnosed in the Santa Lucía Ophthalmology Hospital between October 2007 and September 2013. The annual frequencies of MKM are shown in Fig. 1. No significant differences were found among the percentages of MKM in microbiologically demonstrated corneal abscesses, across the six years of this study.
Annual frequency of MKM considering all causes of corneal lesion and corneal abscess with positive cultures. No significant differences were found between the percentages of MKM in microbiologically proven corneal abscesses, across the 6-year study period. *Refers to annual period from October to September of the following year.
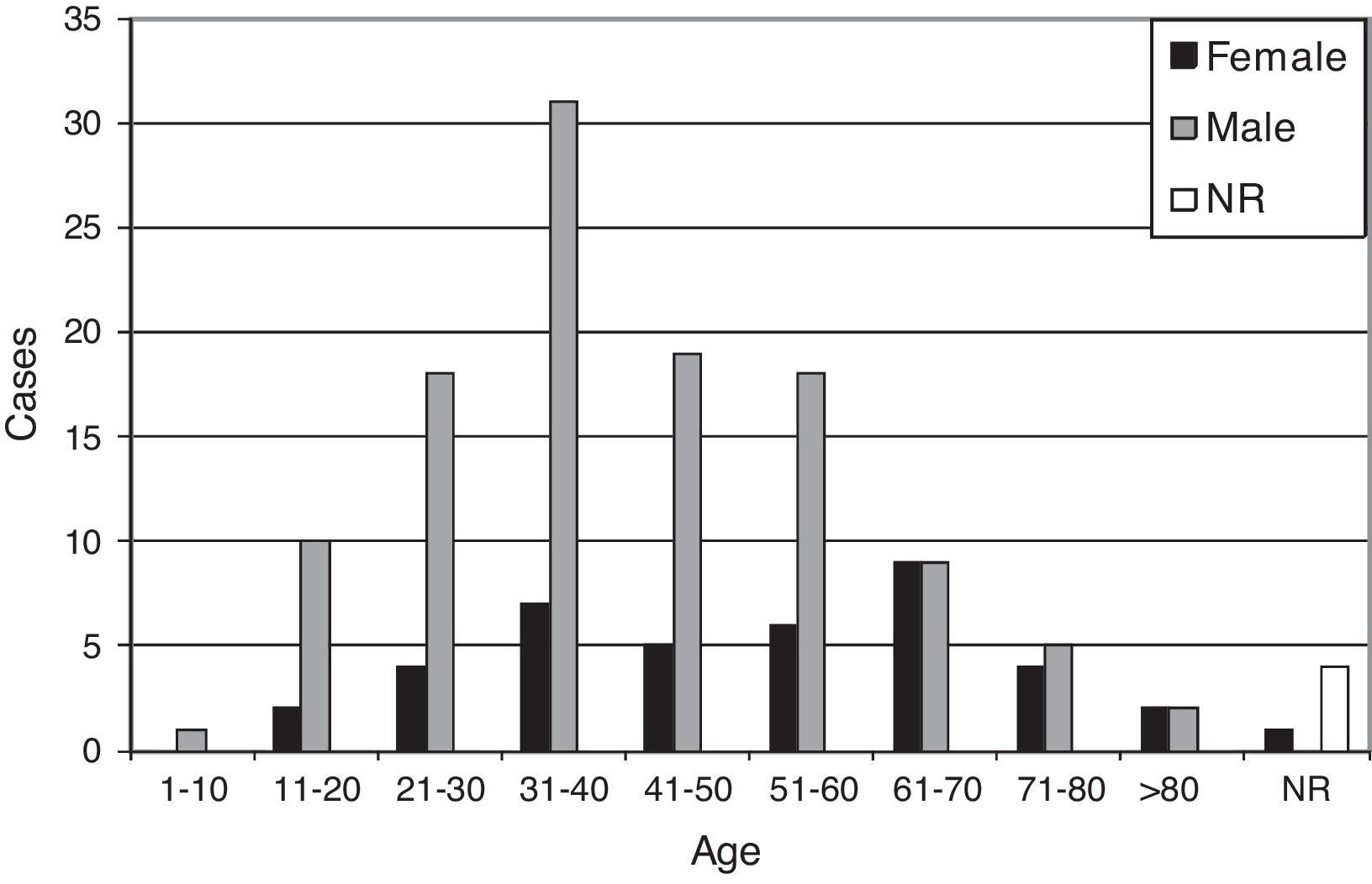
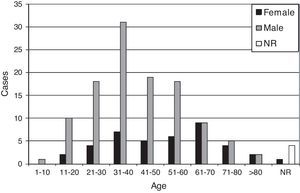
One hundred and thirteen out of 157 MKM cases (72%) corresponded to male patients, while 40 cases (25.5%) were from female patients (with a male-to-female ratio of 2.8:1), which ranged in age from 10 to 95 years old (mean: 45.4, median: 43.5, standard deviation: 18.8). Age and gender are shown in Fig. 2.
