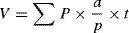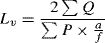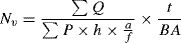Aging is an irreversible process associated with decreased biological functions that can lead to the reduction of reproductive organs capacities in males and females. Paternal age is a significant predictor of offspring health and development. So, the aim of this study was to evaluate the effects of vitamin C on histopathological and biochemical testicular changes following aging process with a focus on stereological methods.
Material and methodsFor this study, 48 adult male NMRI mice were divided into two control and experimental groups. Mice in experimental group were supplemented with vitamin C (150mg/kg) including 24-h interval by oral gavage for 33 weeks. Same regime was performed for animals in control group except that vitamin C was replaced by water. Then, right testes were extracted for stereological and left testes were used for molecular analyses on weeks 8, 12, and 33.
ResultsOur findings showed low semen quality, decreased level of serum Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), and testosterone along with increased reactive oxygen species (ROS) production and higher apoptotic gene expression following aging. Stereological studies showed that the volume of testes, the length of seminiferous tubules, and the number of spermatogenic and none-spermatogenic cells decreased significantly during aging. Also, vitamin C consumption for 33 weeks significantly improved biochemical and histological indices. The impact of aging on male reproduction seems to be inevitable worldwide. Therefore, the use of protective and preventive remedies conserving male fecundity is very important and based on our results, vitamin C is a beneficial candidate for improving age-related testicular changes due to aging process.
El envejecimiento es un proceso irreversible asociado a una disminución de las funciones biológicas que puede conducir a la reducción de la capacidad de los órganos reproductivos en hombres y mujeres. La edad paterna es un predictor significativo de la salud y el desarrollo de la descendencia. Por lo tanto, el objetivo de este estudio fue evaluar los efectos de la vitamina C sobre los cambios testiculares histopatológicos y bioquímicos posteriores al proceso de envejecimiento con un enfoque en los métodos estereológicos.
Material y métodosPara este estudio, 48 ratones NMRI machos adultos se dividieron en dos grupos de control y experimentales. Los ratones del grupo experimental se suplementaron con vitamina C (150mg/kg), incluido un intervalo de 24 horas mediante sonda oral durante 33 semanas. Se realizó el mismo régimen para los animales del grupo de control, excepto que se reemplazó la vitamina C por agua. Luego, se extrajeron los testículos derechos para estereología y los testículos izquierdos se utilizaron para análisis moleculares en las semanas 8, 12 y 33.
ResultadosNuestros hallazgos mostraron baja calidad del semen, disminución del nivel de hormona luteinizante, hormona estimulante del folículo y testosterona en suero, junto con una mayor producción de especies reactivas de oxígeno (ROS) y una mayor expresión de genes apoptóticos después del envejecimiento. Los estudios estereológicos mostraron que el volumen de los testículos, la longitud de los túbulos seminíferos y el número de células espermatogénicas y no espermatogénicas disminuyeron significativamente durante el envejecimiento. Además, el consumo de vitamina C durante 33 semanas mejoró significativamente los índices bioquímicos e histológicos. El impacto del envejecimiento en la reproducción masculina parece ser inevitable en todo el mundo. Por lo tanto, el uso de remedios protectores y preventivos que conserven la fecundidad masculina es muy importante y según nuestros resultados, la vitamina C es un candidato beneficioso para mejorar los cambios testiculares relacionados con la edad debido al proceso de envejecimiento.
Aging is a progressive irreversible process accompanied by a decline in biological functions of the body that can result in physical, psychological, and social disabilities.1,2 Reproductive organs capacities decline in both males and females by aging process, although it has been reported that the changes are milder in men compared to women.3–5
It is important to note that the average of paternal age has been increasing in the different countries because of life style changes.6 Hence, progressive paternal age can cause several fertility complications in couple and newborn. Lower testicular volume, diminished number of germ cells and Sertoli cells, testicular atrophy, and lower quality of semen are some pathologic changes during aging process.5,6 Thickening of the basement membrane and increased rate of aneuploidy in post-meiotic spermatogenic cells are reported following aging in men. Oxidative stress, apoptosis, androgen insufficiency, and chronic low-grade inflammation are some mechanisms of pathologic alterations in testis during aging.5,7–9
Ascorbic acid or vitamin C is a water-soluble vitamin with different therapeutic properties including antioxidant, anti-inflammatory, and immune-supporting effects that plays an essential role in prevention of cell and tissue injuries.10,11 Nevertheless, to the best of our knowledge, scarce studies have been performed around the effect of vitamin C on testicular stereological markers during aging process in male mice. For that reason, in the present study, we aimed to evaluate the influence of vitamin C on spermatogenesis and multiple indices of testicular tissue.
Material and methodsAnimalsProtocol of the present study was reviewed and confirmed by Ethics Committee at Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.MEDICAL.REC.1399.833). In this study, 48 adult 5–6-week-old male NMRI mice weighing 25–30g were obtained from Iran Pasteur Institute. Mice were kept in animal house under standard conditions (22±2°C and 12-h light/dark) and provided with free access to water and food. Then, mice were divided into two control and experimental groups. Animals in experimental group were supplemented with vitamin C (150mg/kg) 12,13including 24-h interval by oral gavage for 33 weeks. Vitamin C (l-ascorbic acid; Sigma, St. Louis, MO, USA) was prepared by diluting in warm water. Same regime was followed for animals in control group except that vitamin C was replaced by water. On weeks 8, 12, and 33, after 12h of fasting, mice were anesthetized under general anesthesia with ketamine (80mg/kg) and xylazine (10mg/kg) intraperitoneally, then blood samples were collected from the heart, mice were euthanized sacrificed, and finally, the right testes were extracted for stereology analysis and left testes were used for molecular and cellular analyses.
Semen analysisAt the end of the study, sperm samples were obtained from the tail of epididymis and then moved to the 1mL of Ham's F-10 media (Sigma–Aldrich Product Number N6635). Subsequently, to observe sperm motility, 10μL of sample was placed on a slide after being incubated at 37°C for 20min. Finally, counting chamber was used for sperm count. Furthermore, Eosin-Nigrosine staining method was used to evaluate sperm viability and sperm morphology.
