The paucity of literature is addressed regarding the correlation between open field as an individual behavioral trait on reproductive capacity in animals.
Materials and methodsTo address this, Nine-month-old indigenous roosters were housed in individual cages. Each animal was observed twice a week for ten minutes before feeding in an open field apparatus for two weeks (7:00–12:00PM).
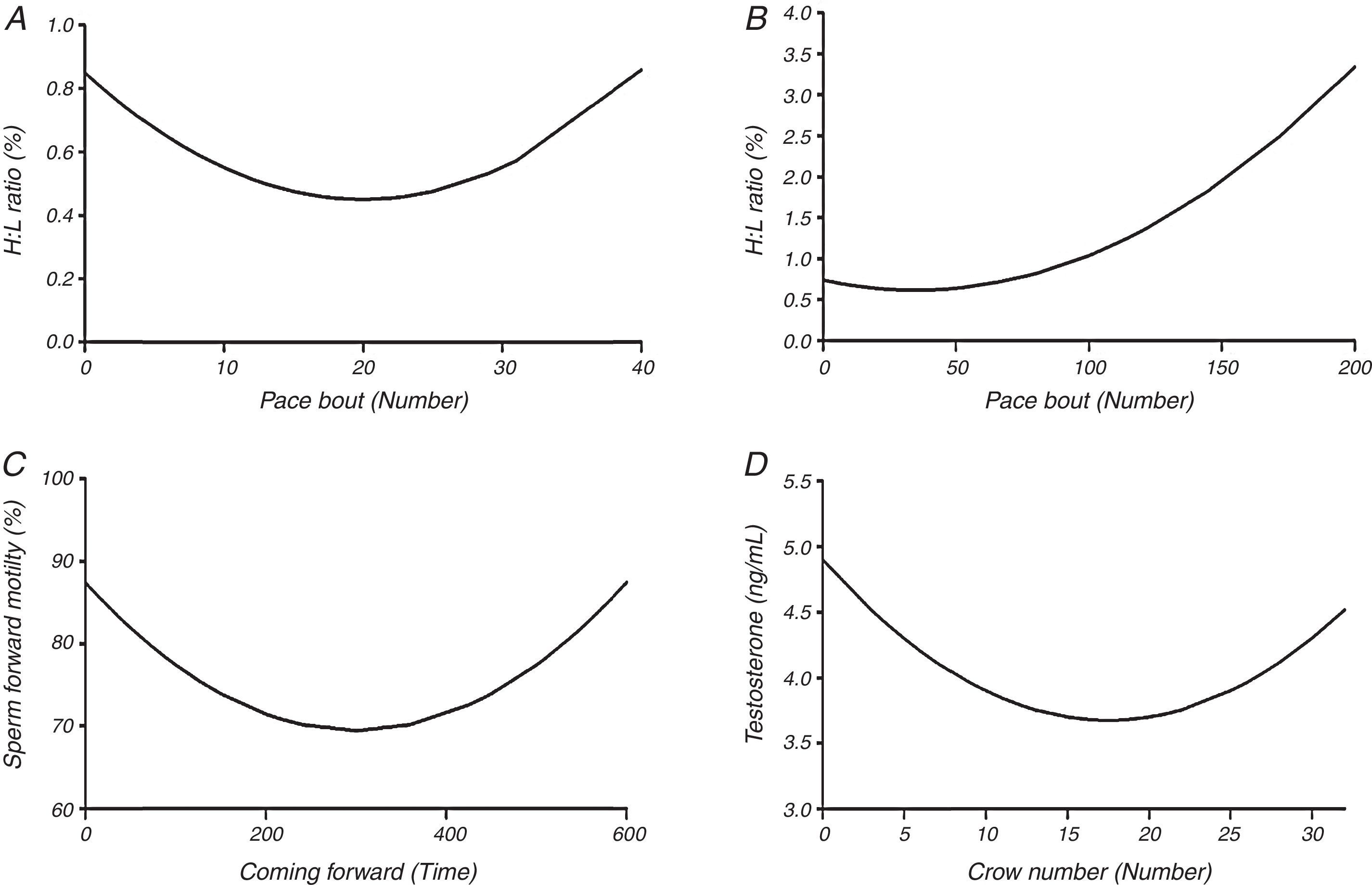
ResultsInterestingly, it was found that rooster's semen characteristics were correlated with their open field behavior. On the other hand, plasma glucose level as a blood attribute was more correlated with semen characteristics. The open field monitoring also revealed that the roosters with the lowest delay to their first pace had the highest sperm forward motility and lower sperm abnormality. The heterophil to lymphocyte ratio (H:L) was found to be low when pace bout and pace numbers were 20 and 35, respectively. The negative correlation between H:L ratio and semen characteristics (live sperm percentage, sperm concentration, and membrane integrity) may be an indication of poor reproductive performance in fearful roosters with higher H:L ratio.