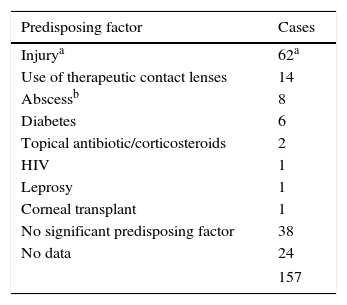
The most common predisposing factors for developing MKM were injury to the eye (62/157, 39.5%) followed by the use of therapeutic contact lenses (14/157, 8.9%), previous herpetic abscesses (8/157, 5.1%) and diabetes (6/157, 3.8%) (Table 2). Forty-one injured patients were able to identify the causing object: 24 (58.5%) reported injuries with plant material, 4 with insects (9.8%), 3 with construction materials (7.3%), 2 with dust (4.9%), 2 with metal (4.9%) and the remaining injuries with animal material, glass, hair, stone, plastic and a gun (one each).
Predisposing factors of the MKM patients.
| Predisposing factor | Cases |
|---|---|
| Injurya | 62a |
| Use of therapeutic contact lenses | 14 |
| Abscessb | 8 |
| Diabetes | 6 |
| Topical antibiotic/corticosteroids | 2 |
| HIV | 1 |
| Leprosy | 1 |
| Corneal transplant | 1 |
| No significant predisposing factor | 38 |
| No data | 24 |
| 157 | |
MKM: Mycotic keratitis due to mould.
Among the 62 patients who had an injury as predisposing factor, 54 (87.1%) were males and 8 (12.9%) were females; the former ranged in age from 31 to 40 years old and the latter from 51–60 to 71–80 years old. However, amongst the 71 patients who had other predisposing factors or who were unable to identify the cause, 42 (59.2%) were males, 29 (40.8%) were females, and ages ranged from 41 to 60 years old, and from 61 to 70 years old, respectively. Significant gender differences were found in injury cases (p=0.0007); however, no significant differences were found in non-injury cases.
No significant predisposing factor was identified in 38 cases; however, 18 of these cases were related to plant manipulation, i.e. people living in rural areas, gardeners, greengrocers and sawmill workers; 7 cases were related to outdoor activities, i.e. waste pickers or construction and shipyard workers (Table 2).
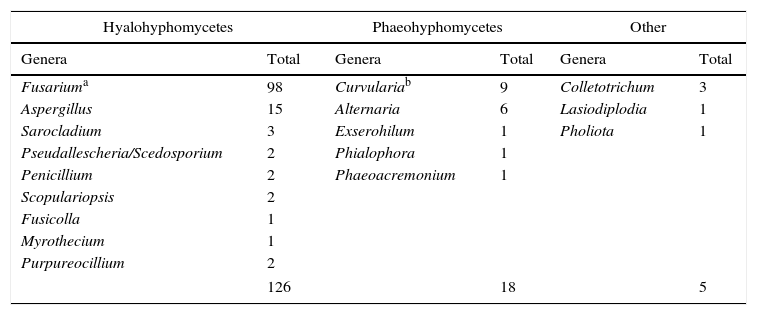
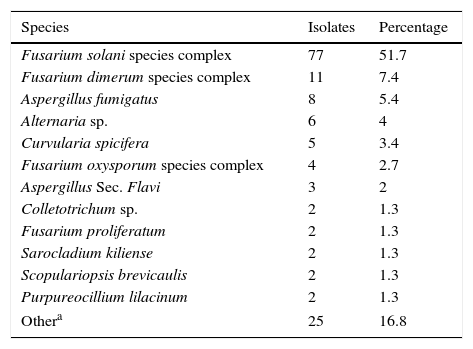
Genera distribution of MKM aetiological agents is shown in Table 3. Hyaline fungi were the most frequently isolated (127/150, 84.7%), followed by pigmented fungi (18/150, 12%) and others (5/150, 3.3%). Frequencies of hyaline and dematiaceous fungi were not significantly different between injured and non-injured patients. The predominant genera of hyalohyphomycetes were Fusarium followed by Aspergillus, while Curvularia (including some ex-Bipolaris species) and Alternaria were the predominant genera for phaeohyphomycetes (Table 3). Coincidentally, they were also the most prevalent genera: Fusarium (99/150, 66%), Aspergillus (15/150, 10%), Curvularia (9/150, 6%) and Alternaria (6/150, 4%). The most frequently isolated agent was Fusarium solani species complex (FSSC) (77 cases, 51.7%), followed by Fusarium dimerum species complex (FDSC) (10 cases, 6.7%) and Aspergillus fumigatus (8 cases, 5.4%) (Table 4). Other isolated agents showed lower rates (Table 4).