Measurement of serum levels of Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), and testosteroneBlood samples were centrifuged at 6000 G for 5min at 4°C and then stored at −80°C until the test was performed. Then, mouse specific ELISA kit was utilized to measure the serum levels of LH, FSH, and testosterone (catalog No. CSB-E11162r).
Reactive oxygen species (ROS) in testicular tissueAfter isolation of testis cells with trypsin EDTA solution, the samples were washed in Phosphate-buffered saline (PBS) and centrifuged at 1200rpm for 5min at 4°C. Next, 20μM in a 100μL aliquot DCFDA fluorogenic dye was added to the samples and then the samples were stored in a 37°C incubator for 45min. Finally, analysis of the specimens was conducted by a flow cytometer with a wavelength of 495nm.14
Glutathione peroxidase (GPx) content assessmentsGPx assay kit (Zelbio GmbH) was used for determining GPx in the testis tissue samples with 5U/mL sensitivity (5kU/L). This assay examined the GPx activity unit as the content of the sample which would catalyze decomposition of 1μmol of GSH to GSSG in 1min. Aliquots of the testicular cells suspension (0.5mL) that have been formerly stained with OPA and NEM probe (5μM), were isolated from incubation medium centrifugation at 1000rpm for 1min. In the next stage, in order to completely remove the fluorescent dye, cell pellet was suspended in 2mL of the fresh incubation medium in two consecutive steps. Finally, all samples were assessed in the quarts cuvettes with the Shimadzu RF5000U fluorescence spectro-photometer adjusted at 495nm excitation and 530nm emission wave-length.15
Tissue preparationAt the end of study, testicular tissue samples were isolated and preserved in Bouin's fixative solution for 48h. Next, the samples were processed in a series of alcohol and xylene solutions and finally, embedded in paraffin blocks. In order to stereological analyses, two types of histological serial sections were prepared using a microtome (Leica RM2125 RTS, Germany). The Systematic Uniform Random Sampling (SURS) was applied to choose 10 sections of each sample by picking a random figure in the range of 1–10 and the selected samples were stained with hematoxylin and eosin (H&E, Sigma, USA). Serial sections with a thicknesses of 5 and 25μm were used to estimate the volume and the cell number of testis, respectively. Testicular cells were morphologically detectable. So that the Leydig cells as polyhedral cells with spherical nucleus and eosinophilic cytoplasm, were positioned in the interstitial tissue of seminiferous tubules. The Sertoli cells with a huge, basal, and oval pale nucleus were located at the base of the epithelium of seminiferous tubules. The dome-shaped spermatogonia with a dark or light round nuclei were placed at the base of the epithelium. Primary spermatocytes as the largest cells of seminiferous tubules were situated at the middle of germinal epithelium and eventually, the spermatids with round or spherical morphology were located toward the seminiferous tubule lumen.
Volume of testisThe total volume of testis was assessed using the Cavalieri method and following formula.16,17
where ΣP is the total points hitting the testis sections, a/p is the area associated with each point, and t is the distance between the sampled sections.Length of seminiferous tubulesIn order to estimate the length of seminiferous tubules and the length density of seminiferous tubules, following equation was used:16,17
where ΣQ is the total number of the seminiferous tubules, a/f is the area per frame, and ΣP is the total points superimposed on testis tissue. After that, the total length of seminiferous tubules was estimated by multiplying the length density (Lv) by the total volume of testis.Testis cell numberTestis cell number was estimated using the optical dissector method.18 Numerical density (Nv) of testis cells was calculated using the following formula:
where ΣQ is the number of cells, ΣP is the number of counting frame grid in all fields, a/f is the area of frame, h is the dissector height, BA is the thickness of microtome section, and t is the real section thickness.Analysis of caspase-3, Bax, and Bcl-2, expression using real-time PCRThe real-time PCR method was carried out to determine the expression rates of caspase-3, Bax, and Bcl-2 genes. For this purpose, the total RNA was extracted from testis samples using RNA extraction kite (Life Technologies, Gent, Belgium) according to the manufacturer's instruction. The concentration of extracted RNA was determined with spectrophotometer (Pico drop Real-Life) and total RNA suspended in 10μl of DEPC water was stored at −80°C.19 Following extraction of all RNA samples, DNase I (Roche, Basel, Switzerland) was used to remove contamination induced by genomic DNA. At that point, a commercial kit (Fermentas, Lithuania) was utilized in order to synthesize cDNA at 42°C for 60min in accordance with manufacturer instructions. Finally, real-time PCR was applied based on QuantiTect SYBR Green RT-PCR kit (Takara Bio Inc, Japan) for quantification of relative expression of genes. All pairs of reverse and forward primer were also designed by Primer 3 Plus software using an exon-exon junction method for separation of cDNA from genomic DNA. Prior to that, PCR primers were tested using Primer-Blast tool available at the website, www.ncbi.nlm.nih.gov/tools/primer-blast20(Table 1).
Real time PCR primer sequences for the studied genes.
| Genes | Primer sequences |
|---|---|
| GAPDH | F: CTCAAGATTGTCAGCAATGCR: CAGGATGCCCTTTAGTGGGC |
| Caspase3 | F: AGCTTCTTCAGAGGCGA CTAR: GGACACAATACACGGGATCT |
| BAX | F: AGGATAGAGCAGGGAGGATGGR: TGGTAGCAAAGTAGAAGAGGG |
| BCL2 | F: AGGAATGTGTGGAATGTGGAGAR: AGATGAATGGTAGAGGGTGTGA |
The quantitative data were extracted from five independent samples (n=5) and presented as mean±SD. All statistical analyses were performed using the SPSS software, version 20. Statistical significance was analyzed by two-way repeated measures ANOVA. Statistical significance was set at P<0.05.
