Infections caused by Fusarium are difficult to treat because these fungi show in vitro and in vivo resistance to practically all the antifungal agents available, which explains the high mortality rates. An attempt to overcome fungal resistance is the combination of antifungal agents, especially those with different mechanisms of action.
AimsEvaluate the in vitro interactions of combinations of voriconazole or itraconazole with other antifungal agents against 32 isolates of Fusarium spp.: Fusarium chlamydosporum, Fusarium oxysporum, Fusarium proliferatum and Fusarium solani.
MethodsDrug interactions were assessed by a checkerboard microdilution method that also included the determination of the MIC of each drug alone according to CLSI (Clinical and Laboratory Standards Institute) document M38-A2, 2008.
ResultsThe best combinations were voriconazole+terbinafine which showed synergism against 84% of Fusarium strains. Other synergistic combinations were voriconazole+itraconazole (50%), voriconazole+fluconazole (50%), voriconazole+miconazole (38%), voriconazole+flucytosine (22%) and voriconazole+ketoconazole (25%). The synergisms observed with itraconazole combinations were itraconazole+terbinafine (25%) and itraconazole+flucytosine (9.37%). The antagonisms observed were: voriconazole+fluconazole (3%) and itraconazole+flucytosine (12.5%).
ConclusionsThe synergism showed by voriconazole+terbinafine was remarkable. To better elucidate the potential usefulness of our findings, new in vivo and in vitro studies deserve be performed.
Las micosis causadas por Fusarium son difíciles de tratar porque tanto in vivo como in vitro estos hongos muestran resistencia a casi todos los fármacos antimicóticos disponibles, lo que explica las altas tasas de mortalidad. Una tentativa de resolver la resistencia fúngica es combinar los fármacos antifúngicos, en especial los preparados con mecanismos de acción diferentes.
ObjetivosValorar las interacciones in vitro de la combinación de voriconazol o itraconazol con otros fármacos antimicóticos frente a 32 aislamientos de Fusarium spp: Fusarium chlamydosporum, Fusarium oxysporum, Fusarium proliferatum y Fusarium solani.
MétodosLas interacciones farmacológicas se valoraron con el método de microdilución en tablero de ajedrez, que también incluyó la determinación de la concentración inhibitoria mínima de cada fármaco por separado de acuerdo con el documento M38-A2, 2008, del Clinical and Laboratory Standards Institute.
ResultadosLas mejores combinaciones fueron voriconazol+terbinafina que mostró sinergia frente al 84% de los aislamientos de Fusarium. Otras combinaciones sinérgicas fueron: voriconazol+itraconazol (50%), voriconazol+fluconazol (50%), voriconazol+miconazol (38%), voriconazol+flucitosina (22%) y voriconazol+ketoconazol (25%). Las sinergias observadas con las combinaciones de itraconazol fueron itraconazol+terbinafina (25%), e itraconazol+flucitosina (9,37%). Los antagonismos observados fueron voriconazol+fluconazol (3%) e itraconazol+flucitosina (12,5%).
ConclusionesLa sinergia observada con voriconazol+terbinafina fue extraordinaria. Para dilucidar mejor la utilidad potencial de los hallazgos del presente estudio, han de realizarse nuevos estudios tanto in vivo como in vitro.
Fusarium species are a common soil saprophyte and important plant pathogens,13 and they may occasionally cause infection in animals.15 In humans, Fusarium species cause a broad spectrum of infections, including superficial, locally invasive, and disseminated disease.22 The infections in severely immunocompromised patients are associated with a poor clinical response to antifungal therapy and high mortality rates.9,23,28 So, the successful outcome is determined by the degree of immunosuppression and the extent of the infection.23,26 Due to its lesser frequency than other mycosis, as aspergilosis and candidiasis, the optimal treatment of fusariosis has not been established.7,28
This scenario is usually worsened because Fusarium infections are relatively resistant to treatments with antifungal agents, also confirmed by the susceptibility tests.4,5,17,26 In general Fusarium species are resistant to echinocandins whilst susceptibility to amphotericin B, voriconazole and posaconazole are variable.9 Therefore the use of a drug alone to treat fusariosis has not changed the mortality rates, which has remained high among immunocompromised patients.7 Combination therapy could be used for improving the efficacy of antimicrobial therapy for difficult-to-treat infections, expanding the antifungal activity.1,18 Salvage therapy combining amphotericin B+voriconazole has been used with good response.9
In general, the in vitro studies with associations of antifungal agents combines agents from different classes but previous studies have also reported synergism between two azoles as fluconazole+miconazole20 or fluconazole+itraconazole.31 Here we also explored this idea but focusing on voriconazole combined with azoles, terbinafine and flucytosine against 32 Fusarium isolates. The effects of the combinations between itraconazole plus terbinafine or flucytosine were also investigated.
