The aspartic proteases, also called aspartyl and aspartate proteases or acid proteases (E.C.3.4.23), belong to the endopeptidase family and are characterized by the conserved sequence Asp-Gly-Thr at the active site. These enzymes are found in a wide variety of microorganisms in which they perform important functions related to nutrition and pathogenesis. In addition, their high activity and stability at acid pH make them attractive for industrial application in the food industry; specifically, they are used as milk-coagulating agents in cheese production or serve to improve the taste of some foods. This review presents an analysis of the characteristics and properties of secreted microbial aspartic proteases and their potential for commercial application.
Las aspartil-proteasas, también denominadas aspartato-proteasas o proteasas ácidas (E.C.3.4.23), pertenecen a la familia de las endopeptidasas, que se caracterizan por una secuencia conservada de Asp-Gly-Thr en su sitio activo. Estas enzimas se encuentran distribuidas en una amplia variedad de microorganismos, donde desempeñan funciones importantes en la nutrición y la patogenia, además de poseer otras características, como alta actividad catalítica y estabilidad en pH ácido, lo que las vuelve atractivas para su uso en industrias como la alimentaria, específicamente en la industria láctea, como agentes coagulantes para la elaboración de quesos o para mejorar el sabor de ciertos alimentos. En la presente revisión se lleva a cabo un análisis de las características y propiedades de las aspartil-proteasas secretadas por hongos y su potencial para aplicaciones comerciales.
The term protease is a synonym for peptidase; i.e., proteolytic enzymes or peptide hydrolases that catalyze the cleaving of peptide bonds in proteins, which they digest into peptides and/or amino acids. Proteolysis is an essential process for life; in fungi, some proteases are important in the formation and germination of spores, in pathogenesis and in post-translational regulation.46,47,62,74
The nomenclature for all proteases is found in the MEROPS database,66 in which they are primarily classified as cysteine, metallo-, serine, threonine and aspartic. The latter are commonly called acid proteases and are endopeptidases with aspartic acid residues at their active site.62 Aspartic proteases that are excreted are called secreted aspartic proteases (SAPs).30 These enzymes are produced by several microorganisms, in which they perform important functions related to nutrition and pathogenesis and have characteristics that make them attractive for industrial applications.5,45 This review analyzes the characteristics and properties of SAPs from fungi and discusses their importance and applications.
Clans and familiesThe acid proteases, commonly called aspartic proteases (E.C.3.4.23), are found in animals, fungi, plants, protozoa, bacteria and viruses.29 Barrett et al. grouped the peptidases into families and clans based on their primary and tertiary structures.7 Each family includes peptidases that are characterized by a statistically significant relationship among their amino acid sequences in the active site. A clan is a group of families whose members have evolved from the same ancestral protein but have diverged to such a degree that their primary structures are no longer comparable.28 When these structures are not available, the proteases are categorized by the order of the residues in the active site in the polypeptide chain and by the motif sequence adjacent to the catalytic site. Each clan is labeled with two letters, with the first letter representing the type of catalyst that characterizes the families included in the clan. Some families, however, cannot yet be assigned to a clan, so when a formal assignation is required, they are described as belonging to ‘clan A-’. In this way, each family is identified by a letter that represents the catalytic group that contains the enzyme, together with a unique number that leads some families to be further divided into sub-families because there is no proof of an ancient divergence among them. This classification includes both intracellular and extracellular aspartic proteases.66 The latter are produced and secreted into the medium through secretion pathways characteristic of each organism, as explained in the following section.