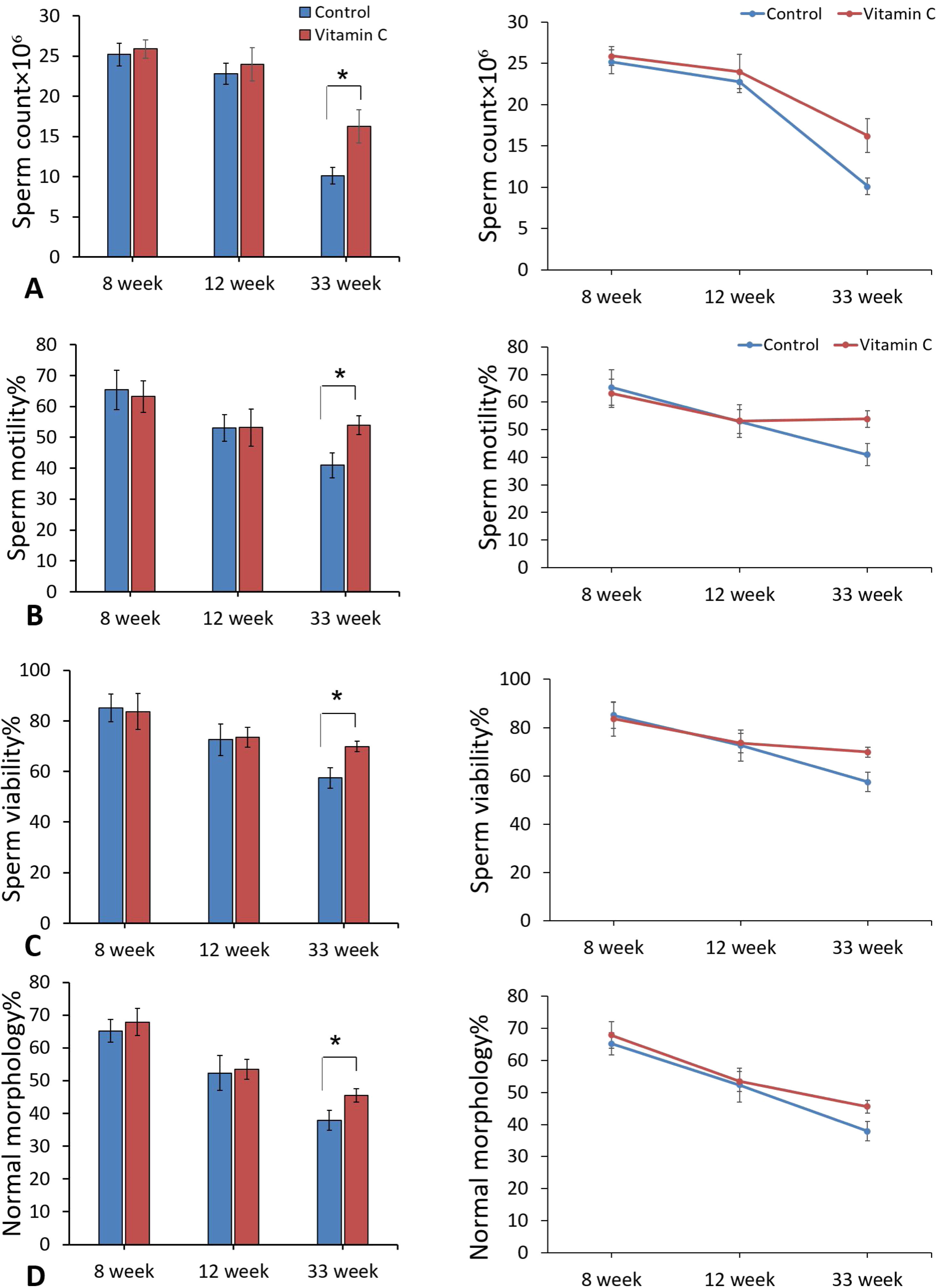
ResultsSperm parametersComparison of semen analysis between experimental and control groups showed a significant improvement in sperm parameters including sperm count, motility, viability, and normal morphology in experimental group compared to the control group after 33 weeks (P<0.05). On the other hand, improvement in sperm parameters was observed after 8 and 12 weeks, but these changes were not significant (Fig. 1).
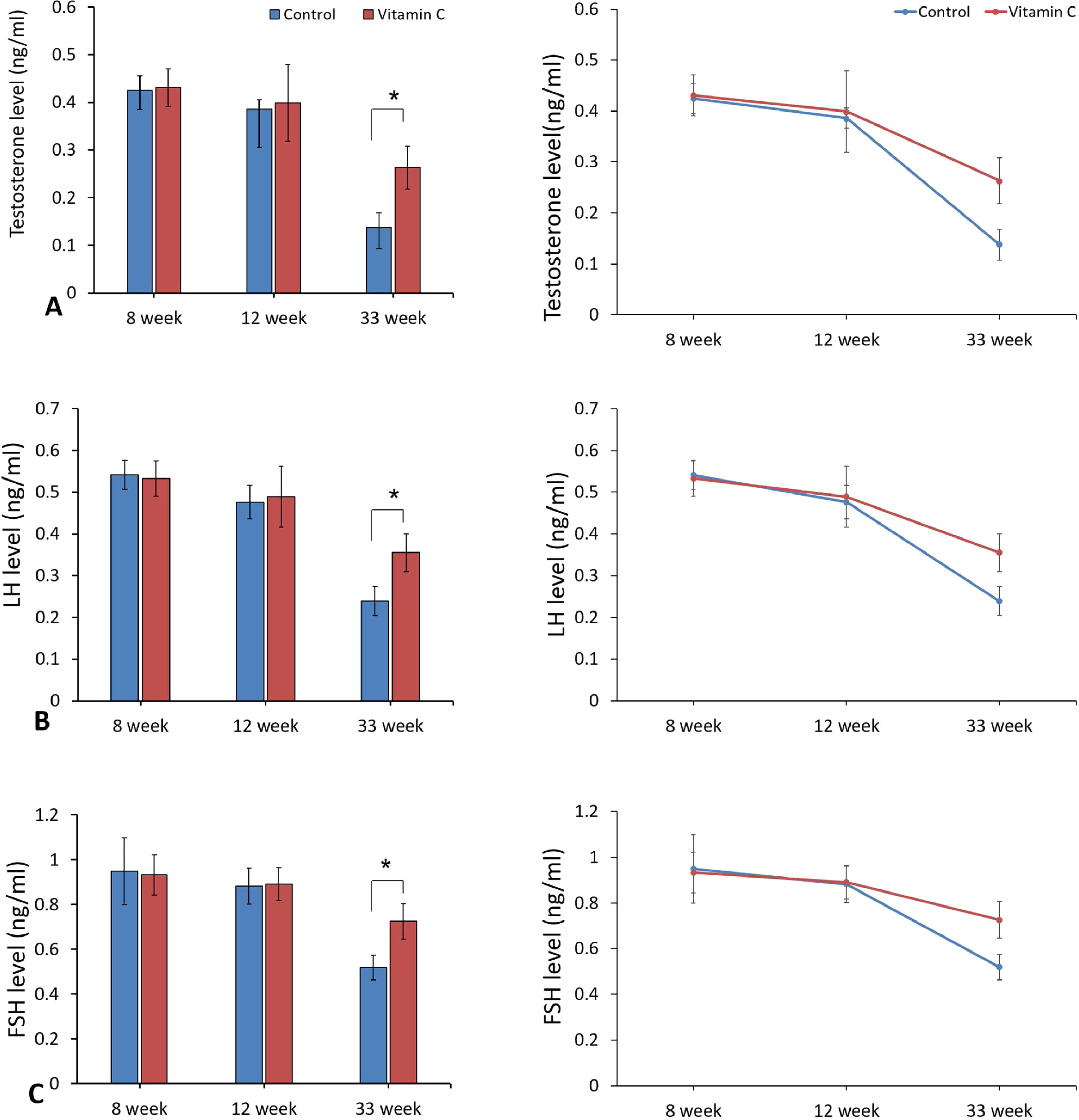
Hormonal analysisAs represented in Fig. 2, hormonal analyses showed that the level of reproductive hormones (LH, FSH, and testosterone) were improved considerably in experimental group compared to the control group after 33 weeks (P<0.05), although there was no significant difference between groups after 8 and 12 weeks (P>0.05).
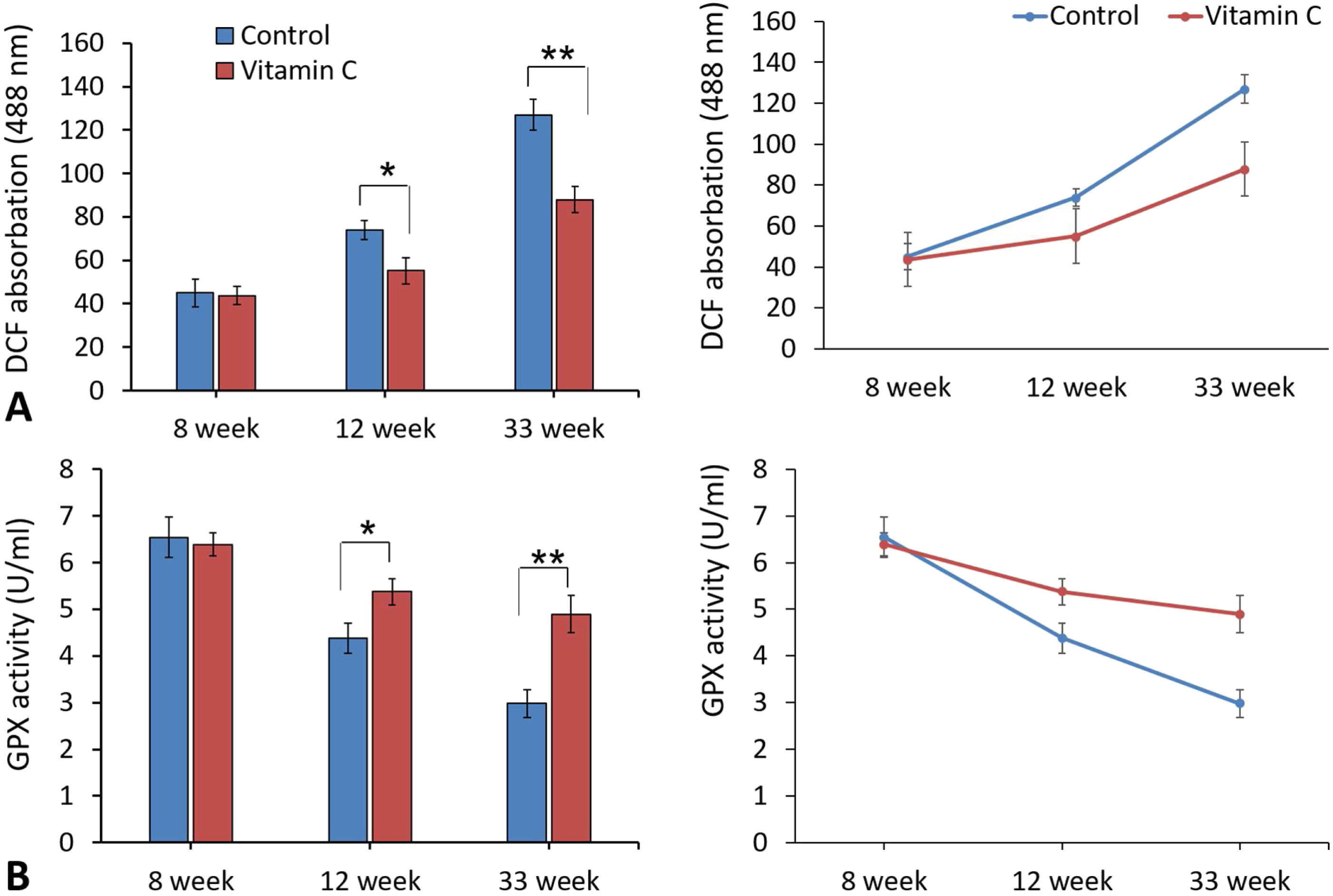
ROS assessment in testicular tissueEvaluation of ROS statues in testicular tissue displayed a significant reduction in DCF absorption in experimental group compared to the control group after 12 and 33 weeks. In addition, there was a significant difference of GPx activity between experimental and control groups following 12 and 33 weeks (Fig. 3).