Materials and methodsThirty-two clinical Fusarium strains were tested in this study: Fusarium chlamydosporum (4), Fusarium oxysporum (8), Fusarium proliferatum (2), Fusarium solani (17) and F. solani ATCC 36031 (1). The strains were obtained from different sources, including blood culture (n=15), tissue biopsy (n=10), cornea (n=3), sediment of dialysate from CAPD (n=2), fissures in interdigital area (n=1), and contact lens solution (n=1). Isolation and identification of the isolates were performed by standard microbiological and molecular techniques. Molecular analysis was performed to confirm the identity of each Fusarium isolate. A DNA fragment comprising an internal transcribed spacer (ITS), which was amplified using primers ITS1 (5′-TCCGTAGGTGA-ACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGAT-ATGC-3′).25 The amplified fragments were sequenced, and the sequences were compared with DNA sequences of Fusarium obtained from GenBank which accession numbers were the ones that follow: F. chlamydosporum (HQ696899, HQ696900, HQ696908, HQ696909), F. oxysporum (HQ696898, HQ696897, HQ696896, HQ696895, HQ696894, HQ696893, HQ696892, HQ696891), F. proliferatum (HQ696886, HQ696887), F. solani (HQ696874, HQ696875, HQ696876, HQ696877, HQ696878, HQ696879, HQ696880, HQ696881, HQ696882, HQ696883, HQ696884, HQ696885, HQ696901, HQ696902, HQ696903, HQ696904, HQ696905).
Antifungal drugs were obtained as standard powders of known potency. Voriconazole (VCZ) (Pfizer, Brazil), terbinafine (TBF) (Sigma Pharma, Brazil), itraconazole (ITZ) (Cristalia, Brazil), miconazole (MCZ) (Cristalia, Brazil), and ketoconazole (KTZ) (Cristalia, Brazil) were dissolved in dimethylsulfoxide (Sigma Chemical Co.) while fluconazole (FCZ) (Medley, Brazil) and flucytosine (FCY) (Hoffman La Roche, Switzerland) were dissolved in distilled sterile water to make stock solutions. Final dilutions were made in RPMI 1640 with glutamine and without sodium bicarbonate (Gibco BRL–Life Technologies) buffered to pH 7.0 with MOPS 0.165M (Sigma Chemical Co.) and adjusted with 0.1M NaOH. The final concentration ranges were 0.25–32μg/mL for flucytosine, 0.5–64μg/mL for fluconazole and 0.125–16μg/mL for miconazole, ketoconazole, itraconazole, voriconazole and terbinafine.
Drug interactions were assessed by a checkerboard microdilution method that also included the determination of the minimum inhibitory concentration (MIC) of each drug alone according to CLSI M38-A2 (Clinical and Laboratory Standards Institute; CLSI, 2008)10 recommendations with serial twofold dilutions of drug combination. Candida parapsilosis ATCC 22019, Candida krusei ATCC 6258 and Aspergillus flavus ATCC 204304 were included as quality control strains for the determination of the MICs.
The isolates were cultured on potato dextrose agar slants at 35°C for 3 days and at 25°C for the following 4 days. The inoculum was prepared by scraping the surface of 7 days old fungal colonies from the agar growth using a loop and suspending the material in sterile saline solution. The density of conidial suspension was adjusted to a 70% transmittance at 530nm. After this, the suspensions were diluted 1:50, and then 1:2 when 100μL were inoculated in the wells containing an equal volume of drugs.
Aliquots of 50μL of drug A plus 50μL of drug B at a concentration four times the targeted final concentration were dispensed in the wells in order to obtain a two-dimensional checkerboard. Conidial suspensions were prepared as described above, and 100μL of the inoculum were added to the wells. The microtiter plates were incubated for 48h at 35°C. For each strain tested, a positive (inoculum diluted) and negative control (only RPMI) were performed.