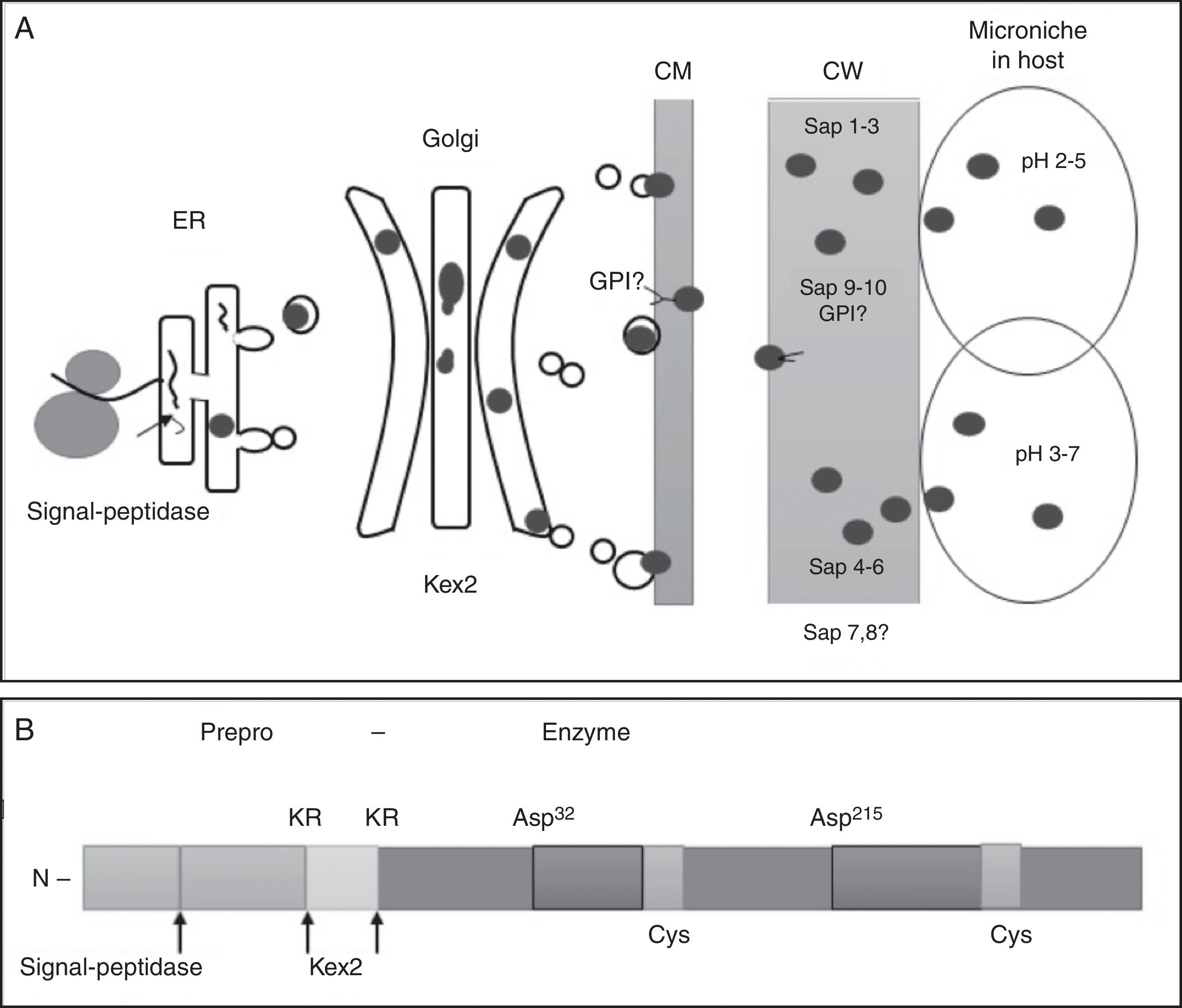
Secretion pathwaysThe secretion pathway for the SAPs in fungi has been best studied in Candida albicans. This process begins when the recently synthesized mRNA is transferred to the cytoplasm, translated into the pre-proenzyme and then transferred to the rough endoplasmic reticulum, where the signal peptide N-terminal is removed by a peptidase. The prepeptide or signal peptide with 16–18 residues of amino acids is required to enter the secretory pathway that transports the protein through the endoplasmic reticulum.56 The proenzyme is transferred to the Golgi apparatus, where it undergoes further processing by a Kex2 proteinase, which recognizes Lys-Arg sequence. There is evidence that in the absence of this enzyme, some SAPs are autocatalytic and process themselves into mature enzymes.36 Before processing by Kex2, the propeptide performs an important function in the proper folding of the domains and in keeping the zymogen inactive.15 Once packed into the secretory vesicles, the enzymes are transported to the plasma membrane, where it may later be incorporated into the cell wall or released to the extracellular space (Fig. 1).30