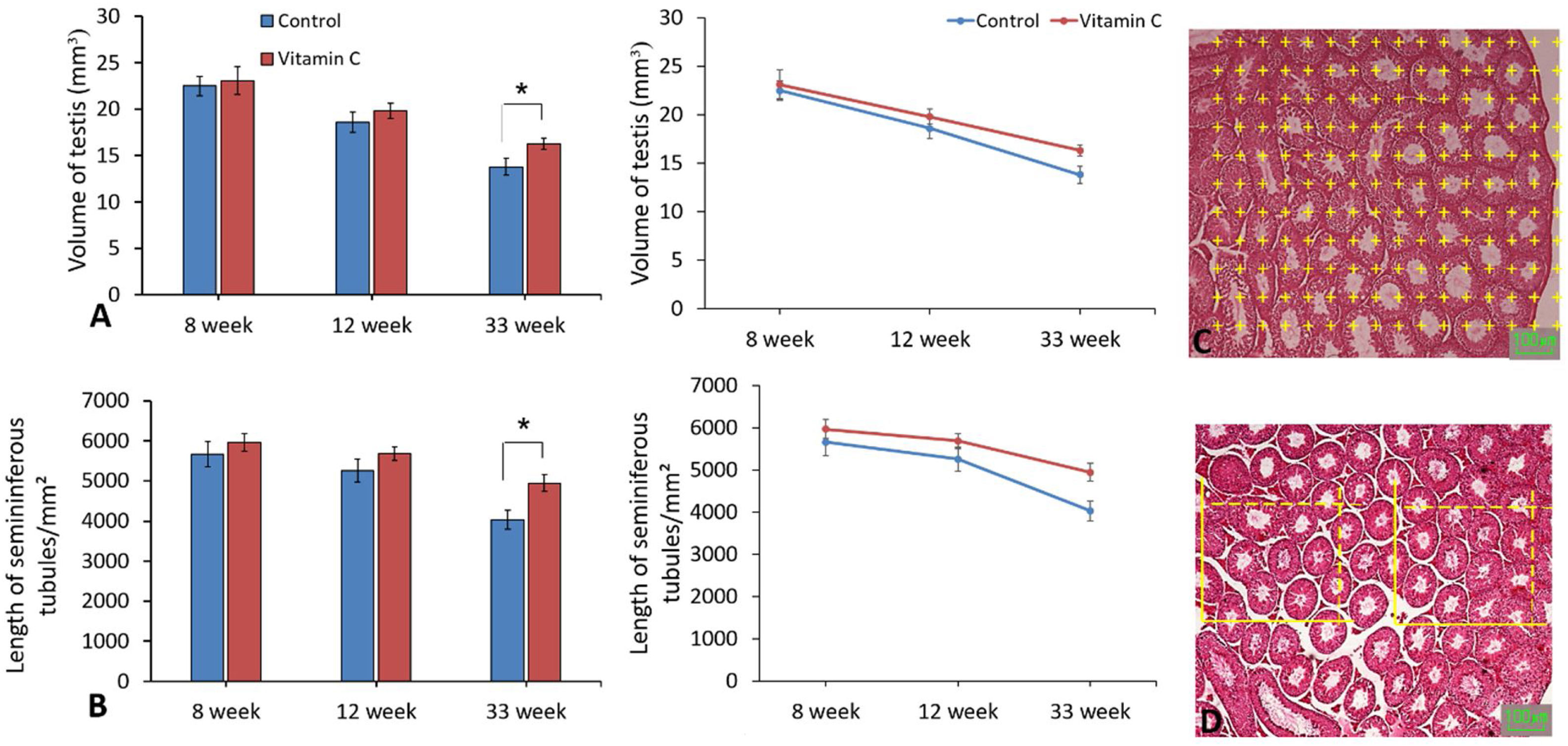
Stereological evaluationsAs demonstrated in Fig. 4, stereological assessments indicated significantly perfection in testis volume and also length of seminiferous tubules in experimental group compared to the control group after 33 weeks (P<0.05), while there was no substantial difference between groups after 8 and 12 weeks (P>0.05).
The effect of vitamin C on testis volume and seminiferous tubules length in mouse models of aging. (A and B) Mean±SD of the testis volume, seminiferous tubules length of testis in the study groups as compared by the ANOVA and LSD; (*P<0.05). (C) Photomicrograph of the testis stained with H&E, we superimposed a point grid and counting frame over the photomicrograph to measure the volume of testis and seminiferous tubules length.