ConclusionsThe data suggested a relationship between open field behavior indices and some reproductive parameters in roosters. The results might be applicable for selection of more reproductive animals. Hence, the rooster may also be useful model for similar studies in other species.
Existe poca literatura que analice la relación entre el comportamiento individual en animales en campo abierto y su capacidad reproductiva.
Material y métodosPara cubrir esta laguna se trabajó con gallos de 9 meses de edad que fueron encerrados en jaulas individuales. Cada animal se observó 2 veces a la semana, durante 10min, en una zona de campo abierto durante 2 semanas, antes de la alimentación (07:00-12:00 p.m.).
ResultadosCuriosamente, se encontró que la característica del semen de los gallos se correlacionaba con su comportamiento en campo abierto. Por otro lado, el nivel de glucosa en plasma, como un atributo de la sangre, se correlacionaba con las características del semen. La monitorización en campo abierto también reveló que los gallos con la menor demora en su primer canto tuvieron la mayor motilidad de los espermatozoides y la menor alteración del esperma. El nivel más bajo de la relación entre heterófilos y linfocitos (H: L) fue encontrado cuando los números de cantos y el ritmo eran 20 y 35, respectivamente. La correlación negativa entre el índice H:L y las características del semen (porcentaje de espermatozoides vivos, concentración de espermatozoides, e integridad de la membrana) puede ser un indicio de mal desempeño reproductivo de gallos con mayor índice H:L.
ConclusionesLos datos indican que existe relación entre los índices de comportamiento en campo abierto y algunos parámetros reproductivos de los gallos. Los resultados podrían ser aplicables para la selección de los animales más reproductivos. El gallo también puede ser útil como un modelo para estudios similares en otras especies.
Stressful situations, through psychological and physiological disturbances, affect fertility in humans1 and animals.2 Many factors such as individual condition, emotional strength, coping strategy, and race might affect fertility. It has been found that, Distress and fear affect both the health and welfare of farm animals.3 Open field behavior is a measure of fear, and it is generally accepted that immobility in the open field is an indicative of fearfulness, while locomotor activity indicates the lack of fear.4,5 In caged laying hens, startle reflexes, induced by sudden noises or frightening visual stimuli, may cause physical damage to limbs, sudden discharge of energy and even death; furthermore, increased corticosterone secretion due to fear and stress may cause immunosuppression.6 The profile of leucocytes is changed in the chicken due to stress. Except for the evaluation of corticosterone level for assessing the stress level in poultry, determination of the ratio of heterophils to lymphocytes (H:L) can be used7,8 which is highly heritable.9,10 Fasting, frustration, water deprivation and crowding increase the H:L ratio.11 Determining the H:L ratio has several advantages in comparison with measuring corticosterone levels; including the lower cost and need for smaller sample volume.12 Therefore we used H:L ratio to determine the stress levels.