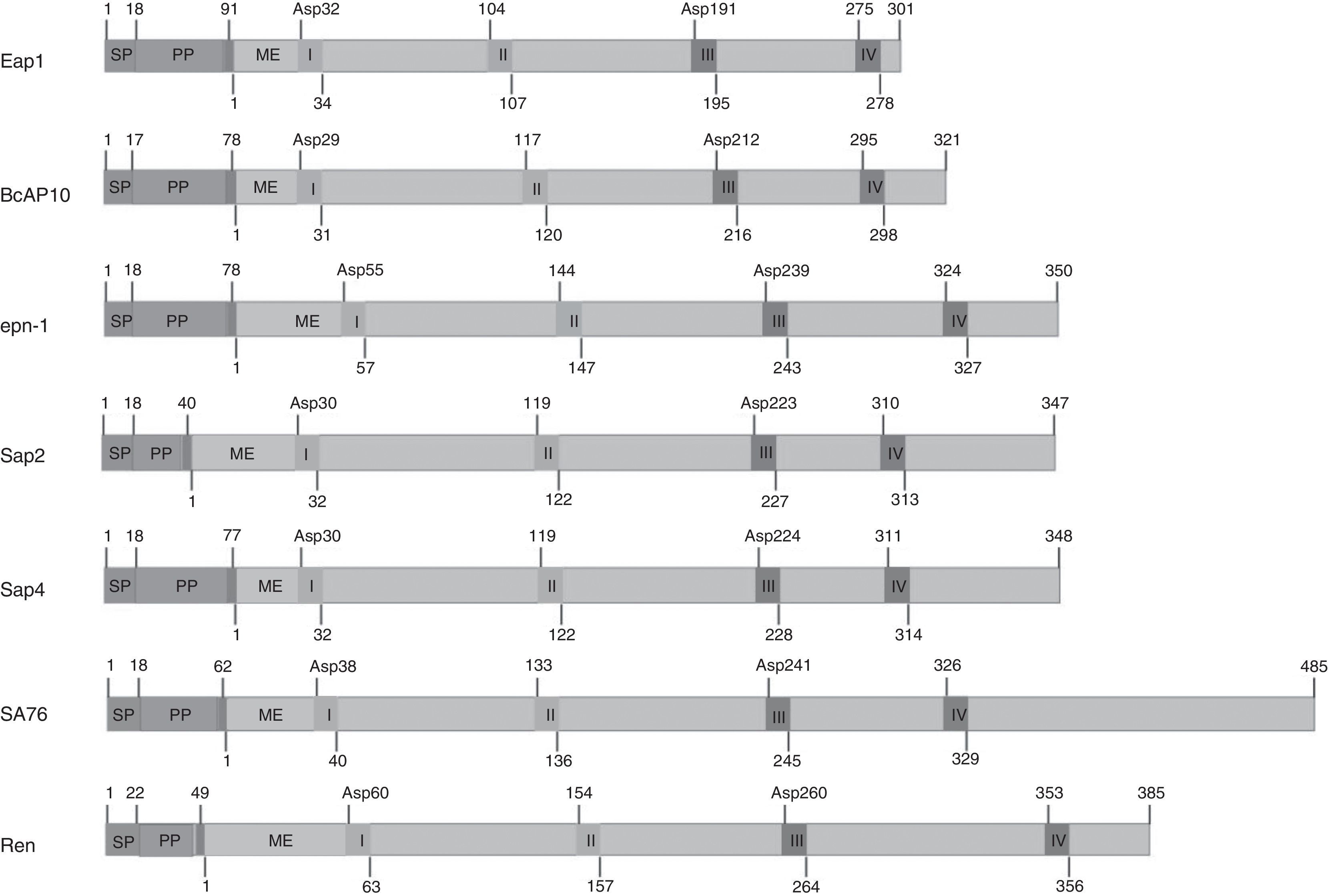
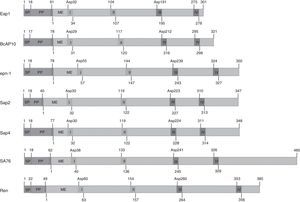
Biochemical propertiesMost SAPs in fungi are synthesized as zymogens, or inactive precursors, which likely provide protection from proteolysis. The zymogen is converted into an active enzyme by a change in pH, which is sufficient to trigger the autocatalytic conversion mechanism.29,73 These enzymes are characterized by the presence of two aspartic residues at the catalytic site. Fig. 2 presents the motifs sites of different fungal SAPs. The predicted zymogens vary in length depending on each fungus. All proteins appear to contain a signal peptide (SP) followed by a propeptide region (PP). All mature enzymes contain the hallmark active site motif Asp-Thr-Gly (I) and its accompanying motif, Gly-Hydrophobic-Hydrophobic-Gly (II), to form the first psi loop. The sequences Asp-Thr/Ser-Gly (III) and Ile-Hydrophobic-Gly-Asp/Gln/Asn (IV) form the second psi loop.