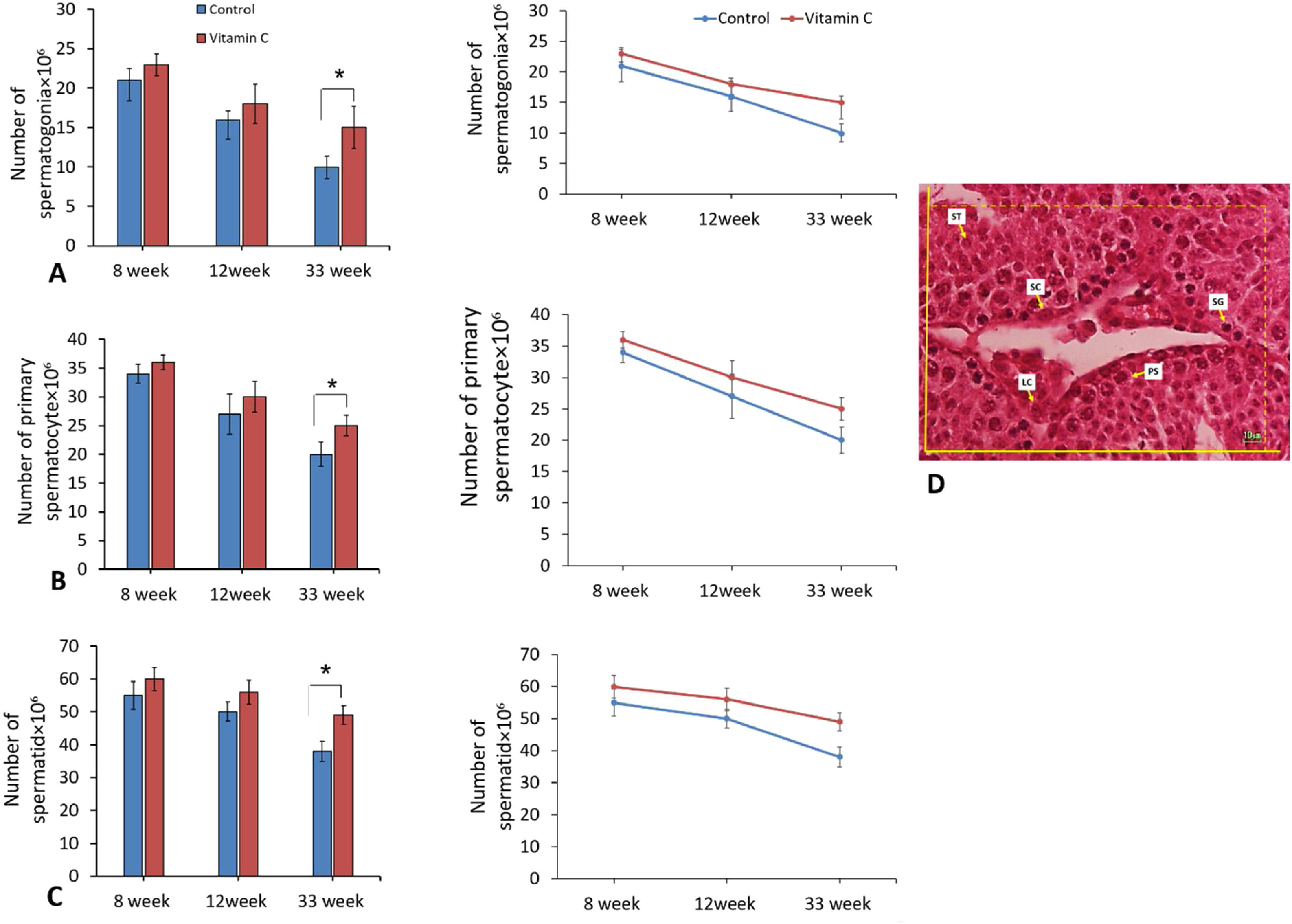
In addition, the number of spermatogenic cells including spermatogonia, primary spermatocyte, and spermatids displayed significant difference between groups after 33 weeks (P<0.05). On the other side, there was no considerable difference between groups regarding to the number of spermatogenic cells after 8 and 12 weeks (P>0.05) (Fig. 5).
The effect of vitamin C on total number of spermatogenic cells in mouse models of aging. (A–C) Mean±SD of the total number of spermatogonia, total number of primary spermatocyte, and total number of round spermatid of testis in the study groups as compared by the ANOVA and LSD; (*P<0.015). (D) Photomicrograph of the testis stained with H&E, 40×. SG (spermatogonia), PS (primary spermatocyte), ST (round spermatid), SC (Sertoli cell), LC (Leydig cell).
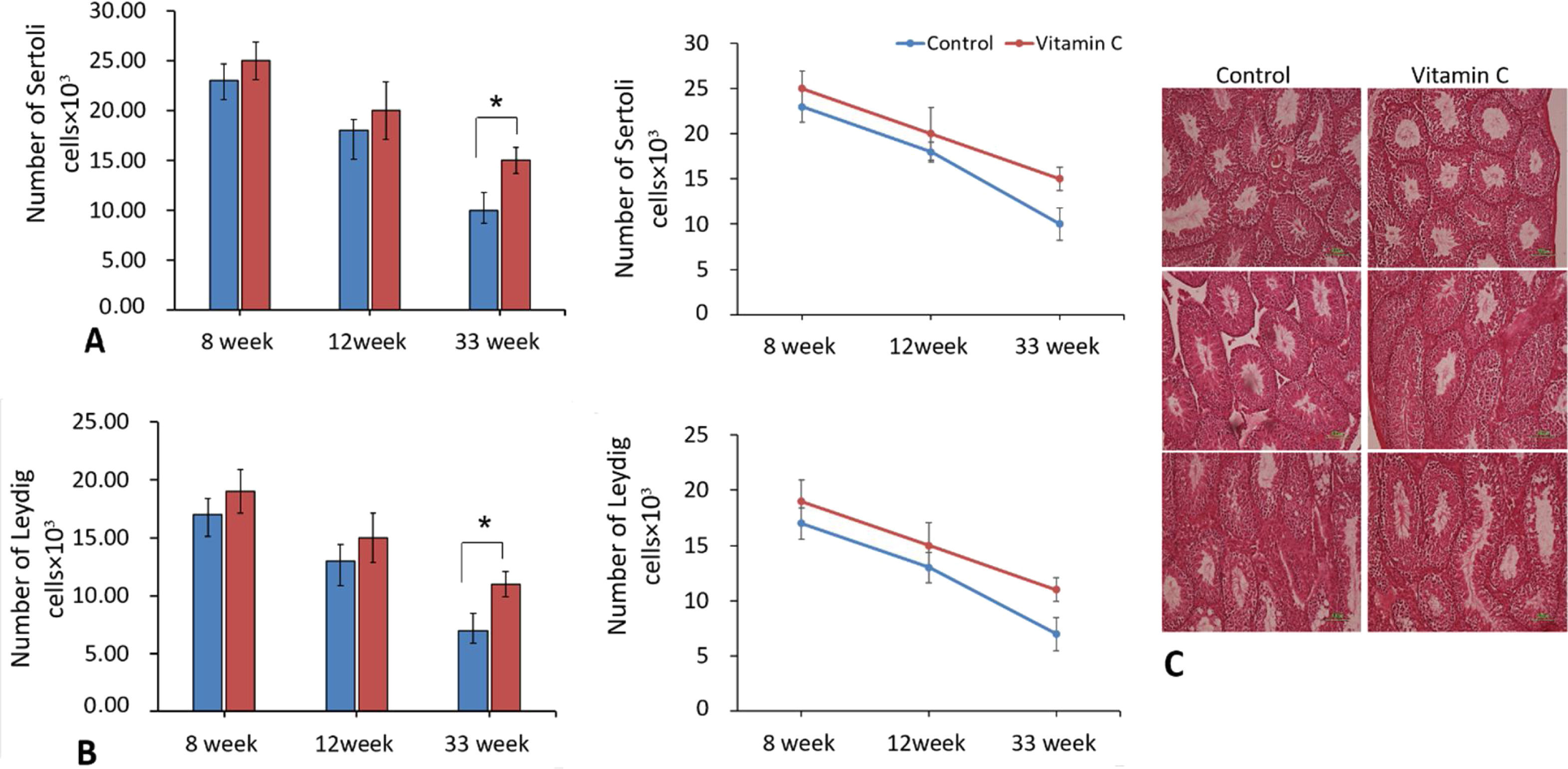
The comparison of number of Sertoli and Leydig cells in testicular tissue of different groups revealed significant difference between them after 33 weeks (P<0.05). Although there was an improvement in Sertoli and Leydig cell number after 8 and 12 weeks, it was not significant (Fig. 6).