The MIC endpoints for the antifungal agents alone were: (a) the lowest concentration of drug that produced complete inhibition (100%) of the fungal growth for voriconazole and itraconazole; (b) the lowest concentration that produced 80% of growth inhibition for tests with terbinafine and (c) the lowest concentration that inhibited 50% of the fungi growth for tests with flucytosine, ketoconazole and miconazole. As all combined MICs contained voriconazole or itraconazole, in the combinations tests the MICs were considered as the lowest concentration that produced 100% of growth inhibition. Off scale MICs were converted to the next-higher twofold concentration.
Drug interactions were evaluated using a checkerboard titration method for the following combinations: VCZ+TBF, VCZ+ITZ, VCZ+FCZ, VCZ+MCZ, VCZ+FCY, VCZ+KTZ, ITZ+TBF and ITZ+FCY. The fractional inhibitory concentration index (FICI) was used to classify drug interaction. The FICI was calculated using the following formula: (MIC of drug A in combination/MIC of drug A alone)+(MIC of drug B in combination/MIC of drug B alone). Drug interactions were considered as synergistic for FICI≤0.5, not interactive (indifferent) when the FICI was between 0.5 and 4 and antagonistic for FICI>4.18
ResultsTotal absence of activity against all Fusarium strains studied was observed for flucytosine (MIC>32μg/mL), fluconazole (MIC>64μg/mL) and itraconazole (MIC>16μg/mL) when tested alone. The susceptibility to the rest of the antifungal agents showed the following ranges: ketoconazole (2–6μg/mL), miconazole (4–16μg/mL), terbinafine (2–16μg/mL) and voriconazole (2–8μg/mL).
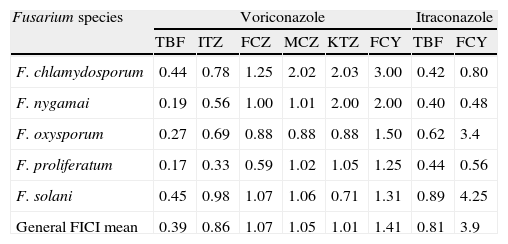
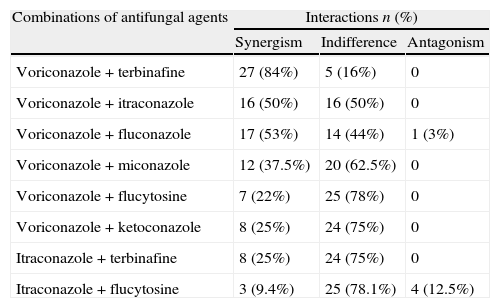
The results of the checkerboard analysis (FICI means) for voriconazole and itraconazole combinations against Fusarium strains are summarized in Table 1. The combination VCZ+TBF showed synergism for 27 strains (84%). Indifference was observed in one strain of F. chamydosporum, one strain of F. oxysporum and three strains of F. solani, totaling 16% of indifferent interactions. Antagonisms were not observed for this combination. The interactions obtained by the combinations are shown in Table 2.
Geometric mean fractional inhibitory concentration indexes (FICIs) for voriconazole and itraconazole in association with antifungal agents against Fusarium species.
| Fusarium species | Voriconazole | Itraconazole | ||||||
| TBF | ITZ | FCZ | MCZ | KTZ | FCY | TBF | FCY | |
| F. chlamydosporum | 0.44 | 0.78 | 1.25 | 2.02 | 2.03 | 3.00 | 0.42 | 0.80 |
| F. nygamai | 0.19 | 0.56 | 1.00 | 1.01 | 2.00 | 2.00 | 0.40 | 0.48 |
| F. oxysporum | 0.27 | 0.69 | 0.88 | 0.88 | 0.88 | 1.50 | 0.62 | 3.4 |
| F. proliferatum | 0.17 | 0.33 | 0.59 | 1.02 | 1.05 | 1.25 | 0.44 | 0.56 |
| F. solani | 0.45 | 0.98 | 1.07 | 1.06 | 0.71 | 1.31 | 0.89 | 4.25 |
| General FICI mean | 0.39 | 0.86 | 1.07 | 1.05 | 1.01 | 1.41 | 0.81 | 3.9 |
FCZ, fluconazole; FCY, flucytosine; ITZ, itraconazole; KTZ, ketoconazole; MCZ, miconazole; TBF, terbinafine.