Elements and motifs present in different fungal SAPs. SAPs from plant pathogens: Eap1, BcAP10 and epn-1 (Sporisorium reilianum, Botrytis cinerea and Cryphonectria parasitica respectively). SAPs from human pathogen: Sap2 and Sap4 (Candida albicans). SAP from mycoparasitic fungus: SA76 (Trichoderma harzianum). SAP from important industrial fungi: Ren (Rhizomucor miehei). The numbers indicate the length in aminoacids or its position. SP, PP and ME indicate the signal peptide, the propeptide region and the beginning of the mature protein respectively. The two-aspartic residues at the catalytic site are shown. I (Asp-Thr-Gly) and II (Gly-Hydrophobic-Hydrophobic-Gly) form the first psi loop. III (Asp-Thr/Ser-Gly) and IV (Hydrophobic-Gly-Asp/Gln/Asn) form the second psi loop.
The molecular weights of SAPs are in the range of 30–45kDa.62 They also show affinity for hydrophobic amino acids, usually phenylalanine.35
Acid pH is the optimum one for the activity of SAPs, that display their greatest activity at pH 3–4 with isoelectric points of 3–4.5.62 These enzymes are active in a wide range of temperature, such as the aspartic protease of the psychrotolerant yeast Candida humicola, which is active in a range of 0–45°C.65 Proteases with activity at high temperatures have been described.13
In general, the aspartic proteases perform better on peptide bonds of amino acids with lateral hydrophobic chains (Leu-Tyr, Phe-Phe, Phe-Tyr, etc.), but their affinity for the substrate varies substantially; pepsin degrades most of the proteins into small peptides, but the enzymes utilized in cheese production coagulate the milk through a process of selective excision of the Phe105-Met106 peptide bond of κ-casein.6 The sequencing and structural comparison of these enzymes suggest that two residues of aspartic acid may be responsible for conferring specificity to the fungal SAPs that degrade substrates which contain Lys at position 1.62
Crystallographic studies have demonstrated that these enzymes are bilobed molecules in which the active site is located between the lobes, and each lobe contributes one of the two residues of aspartic acid that are essential for the catalytic activity (psi loops).62
The catalytic mechanism involves residues of aspartic acid at the active site of the peptide chain, bound to a water molecule that acts as a nucleophile.7 There are no functional groups on these enzymes that provoke a nucleophilic attack against the carbonyl of the peptide bond that is to be cleaved, so there is no covalent intermediate between the enzyme and the substrate.59
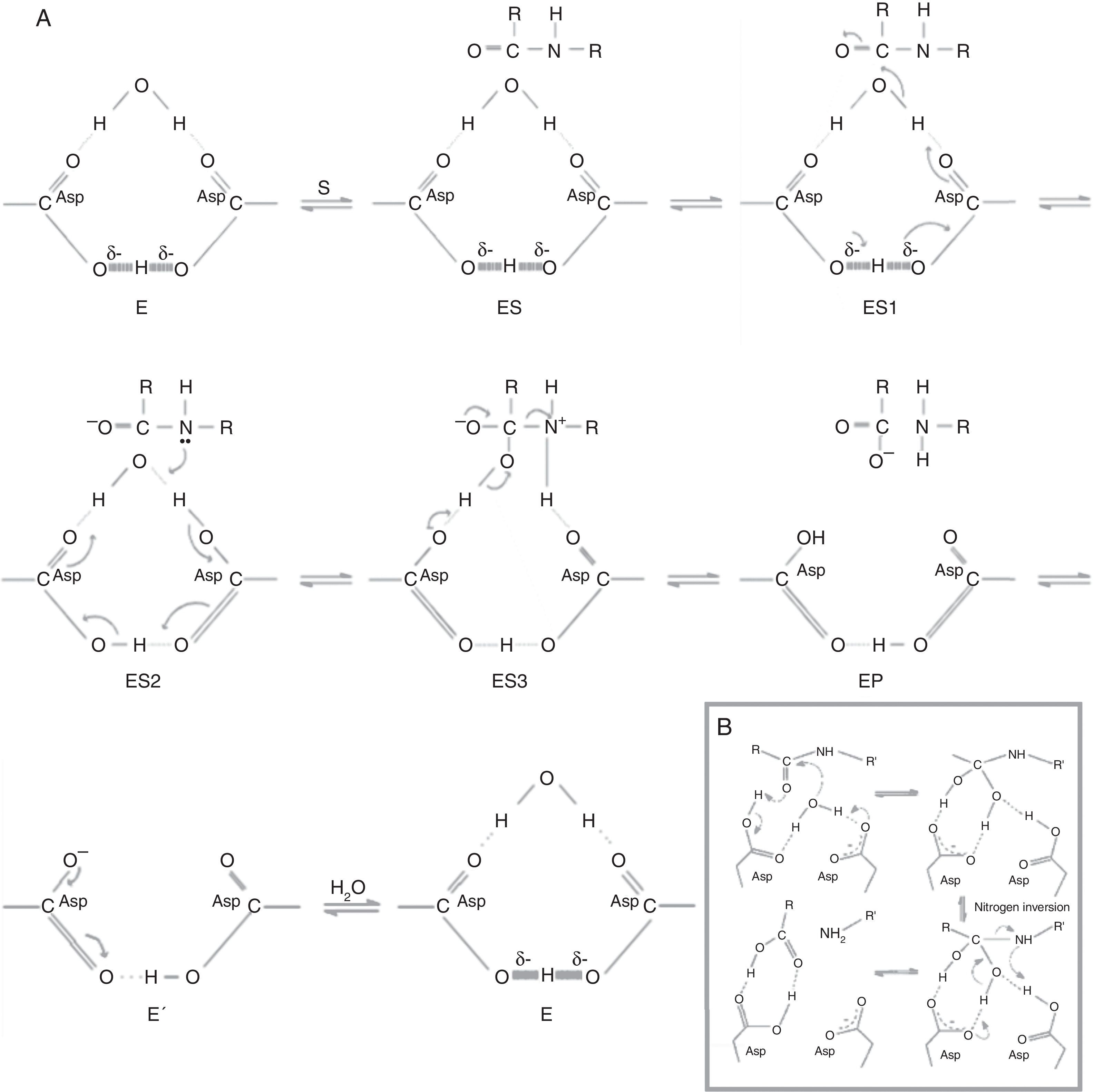
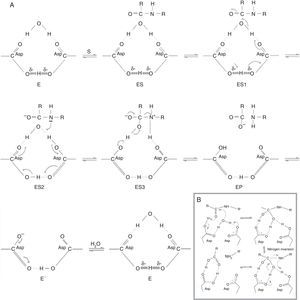
Fig. 3A shows how catalysis begins with the structure of the cycled active site (E), to which the substrate is bound (ES), and then, the electrons extend in a counterclockwise movement through the sensitive carbonyl (ES1) form to generate an intermediate bound to a diprotonated form of the enzyme (ES2). In the next step, the clockwise movement of the two electrons generates an intermediate zwitterion bound to the monoprotonated form of the enzyme (ES3). Finally, the zwitterion collapses, releasing the carboxyls from the enzyme (EP). This completes the chemical processing of the substrate but not that of the enzyme. The regeneration of the latter is completed when it is deprotonated and rehydrated (E′).53