The effect of vitamin C on total number of Sertoli cells and Leydig cells in mouse models of aging. (A and B) Mean±SD of the total number of Sertoli cells, and total number of Leydig cells of testis in the study groups as compared by the ANOVA and LSD; (*P<0.05). Photomicrograph of the testis stained with H&E (10×), on 8, 12, and 33 weeks.
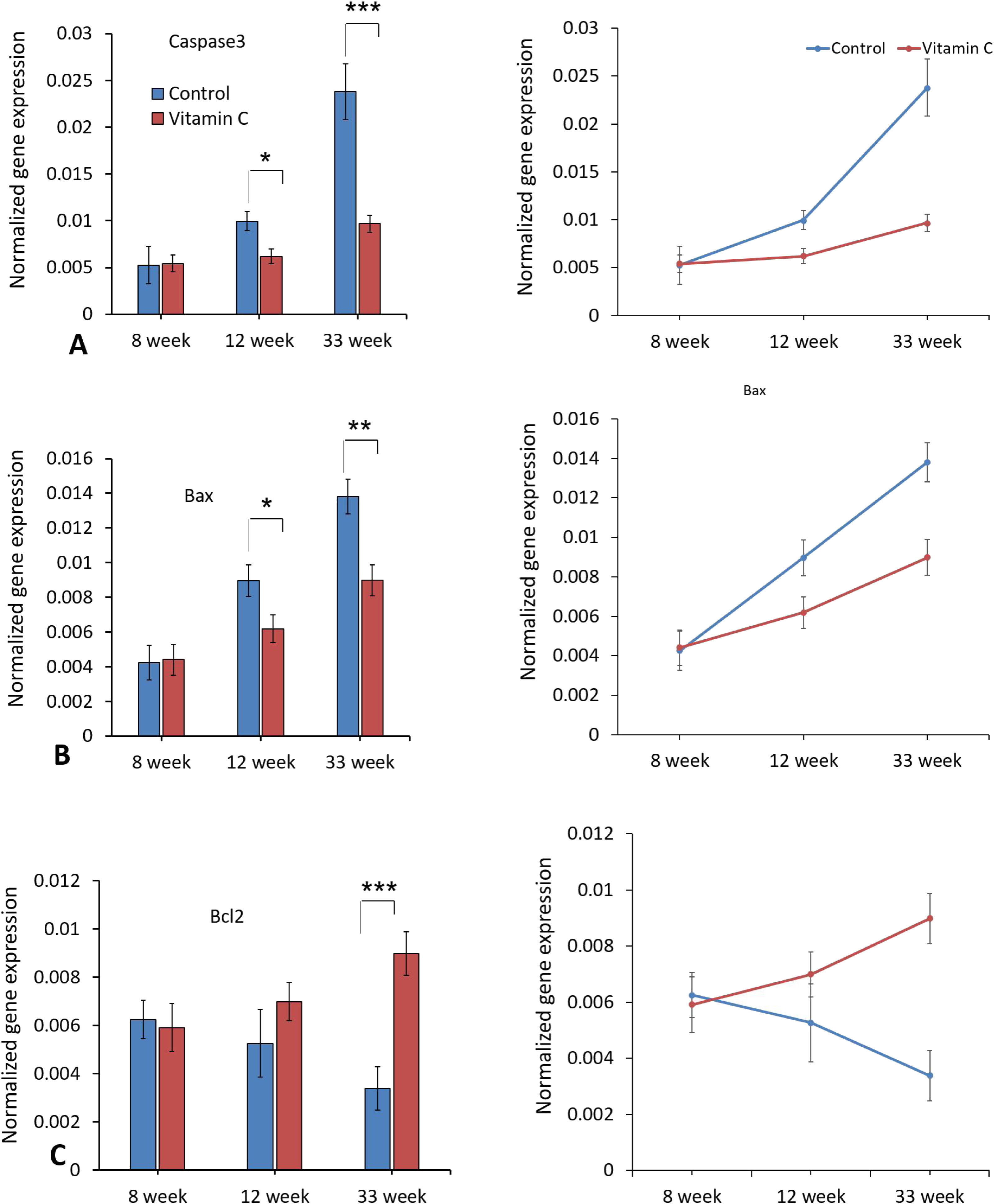
As demonstrated in Fig. 7, the expression levels of Caspase-3 and Bax genes were decreased significantly in experimental group compared to the control group (P<0.05), while the expression level of Bcl2 gene was increased considerably after 33 weeks (P<0.05). Moreover, there was no significant difference in apoptotic gene expression between groups after 8 and 12 weeks.
DiscussionIn the present study, we investigated potential impacts of vitamin C consumption on testicular damages following aging in NMRI mice. In our model of testicular aging, we displayed low semen quality, decreased levels of serum reproductive hormones such as LH, FSH, and testosterone, increased ROS production, and also high expression of apoptotic genes. Stereological experiments also showed that the volume of testis, the length of seminiferous tubules, and the number of spermatogenic and none-spermatogenic cells decreased significantly during aging process.
We observed that vitamin C administration for 33 weeks improved all of the above-mentioned indicators.