Genera distribution of 149 moulds causing MKM from October 2007 to September 2013. Mixed cultures (3) have been excluded from the table.
| Hyalohyphomycetes | Phaeohyphomycetes | Other | |||
|---|---|---|---|---|---|
| Genera | Total | Genera | Total | Genera | Total |
| Fusariuma | 98 | Curvulariab | 9 | Colletotrichum | 3 |
| Aspergillus | 15 | Alternaria | 6 | Lasiodiplodia | 1 |
| Sarocladium | 3 | Exserohilum | 1 | Pholiota | 1 |
| Pseudallescheria/Scedosporium | 2 | Phialophora | 1 | ||
| Penicillium | 2 | Phaeoacremonium | 1 | ||
| Scopulariopsis | 2 | ||||
| Fusicolla | 1 | ||||
| Myrothecium | 1 | ||||
| Purpureocillium | 2 | ||||
| 126 | 18 | 5 | |||
Different species recovered from MKM cases from October 2007 to September 2013. Mixed cultures (3) have been excluded from the table.
| Species | Isolates | Percentage |
|---|---|---|
| Fusarium solani species complex | 77 | 51.7 |
| Fusarium dimerum species complex | 11 | 7.4 |
| Aspergillus fumigatus | 8 | 5.4 |
| Alternaria sp. | 6 | 4 |
| Curvularia spicifera | 5 | 3.4 |
| Fusarium oxysporum species complex | 4 | 2.7 |
| Aspergillus Sec. Flavi | 3 | 2 |
| Colletotrichum sp. | 2 | 1.3 |
| Fusarium proliferatum | 2 | 1.3 |
| Sarocladium kiliense | 2 | 1.3 |
| Scopulariopsis brevicaulis | 2 | 1.3 |
| Purpureocillium lilacinum | 2 | 1.3 |
| Othera | 25 | 16.8 |
MKM: Mycotic keratitis due to mould.
Includes 25 isolated species, each just once: Aspergillus calidoustus, Aspergillus sp., Aspergillus sydowii, Aspergillus Sec. Terrei, Colletotrichum dematium, Curvularia geniculata, Curvularia hawaiiensis, Curvularia lunata, Curvularia sp., Exserohilum sp., Fusarium sp., Fusarium lateritium, Fusarium incarnatum-equiseti species complex, Gibberella fujikuroi species complex, Fusicolla merismoides, Lasiodiplodia theobromae, Myrothecium sp., Penicillium oxalicum, Penicillium sp., Phaeoacremonium parasiticum, Phialophora bubakii, Pholiota sp., Pseudallescheria sp., Sarocladium strictum, Scedosporium dehoogii.
No significant differences were found in MKM cases caused by all moulds, hyaline and pigmented, between seasons of the year (data not shown).
During the 6-year period of the study, we detected only 15 cases of keratitis caused by yeasts (80% caused by Candida albicans) mostly in HIV+ patients, patients with diabetes and contact lenses users (data not shown).
DiscussionPrimary diagnosis of MKM is made by detecting fungal elements by direct microscopic observation of corneal scrapings. Although calcofluor white stain appears to improve sensitivity of microscopic examination (91.4%), it requires the use of specific equipment such as a fluorescence microscope.9 The most widely used staining method is KOH 10–30%, with sensitivity values of 86–91%, while Giemsa and Gram stains appear to have slightly lower values (85.1 and 88.2%, respectively). In our work, combined observation of GRAM and Giemsa stains allowed us to confirm 96.2% of the cases, 93.6% of which were concordant with culture development, showing the significance of this test as a rapid diagnostic method. These values were higher than those reported in the literature (between 66 and 86.3%). Such high percentage is due to repeated scraping, which allowed us to confirm the diagnosis when direct microscopic examination was not in agreement with culture results. This was also possible thanks to the fact that hospital care is public and free, which encouraged patients to return for treatment.