Chemical and kinetic mechanisms of aspartic protease catalysis. (A) Modified from Northrop.53 E: cycled active site; ES: substrate bound to active site; ES1: substrate bound to active site in where the electrons extend in a counterclockwise movement through the sensitive carbonyl; ES2: intermediate bound to a diprotonated form of the enzyme; ES3: intermediate zwitterion bound to the monoprotonated form of the enzyme; EP: product release; E′: deprotonation and rehydration of the enzyme. (B) Modified from Veerapandian et al.82
Fig. 3B shows another mechanism, one in which a water molecule is bound to the two aspartic residues where it acts as a nucleophile that attacks the carbonyl carbon of the bound peptide. This leads to the formation of a tetrahedral intermediate that is stabilized by hydrogen bonds. The fission of the peptide bond (CN), is accompanied by a proton transfer from the amino group of the Asp with nitrogen inversion. Consequently, the other aspartic acid is negatively charged at this stage and is, therefore, ready for the next catalysis. The major difference between these mechanisms is that the final step, in the previous one, involves the binding of a second water molecule (3A).11,77,82
SAPs inhibitorsThe aspartic proteases are generally inhibited by pepstatin, a peptide produced by Streptomyces.29,35,62 However, there are reports of SAPs that are insensitive to pepstatin but sensitive to diazoacetyl-dl-norleucine methyl ester (DAN) and 1,2-epoxy-3-(p-nitrophenoxy)propane (EPNP) in the presence of copper ions.79
The aspartic protease inhibitors are grouped as follows: Kunitz-type inhibitors, which are widely distributed in plants, having been described in legumes, cereals and solanaceae33; Ascaris inhibitors44 isolated from the hemolymph of Apis mellifera10; and the IA3 inhibitor, which is produced by the yeast Saccharomyces cerevisiae.16 Similarly, inhibitors can be classified into categories according to their molecular nature as protein inhibitors or low molecular weight inhibitors.14
Regulation of the expression of the genes encoding SAPsThe principal factor for the regulation of the expression of the genes that encode the SAPs in fungi is pH.40 The transcription factor involved is called PacC, and it activates gene transcription induced at alkaline pH while repressing the genes induced at acid pH. PacC also plays an important role in meiosis, morphogenesis and tolerance to NaCl-induced stress. In addition, it has been demonstrated to be a determining factor of virulence in fungi that are pathogenic for plants and animals, including C. albicans, Aspergillus nidulans, Sclerotinia sclerotiorum and Colletotrichum acutatum.9,54,78
On the other hand, regulation may be related to nitrogen catabolism because one function of SAPs is to degrade exogenous proteins so they release the amino acids utilized by the microorganism.52 Some transcription factors may be involved, as in the case of the SAPs of Rhizopus oryzae and C. albicans; there are reports of their probable function in supplying nitrogen to the microorganism when this element is exhausted in the medium.19,69