According to the previous conclusions, the factors such as semen volume, total sperm count, sperm motility, and normal morphology of sperm decrease and percentage of DNA fragmentation increases following male aging.5,6 Declined number of Leydig cells and its subsequent reverse impact on gonadotropins secretion is reported previously.21 Increased thickness of basement membrane of seminiferous tubules, reduced seminiferous epithelium as well vascularization of testicular tissue are other important testicular problems in aged males.22 In addition, the frequency of chromosomal aneuploidies was increased in sperm from aged men.9
Elevated ROS production in the testicles of aged male leads to raised lipid peroxidation, DNA damages, apoptosis, and several cellular complications.5 A number of documents have exposed detrimental effects of increased testicular ROS production on sperm quality.23–25 Imbalance between pro and anti-inflammatory elements can be considered as one of the possible mechanisms involved in injuries caused by aging in reproductive organs, especially the testicles. The relation between levels of inflammatory molecules, androgen insufficiency, and the spermatogenic failure following aging has been described previously.7,26,27 Apoptotic markers also have been considered as valuable signs of male fertility.28,29 Reproductive structures undergo cycles of cell proliferation and cell death because of changes in hormonal levels. According to this, during cycles, there is a substantial rise in cell number, which is then declined by a coordinate process of cell death. Current results indicate the existence of a close collaboration between sex hormones and apoptotic pathways.30
Similar to previous works, we displayed low semen quality, increased ROS production, hormonal insufficiency, and increased apoptosis in mice testicles during aging process.
Antioxidant administration can be an appropriate method for prevention and treatment of impairments in ROS scavenging activities.31 Vitamin C is a water soluble antioxidant and its therapeutic effects has been investigated in different studies. Accordingly, vitamin C administration in patients with sepsis or after ischemia/reperfusion can ameliorate organ dysfunctions via antioxidant, anti-inflammatory, and immune supporting properties.32–34 According to the literature review, vitamin C can recover ROS-associated damages. Vitamin C also is able to protect endothelial barrier, enhance function of immune system, and increase antibacterial defense through antioxidant effects.10
In the study performed by Veurink et al., the promising effects of antioxidants such as vitamin C supplementation on longevity of apolipoprotein E (ApoE)-deficient mice with high level of ROS and oxidative stress was assessed. Accordingly, the authors concluded that mice with high fat diet and antioxidant supplementation showed lower mortality rate compared to untreated mice.35 In another study, vitamin C combined with vitamin D was able to decrease lipid peroxidation and percentage of abnormal sperm cells. This regime was also talented to improve sperm count in mice exposed to cadmium.36 In the case of couples with history of recurrent pregnancy loss, vitamin C has been determined to enhance sperm DNA integrity, semen quality, and expression level of protamine genes. As a result, it becomes clear that vitamin C has constructive aspects on pregnancy outcomes of such patients.37
According to the researches, the level of vitamin C in various organs such as heart, liver, kidney, lungs, and skeletal muscles decreased with age, while vitamin C consumption boosts the level of vitamin C in mentioned organs.38 In another study conducted by Iwama et al., it has been shown that the capacity of organs to synthesize vitamin C diminished gradually during mice aging.39 On the other hand, it has been demonstrated that the level of antioxidant production decreases in Leydig cells of rat during aging process.
Rodríguez-González and colleagues indicated that maternal obesity (MO), as a risk factor increases oxidative stress in male rat offspring reproductive system and decreases reproductive ability. According to this, at postnatal days (PNDs) 450 and 650, maternal obesity offspring had lower LH, while testosterone levels were lower at all ages. Also, Testicular malondialdehyde (MDA), ROS concentrations, superoxide dismutase (SOD), and GPx activity were greater in MO offspring at all ages. Furthermore, at PNDs 450 and 650, MO offspring spermatozoa exhibited higher MDA concentrations and lower SOD and GPx activity with reduced sperm concentration, viability and motility, and more sperm anomalies. Eventually, they concluded that MO during gestation increases offspring testicular oxidative stress leading to premature aging of reproductive capacity.40 Besides, the relationship between increased testicular oxidative stress and accelerating reproductive capacity aging in male rat offspring of protein-restricted mothers was found in another study by Rodríguez-González et al. So, it can be supposed that oxidative stress and its consequences are among the causes affecting age-related testicular dysfunction, and vitamin C supplementation is one of the factors that attenuate age-related testicular impairments. According to our knowledge, there is no comprehensive data regarding the effects of vitamin C consumption on molecular and stereological indices of age-related testicular injuries. In our present study, we confirmed beneficial impacts of vitamin C administration in mice testis during aging. We also found that vitamin C administration for 33 weeks significantly improved semen quality (sperm count, motility, morphology, and viability) in mice. In addition, indicators such as apoptotic gene expression and oxidative stress were considerably declined following vitamin C consumption. Stereological studies also disclosed that the volume of testis, the length of seminiferous tubules, and the number of spermatogenic and none-spermatogenic cells were significantly augmented. In accordance with the present study, Maiada Moustafa reported positive effects of vitamin C on the number of seminiferous tubules as a histological aspect of testicular tissue along with hormonal conditions in aged rats.
ConclusionIn conclusion, it seems that paternal age is increasing in the world. Hence, use of protective and preventive regimens preserving male fertility is important. Oxidative stress is a key factor in age-related testicular injuries. According to our outcomes, vitamin C as an antioxidant can have beneficial effects on age-related testicular oxidative stress and injuries and in general, the results of the present study showed that vitamin C can improve the process of spermatogenesis and also sperm quality.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authorship- 1.
The conception and design of the study: FH, NK, and FF.
- 2.
Supervision and data analyses: VE and MAA.
- 3.
Drafting the article: NA, HC, AR, and MJF.
- 4.
Final approval of the version to be submitted: AA, SA, and MS.
The protocol of the present study was reviewed and confirmed by Ethics Committee at Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.MEDICAL.REC.1399.833).
Consent for publicationConsent for publishing the resulting draft of the present research has been confirmed by all co-authors.
FundingThe present study was supported financially by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No. 991733).
Conflict of interestThe authors declare that they have no competing interests.
This study was conducted in collaboration with Shahid Beheshti University of Medical Sciences and Mashhad University of Medical Sciences.