Drug interactions were classified as synergistic when the FICI was less than or equal to 0.5, indifferent when the FICI was greater than 0.5 and less than or equal to 4 and antagonistic when the FICI was greater than 4.
Synergistic, indifferent and antagonistic interactions for combinations of voriconazole or Itraconazole with antifungal agents against 32 clinical Fusarium spp. strains.
| Combinations of antifungal agents | Interactions n (%) | ||
| Synergism | Indifference | Antagonism | |
| Voriconazole+terbinafine | 27 (84%) | 5 (16%) | 0 |
| Voriconazole+itraconazole | 16 (50%) | 16 (50%) | 0 |
| Voriconazole+fluconazole | 17 (53%) | 14 (44%) | 1 (3%) |
| Voriconazole+miconazole | 12 (37.5%) | 20 (62.5%) | 0 |
| Voriconazole+flucytosine | 7 (22%) | 25 (78%) | 0 |
| Voriconazole+ketoconazole | 8 (25%) | 24 (75%) | 0 |
| Itraconazole+terbinafine | 8 (25%) | 24 (75%) | 0 |
| Itraconazole+flucytosine | 3 (9.4%) | 25 (78.1%) | 4 (12.5%) |
When VCZ was combined with ITZ, significant reductions in MIC geometric mean to ITZ (from >16 to 0.125μg/mL) and in MIC geometric mean to VCZ (3.2–1.95μg/mL) were present. This combination was synergistic for 50% of the strains and equally indifferent for all of them.
The mean MIC to FCZ for all isolates have decreased from >64μg/mL to 0.125μg/mL when it was used in combination with VCZ. Here, 53% of the strains tested demonstrated synergism, 44% were indifferent and against one isolate of F. solani this combinations was antagonistic.
When VCZ was combined with MCZ the number of synergistic interactions was 12/32 (37.5%) and 62.5% were indifferent interactions but antagonism was not detected.
The combination VCZ plus FCY demonstrated synergism for only 7 isolates (22%) and the interactions were indifferent for 25 strains (78%). Although the MIC geometric mean for FCY dropped from >32 to 0.125μg/mL when it was combined with the VCZ, this reduction was not significant to promote synergistic activity against the most Fusarium strains.
For the combination VCZ+KTC the predominance of indifference interactions (75%) were observed, while synergistic interactions were observed for 25% (8 of 32) of the strains tested. Synergy activity was observed for all F. chlamydosporum, F. proliferatum, and for only one F. solani; no one F. oxysporum strain was sensible for this combination as well as 94.1% of F. solani strains.
The best result for combinations with ITZ was ITZ+TBF which showed 8/32 (25%) of synergisms but no one antagonism. However, it is important to emphasize that this combination was indifferent for 100% of the F. solani strains and for 75% of the F. oxysporum strains.
With flucytosine the interactions showed 9.3% of synergism, 78.1% of indifference and 12.5% of antagonism. No one synergism was obtained from tests against F. solani and F. oxysporum which showed 11.7% and 25%, respectively of antagonistic interactions.
DiscussionCombined antifungal therapy can be a potential strategy to improve the prognosis of some fungal infections. The in vitro studies evolving drugs associations have reported a lot of combinations including those with antifungal+non-antifungal agents, sometimes showing synergisms.1 So, it seems rational to also explore meticulously the combinations antifungal+antifungal agents mainly against microorganisms difficult to treat as Fusarium species. In order to uncover potent antifungal combinations, the current study evaluated the interactions between VCZ with FCY, FCZ, ITZ, KTZ, MCZ and TBF and also the combinations evolving ITZ plus TBF or FCY against Fusarium strains by checkerboard microdilution assay.
In general our results showed a poor susceptibility of Fusarium for all the drugs tested alone, as previously described by some authors.4,5,26 Despite the high in vitro MIC values, VCZ have been proposed as monotherapy for fusariosis in patients refractory to other drugs.9F. proliferatum demonstrated a major number of synergistic interactions while F. solani, and F. oxysporum showed a more resistant susceptibility pattern.