Fungal microbial SAPsDifferent non-pathogenic fungi produce SAPs; the mucorales, for example, whose members include Rhizopus, Mucor, Rhizomucor, Absidia and Cunninghamella. Rhizomucor pusillus and Rhizomucor miehei, are commonly used in industry as microbial sources of renins, which are aspartic proteases. Some species of Mucor produce SAPs, including Mucor circinelloides f. circinelloides, Mucor circinelloides f. griseo-cyanus, Mucor circinelloides f. janssenii, Mucor circinelloides f. lusitanicus, Mucor genevensis, Mucor hiemalis f. hiemalis, Mucor hiemalis f. luteus, Mucor piriformis f. piriformis, Mucor piriformis f. nanus, Mucor racemosus f. chibinensis, Mucor subtilissimus, Mucor variosporus and Mucor carbonaceus.3,4,8,20,21,23,41,86 In addition, the SAPs produced by some species of Aspergillus have been purified and characterized and now have important applications in the food industry.71 The aspartic protease PsoP1 described for the basidiomycete Piptoporus soloniensis, has been characterized and might be an efficient substitute for chymosin.18
On the other hand, the function proposed for the SAPs in phytopathogens is the facilitation of the penetration and colonization of a host by degrading the proteins present in the cell walls of plants during infection and releasing the amino acids, which constitute the principal source of nitrogen and sulphur.64 These enzymes have been studied in different fungal phytopathogens as virulence factors.27,49,60,61,80
The SAPs of fungal phytopathogens have been studied due to their role in the life-cycle of these microorganisms and their potential applications in biotechnology. For example, aspartic protease activity was found in carrot tissue infected with Botrytis cinereae. This same fungus has a family of genes that encode for aspartic proteases (Bcap1-14), in which the transcripts of Bcap1, Bcap2, Bcap3, Bcap4 and Bcap5 have been detected in infected plant tissues. On the other hand, the product of Bcap8 is expressed constitutively, and a mutation in this gene has no effect on virulence.24,25,49 The acid protease of Fusarium culmorum may play an important role during processes of infection due to its capacity to degrade plant proteins.80Monilinia fructigena produces an aspartic protease that might be involved in the nutrition of this pathogen.27 The gene aspS encodes for the aspartic protease of S. sclerotiorum, which is expressed at the onset of infection in sunflower cotyledons.60 Interruptions of the gene gcsap, which encodes for an extracellular aspartic peptidase in Glomerella cingulata, demonstrate that this enzyme is not required for pathogenesis.57 The extracellular aspartic protease of Sporisorium reilianum have been purified and typified; this enzyme has the ability to coagulate milk.42Stenocarpella maydis is a fungal pathogen of maize that produces an extracellular acid protease in a minimal medium with glucose at acidic pH, in solid-state and submerged fermentation. High levels of activity were also found in solid fermentation on cobs, broken corn and corn leaves.26