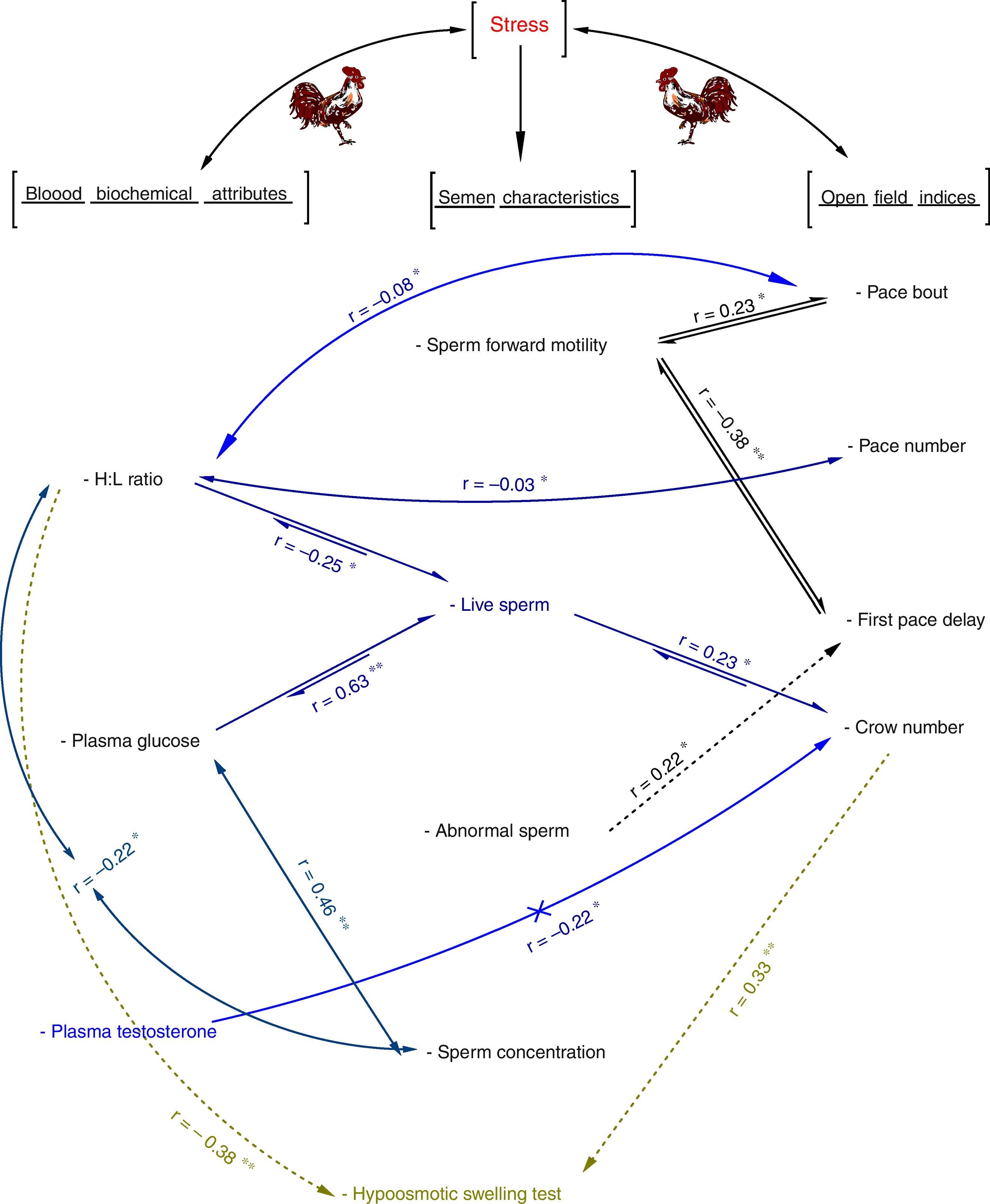
Fear and open field activity are also associated with the reactive and proactive coping strategies; the reactive coper's behavior is directed by current clues in the environment and response stronger to environmental stimuli, whereas the proactive coper's animal seem to behave more, according to their past experiences and easily develop routines.13,14 It is proposed that reactive copers are more adaptive to the changes in the environment, while proactive copers are more comfortable in predictable and unchanging environments.13,15 As previously mentioned, proactive animals establish routines and are more vulnerable to the development of abnormal behaviors.13,14 These animals are also more afraid and have less locomotor activity in the open field.16 Open field activity is heritable, and divergent selection for this trait has been carried out in species such as rabbits,9 mice10,17 and chickens.18 It has been proposed that selection against fear is a more rapid, effective, economical and reliable method in breeding programs.19 Because poultry farming is a very large industry, small changes in fertility, especially in males – due to a smaller number of males and then their greater contribution to flock fertility – could have significant economic impact. It has also been revealed that fear affected sexual behavior in cattle and sheep.20,21 Despite of literature available on the physiological and neurobiological difference between low and high fear index animals,22,23 there have been little, if any, endeavor in elucidating the probable effect of open field as an individual behavioral trait on reproductive capacity in animals. Then this investigation aimed to determine the