The frequency of fungal keratitis, especially in tropical and subtropical regions, may account for more than 50% of all patients with culture-proven microbial keratitis, varying between 13 and 62%.21,30 In South America, it may vary between 11 and 56% in Brazil, and it may reach 26% in Paraguay.12,16 Our study reported 17% of MKM in all microbiologically proven keratitis; this incidence is similar to those reported in the 2005–2006 research of the same hospital.5 Climate, geographical location and economic factors may play a vital role in the wide variation of these percentages. The results in our city (located in 34.604S and 58.382W), with subtropical climate, are similar to those from other regions with similar climate, such as Southeastern Brazil and Paraguay, where frequency remains low.1,12,16 In contrast, tropical regions have reported higher frequencies.2,13,20,22,27,36
No significant differences were detected in annual MKM frequencies across the study period, suggesting that MKM incidence remains constant over time within the hospital's area of coverage, which includes spontaneous demand from the City of Buenos Aires and suburb, as well as patients referred from other regions of the country.
A preponderance of males (male-to-female ratio 2.8:1) was observed in this series although males developed corneal ulcers more commonly in midlife and females acquire the disease at an advanced age. Male preponderance has been reported by most previous studies.9,30 However, significant differences were found when comparing gender in trauma and non-trauma cases, showing that males were more affected by traumatic events than females. This might be explained by the fact that trauma cases among men of working age are frequently work-related.
In this study, injury was the main predisposing factor causing more than one-third of the cases. This is in accordance with several reports all around the world, although the percentage may vary between 30 and 60%.3,6,10,11,14,16,19,21,23,24,27,28,30,33 This study also documents other factors in connection with MKM such as the use of therapeutic contact lenses, presence of herpetic abscesses and diabetes. Very few cases with ocular surgery or previous use of corticosteroids were diagnosed, although these predisposing factors have been widely documented.6,19,30 It is worth noting that patients treated in this free and public healthcare center usually come from low-income homes with limited access to expensive medical practices such as corticosteroid treatments, surgery, and contact lenses.
Trauma with plant material was the most frequent cause of injury, followed by trauma caused by insects, construction materials and others, in keeping with reports by several other authors.10,30
No significant predisposing factor was detected in a quarter of the cases (37). However, 18 of these patients were involved in plant handling (i.e. farmers, gardeners, rural area denizens, greengrocers, and sawmill and stable workers).
As reported in other studies, the presence of hyaline fungi was more frequent (86.6%) than pigmented ones (11.8%).9,12,26 However, no significant differences were found between hyaline and pigmented percentages in trauma and non-trauma patients, suggesting that this characteristic is unrelated to this predisposing factor.
Two-thirds of the cases were caused by the genus Fusarium and one-tenth by Aspergillus, results that are similar to those reported in Southeastern Brazil (climatic characteristics shared with Buenos Aires).12 Research from other regions also reported Fusarium and Aspergillus as the most frequent agents, though the former shows lower rates (35–55%) and the latter usually higher ones (18–30%).6,9,11,15,26,33Curvularia species were the commonest cause of pigmented MKM, in accordance with other authors; however, in this study, the genera Curvularia included the former Bipolaris spicifera and Bipolaris hawaiiensis.26
This is the first report that shows Pholiota sp. as a causative agent of human MKM in a 39-year-old male who suffered a corneal lesion due to a piece of plant material. Once the material was extracted and the corneal tissue was scraped, direct examination and culture allowed MKM diagnosis. Pholiota sp. anamorph morphologically resembles several genera of Basidiomycota arthroconidial state, as well as the characteristics of culture, appearance, color and growth rate.34 However, molecular tools allowed us to reach an unambiguous identification, showing Pholiota sp. to be the etiologic agent, in agreement with the morphological features. Outcome monitoring was not possible because the patient did not come back for further treatment or follow-up. This result demonstrates that this basidiomycete can cause human infection in immunocompetent hosts.
Although many authors did not report species identification in their MKM studies, our results agree in general with reports by other authors describing FSSC as the most common species.8,11,33
Almost two-thirds of the cases were caused by three species or complexes only (FSSC, FDSC and A. fumigatus); however, the total of 29 species or complexes and 18 genera recovered suggests the enormous diversity of possible causative agents, as others have stated.14,30 Therefore, specifically trained mycology professionals are necessary at clinical laboratories for an accurate identification of the above microorganisms.
Argentina lacks extensive epidemiological and clinical data on MKM, a serious infection of the eye, which contributes to one of the leading causes of monocular blindness worldwide. While statistics are difficult to obtain, there is evidence of regional variability in both incidence and type of infection.14 This is the first 6-year study performed in Argentina for all the proven MKM cases diagnosed at the Santa Lucía Ophthalmology Hospital between October 2007 and September 2013, and it is a step that leads to a better understanding of the epidemiology and distribution of MKM causal agents in our country.
Conflict of interestThe authors declare that there is no conflict of interest.