SAPs have also been described in fungi that are pathogenic for humans. This is the case of several species of Candida, in which 10, 4 and 3 genes that encode for these enzymes have been cloned from C. albicans, Candida tropicalis and Candida parapsilosis, respectively. These proteolytic activities aid in the colonization and penetration of tissues.45,54,55 Additionally, studies of the yeast C. albicans have demonstrated that these virulence factors are associated with the formation of hyphae, phenotypical changes, adhesion, and evasion of the host immune response.50,55 Various studies have shown that all the SAPs of C. albicans can be expressed in vitro and present a differential pattern of expression depending on the environmental conditions in which the yeast is found.51,55,76 A total of 10 SAPs have been described for this fungus. Of these, it has been shown that numbers 1–8 are related to protein hydrolysis and tissue damage.75 Expression of the genes SAP1-SAP3 is regulated differentially during phenotypical changes because the encoded isoenzymes for these genes are expressed in opaque cells but not in the white phase when WO-1 yeasts are used.48 Reconstituted human epithelium (RHE) and oral and vaginal epithelium have been used to show that the genes SAP1-3 are expressed in the epithelial colonization stage and, later, during tissue damage when infection is evident. This indicates that the proteins SAP1, 2 and 3 play a role in establishing superficial infections.12,55,70 SAP1, SAP3 and SAP8 are more commonly expressed in oral than vaginal infections.50,55 The proteases SAP4-SAP6 are primarily related to systemic infections32; on the other hand, the genes SAP4, SAP5 and SAP6 were expressed only during the yeast-to-hyphal transition at neutral pH. Like SAP2, SAP4, SAP5 and SAP6 are stable at neutral pH. This difference in the optimal pH of the isoenzymes may be essential for this yeast when it infects the vaginal mucosa (acid pH) or the oral cavity (neutral pH). Other studies indicate that the principal SAP gene transcribed during formation of the germinative tube at neutral pH is SAP6.85 Apparently, of the SAP4-6 group, only SAP5 is required to facilitate the colonization, penetration and infection of the mucosa by C. albicans.2,38,51 Expression of the gene SAP8 is strongly induced at 30°C, suggesting that it may be preferentially expressed during superficial infections.50
The dermatophytes are a group of fungi that is responsible for superficial mycoses in humans and animals, where the neutral or alkaline proteases are important virulence factors. However, in a comparative study between the proteins secreted by Microsporum canis and Arthroderma benhamiae in soy protein medium, an aspartic protease of the pepsin family was secreted at acidic pH.72
In the basidiomycetous yeast Cryptococcus sp. S-2, a novel aspartic protease that was purified hydrolyzed several synthetic substrates in a similar way to that observed with pig pepsin A and human pepsin A.63
Additionally, fungi such as Aspergillus fumigatus secrete an aspartic protease that causes hydrolysis of the structural proteins in the lungs, thus performing an important role in invasive aspergillosis.37 In this same fungus, an aspartic protease called PEP1 that shows a high similarity to the aspergillopepsins of Aspergillus niger was identified.68 It has been suggested that the PEP1 of A. fumigatus may be an allergen associated with allergic bronchopulmonary aspergillosis (ABPA) and may play a less important role in tissue invasion. Other SAP called PEP2 acts on the cell wall of A. fumigatus weakening it to allow the fungus to grow.67