association between open field behavioral indices (pace bout, pace number, first pace, peck bout, peck number, first peck, crow number, and coming forward), semen characteristics (sperm forward motility, live and abnormal sperm, sperm concentration, sperm membrane integrity), and some blood biochemical attributes.
As shown in Fig. A.1 (Appendix 1), the association of sperm motility, concentration, viability, abnormality and membrane integrity and the selected blood parameters with open field behavior in Iranian native breeder roosters were determined. Additionally, we determined the effect of environment stress on the H:L index. These findings might be useful in studies concerned with the effect of stress level on animal welfare and fertility.
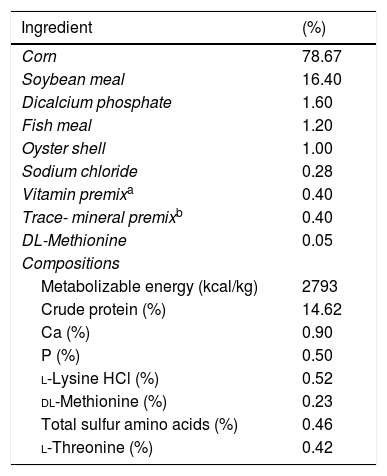
Materials and methodsAnimals and housingOne hundred and eight number of nine-month-old Iranian indigenous roosters (Research Center of Fars Native Chickens, Shiraz, Iran) were housed in individual cages (30cm×40cm×40cm W×L×H), maintained in an environmentally controlled facility (24°C and 14h L:10h D photo schedule) and fed a basal corn-soybean based diet (Appendix 1 – Table A.1).
Open field testEach rooster was monitored for ten minutes in an open field, in a separate windowless room. The open field was a wooden box (125×125cm) divided into 25×25cm dark and white squares to facilitate observation. The box was equipped with a webcam (2.0 – Megapixel, Gigaware, UK) and a microphone and all activities were monitored from another room. All observations were carried out before feeding, between 7:00AM and 12:00PM, twice a week for 10min in an open field apparatus for two weeks. The ethogram is shown in (Appendix 1 – Table A.2).