Combination between VCZ plus TBF showed the best synergism (84%) and antagonism was not observed for this combination. It is in accordance with Ortoneda et al.26 and Córdoba et al.11 who reported the same synergism in 72.7% and 72.4%, respectively. Azor et al.,5 studying the susceptibility of 48 Fusarium strains against 11 antifungal agents, observed that terbinafine was the most active drug against all the species tested, what corroborates our results. The enhancement of the in vitro activity of VCZ when in combination with TBF has been explained by the blockage of ergosterol biosynthesis at different levels.11
In general the in vitro studies focusing associations of antifungal agents have combined drugs from different classes in order to add two mechanisms of action. So, the most studied combinations include polyene+azoles or polyene+pyrimidine analog (flucytosine) or azoles+echinocandins or polyene+echinocandins.18 Combinations among antifungal agents plus non-antifungal agents have also been explored.1 Our study has also included combinations among azoles as VCZ+KTZ, VCZ+MCZ, and VCZ+ITZ. Azole–azole combinations have been employed on a limited number of studies.18 The combination of FCZ plus ITZ in an experimental systemic cryptococcosis and meningitis resulted in improvements in tissue sterilization.31 Another cornerstone for our investigation was Mykami et al.20 who reported synergism between FCZ+MCZ against Candida. It was suggested that the mechanism of this effect would be the direct membrane damage induced by miconazole what might make the membrane more fragile and make easy the entrance of fluconazole into the fungal cell. Here we explored the same idea but focusing voriconazole in combination with other azoles. Among the azole+azole combinations tested, VCZ plus ITZ and VCZ plus FCZ have demonstrated 50% to 53% respectively of synergistic interactions against different species of Fusarium spp. The antagonistic interaction between VCZ plus FCZ (3%) have not been reported because this combination is not common; the mechanisms involved in this interactions seem to be unknown. Combinations of VCZ with MCZ and KTZ have demonstrated indifferent interactions for most strains tested. As far as we know combinations among voriconazole and azoles have not yet been studied against Fusarium.
The combinations azoles+FCY have been lesser studied than amphotericin B+FCY. Although previous investigations of the interactions between FCY with older azoles (miconazole and econazole) had suggested antagonism,14 recent reports of triazole–FCY combinations have indicated synergism or indifference.2,6,16,21,24,30 Here we obtained 22% of synergism by VCZ+FCY against Fusarium, in spite of indifferences being remarkable. Combination between VCZ and FCY has not yet been studied for Fusarium. The most combinations among azoles+FCY have been studied focusing Cryptococcus and Candida because these genera are primarily sensitive to FCY and the addition of a triazole suppresses the emergence of flucytosine-resistant mutants.18
The combination between ITZ and TBF showed 25% of synergism but no antagonism was registered. This is partially in accordance with the results reported by Ortoneda et al.26 who found 36.3% of synergism and 45.4% of antagonism. This combination has been studied by other authors who have noticed synergisms against Candida,8Aspergillus,29 and against two very problematic microorganism, as Scedosporium prolificans19 and Pythium insidiosum.3 The positive interaction may be due to the combined effect of TBF and the azoles on different targets in the ergosterol biosynthesis pathway.26 Our results were similar to the finding pointed by Córdoba et al.11 that this combination did not show synergism against F. solani, even though they have been detected for some isolates of F. oxysporum.
The combinations of FCY+ITZ or KTZ or posaconazole in animal models have resulted in improvements in survival12,27 and tissue clearance31 in cases of cryptococal meningitis. In addition, in a clinical case series of systemic mycosis the outcomes with ITZ+FCY were comparable or better than those for ITZ alone.32 The azole damages the fungal cell membrane, enabling increased uptake of flucytosine. This mechanism may explain potential synergism between azoles and flucytosine.18 Here we showed only 3 (9.4%) synergistic interactions which were against F. chlamydosporum and F. proliferatum that confirm the variability of susceptibility among Fusarium species already suggested by others.11,23,26
Finally, our study demonstrated that the in vitro combination of VCZ against the genus Fusarium can be enhanced by other drugs showing different mechanisms of action including drugs of the same class as azoles. To better elucidate the potential usefulness of our findings, new in vivo and in vitro studies should be performed.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank Camila Donato Mahl and Maria Izabel Azevedo for the technical assistance during molecular identification of the Fusarium spp.