Finally, reports have described that SAPs play an important role in mycoparasitism processes; for example, reports about the fungus Trichoderma harzianum indicate that a protease (SA76) is related to its mycoparasitic activity and shows a 61% identity with the aspartic protease of Gibberella zeae, 37% identity with that of Neurospora crassa, and 33% identity with that of Chaetomium globosum.39 In Trichoderma asperellum, the sequence of a gene that encodes for a protease is homologous to PapA of T. harzianum and to AP1 of Botryotinia fuckeliana. A RT-PCR analysis confirmed that these proteases are induced in vitro when the fungus is confronted with the plant pathogen Rhizoctonia solani.83
Applications for SAPsThe proteases constitute one of the most important groups in the enzyme industry and have diverse applications in a variety of industries, including the production of detergents, foods, pharmaceuticals and leather.62,84 Proteases with high activity and stability at acid pH have important industrial applications, specifically in food industries such as dairy industries in which they are used as milk-coagulating agents in cheese-processing and as flavor enhancers in other foods.62,71,75 The aspartic proteases have been used as microbial coagulants in cheese making.1 The scarcity of calf rennet caused by the increased demand for this product by cheese-makers has led to an intensification of the search for alternatives for coagulating milk.62 The primary function of the proteases in the cheese industry is to hydrolyze the peptide chain Phe105-Met106 or Ser104 and Phe105 to produce κ-casein and macropeptides.17,62 Due to its specificity, chymosin is used preferentially to hydrolyze casein, the substance responsible for its excellent performance in cheese production.34
The proteases produced by the GRAS microorganisms (Generally Recognized As Safe), such as R. miehei and Endothia parasitica, have gradually replaced chymosin in the cheese-making industry. In 1988, the first recombinant chymosin was introduced into the cheese industry for evaluation, and Genecor International soon increased the production of chymosin in A. niger var. awamori to commercial levels.22 Among the alternatives that have been tested as possible microbial substitutes, perhaps the most important are R. miehei and R. pusillus. Other SAPs that have the capacity to coagulate milk have been isolated from Mucor bacilliformis, S. reilianum and Cryptococcus sp. S-2.5,42,63
In addition, some fungal aspartic proteases have been used to degrade proteins that cause turbidity in juices and wine, such as the protease BcAP8 from B. cinerea, and the aspergillopepsin I from Aspergillus saitoi, both used in wine-making because they effectively remove haze-forming proteins, thus reducing bentonite requirements.43,58,75,81 We consider that secreted aspartyl proteases have biochemical characteristics that place them as fungal proteases of interest for study and biotechnological application.
Conflict of interestThe authors declare no conflict of interest.
Virginia Mandujano-González is a fellow of CONACyT México. This work was supported by the Fondo de Ciencia Básica SEP-CONACyT México.