Analysis of semenThe roosters were trained for semen collection during a period of two weeks, semen was collected twice a week from each animal, by the abdominal massage method.24 Sperm concentration was determined in duplicate by a Neubauer hemocytometer. Sperm motility was determined on a scale of 0–5 using a light microscope (400× magnification) by mixing one drop of semen with one drop of 2.9% sodium citrate solution on a warm slide. The percentage of live and morphologically abnormal sperm was determined by observing (1000 × magnification) the sperm in 10 microscope fields (at least 100 sperm) using semen stained with eosin-nigrosin stain.25 Spermatozoa with detached head, malformed head, coiled tail, double tail and protoplasmic droplets were considered as morphologically abnormal ones. The hypoosmotic swelling test (HOST) was used to assess sperm plasma membrane integrity. Briefly, 10μl of semen was mixed with 50μl of a hypotonic solution (100-mOsm NaCl), incubated for 15minutes at 37°C, and a 20-μl sub-sample was studied under oil immersion microscope. Spermatozoa with swollen coiled tails were designated as having intact membranes.26
Heterophil to lymphocyte ratio (H:L)A small drop of blood was withdrawn by puncturing the roosters’ comb with a Pasteur pipette. The blood samples were diluted with a drop of EDTA-containing water (1.5mg/ml) and smeared on a slide. Two slides per rooster were prepared, air-dried, fixed in pure ethanol, and stained with Giemsa for 20min. One-hundred leucocytes, including the heterophils, lymphocytes, monocytes, basophils and eosinophils, were counted on each slide by two independent observers, and the H:L ratio was calculated.27
Plasma glucose and testosterone concentrationsBlood samples were taken (2ml) from the wing vein after carrying out the open field test. Samples were centrifuged for 12min at 963×g and 18°C, and plasma samples stored at −20°C. Plasma testosterone concentration was measured by ELISA using a commercially available kit (Monobind Inc., Costa Mesa; intra-assay coefficient of variation (CV) <10%, inter-assay CV <12% and detection level above 0.14nmol/l).28 Plasma glucose level was determined by a spectrophotometric method (Cobas Mira Chemistry Analyser; Roche, Mannheim, Germany), using a commercially available kit (ZiestChemie Diagnostic, Tehran, Iran).
Statistical analysisNo data transformation was required. The data were analyzed using SAS software.29 Pearson's correlation coefficients were calculated to determine the association of blood parameters and semen characteristics with open field behavior. The multivariate regression model was as follow:
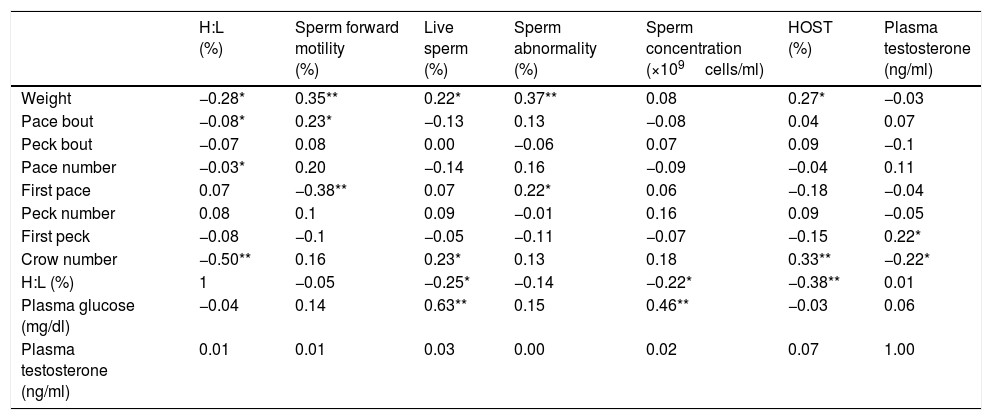
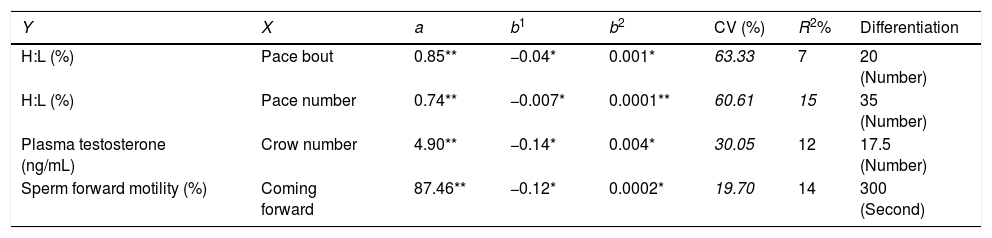
in which, y=blood parameter, and or semen characteristic, a=intercept, b1, b2; regression coefficients of blood parameters and semen characteristics linear (X) and quadratic forms (X2) of open field behavior observations, respectively; and x=open field behavior observations.ResultsThe multivariate regression was calculated for all variables to estimate the level of open field behavioral traits based on the lowest or the highest levels of blood parameters and/or semen characteristics. The first derivative of the multivariate regression function was set equal to zero and the resulted equations were solved (Appendix 1 – Table A.3). The correlation coefficients between open field behavioral parameters, sperm characteristics, and blood parameters are presented in Table 1. It was found that sperm motility was correlated with pace bout (r=0.23, P<0.05) and the first pace delay (r=−0.38, P<0.01). The percentage of live sperm in the semen was correlated with crow number (r=0.23, P<0.05), H:L ratio (r=−0.25, P<0.05) and plasma glucose concentration (r=0.63, P<0.01). Moreover, Sperm abnormality was correlated with the first pace delay (r=0.22, P<0.05). Sperm concentration was correlated with H:L ratio (r=−0.22, P<0.05) and plasma glucose level (r=0.46, P<0.01) and the percentage of HOST-positive spermatozoa was correlated with crow number (r=0.33, P<0.01) and H: L ratio (r=−0.38, P<0.01). A correlation between plasma testosterone level and the crow number was also detected (r=−0.22, P<0.05). No significant correlation was found between testosterone and semen characteristics (Appendix 1 – Table A.3).
Correlation coefficients of open field behavioral parameters with blood and sperm parameters.
| H:L (%) | Sperm forward motility (%) | Live sperm (%) | Sperm abnormality (%) | Sperm concentration (×109cells/ml) | HOST (%) | Plasma testosterone (ng/ml) | |
|---|---|---|---|---|---|---|---|
| Weight | −0.28* | 0.35** | 0.22* | 0.37** | 0.08 | 0.27* | −0.03 |
| Pace bout | −0.08* | 0.23* | −0.13 | 0.13 | −0.08 | 0.04 | 0.07 |
| Peck bout | −0.07 | 0.08 | 0.00 | −0.06 | 0.07 | 0.09 | −0.1 |
| Pace number | −0.03* | 0.20 | −0.14 | 0.16 | −0.09 | −0.04 | 0.11 |
| First pace | 0.07 | −0.38** | 0.07 | 0.22* | 0.06 | −0.18 | −0.04 |
| Peck number | 0.08 | 0.1 | 0.09 | −0.01 | 0.16 | 0.09 | −0.05 |
| First peck | −0.08 | −0.1 | −0.05 | −0.11 | −0.07 | −0.15 | 0.22* |
| Crow number | −0.50** | 0.16 | 0.23* | 0.13 | 0.18 | 0.33** | −0.22* |
| H:L (%) | 1 | −0.05 | −0.25* | −0.14 | −0.22* | −0.38** | 0.01 |
| Plasma glucose (mg/dl) | −0.04 | 0.14 | 0.63** | 0.15 | 0.46** | −0.03 | 0.06 |
| Plasma testosterone (ng/ml) | 0.01 | 0.01 | 0.03 | 0.00 | 0.02 | 0.07 | 1.00 |
* and ** indicate the significance of correlation coefficients at 5 and 1 percent probabilities, respectively.
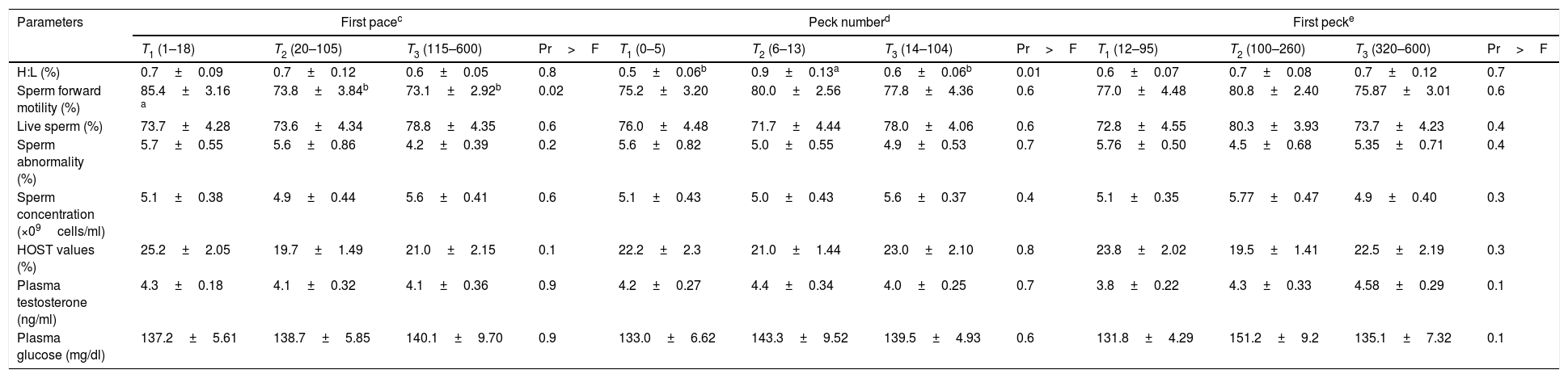
It was found that the first peck and peck number as well as coming forward were not associated with blood parameters or semen characteristics (P>0.05) (Appendix 1 – Table A.3). There was a non-linear association between parameters given in Fig. 1. The H:L ratio was significantly associated with the pace bout and pace number (P≤0.05), where the lowest H:L ratio was found when pace bout and pace number were 20 and 35, respectively (Fig. 1A and B). There was an association between testosterone concentration and crow number as well as sperm forward motility and coming forward behavior (P≤0.05) (Appendix 1 – Table A.3). The lowest level of testosterone concentration (3.6ng/mL) and sperm forward motility (69.5%) were found when crow number was 17.5 and coming forward was 300s, respectively (Fig. 1C and D).
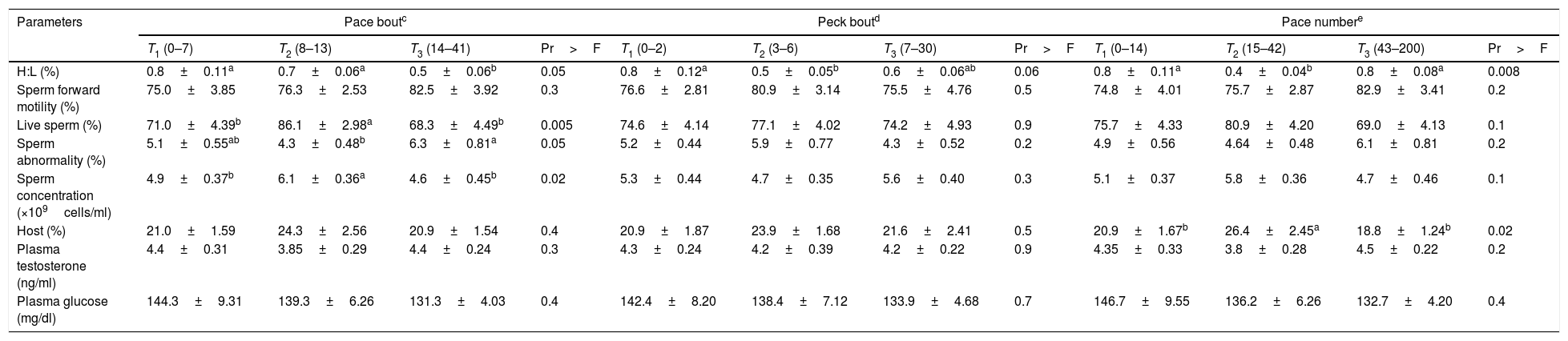
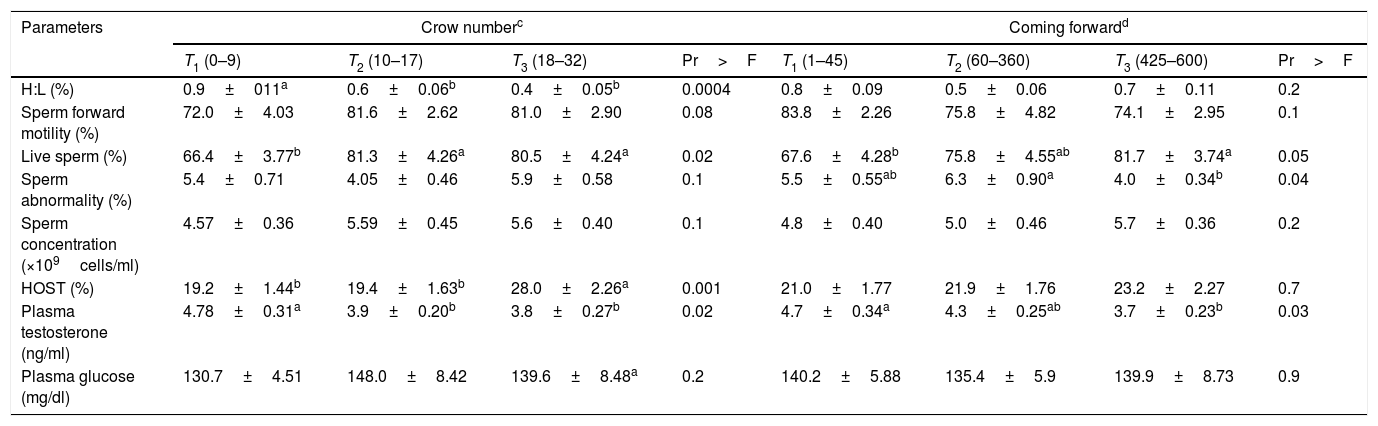
Interestingly, the sperm abnormali percentage was significantly higher in animals with the highest pace bout (Table 2; T3). Live sperm percentage and HOST values were significantly higher in roosters with the highest crow number (Table 4; T3). Sperm forward motility was higher in roosters with shorter delays to the first pace (Table 3; T1). Plasma testosterone concentration was negatively correlated to crow number (r=−0.22, P<0.05) (Appendix 1 – Table A.3), and was higher in birds with lowest crow number and coming forward activity (Table 4; T1).
Relationship of blood and sperm parameters with pace-bout, peck-bout and pace number in roosters (Mean±SE; n=108).
| Parameters | Pace boutc | Peck boutd | Pace numbere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 (0–7) | T2 (8–13) | T3 (14–41) | Pr>F | T1 (0–2) | T2 (3–6) | T3 (7–30) | Pr>F | T1 (0–14) | T2 (15–42) | T3 (43–200) | Pr>F | |
| H:L (%) | 0.8±0.11a | 0.7±0.06a | 0.5±0.06b | 0.05 | 0.8±0.12a | 0.5±0.05b | 0.6±0.06ab | 0.06 | 0.8±0.11a | 0.4±0.04b | 0.8±0.08a | 0.008 |
| Sperm forward motility (%) | 75.0±3.85 | 76.3±2.53 | 82.5±3.92 | 0.3 | 76.6±2.81 | 80.9±3.14 | 75.5±4.76 | 0.5 | 74.8±4.01 | 75.7±2.87 | 82.9±3.41 | 0.2 |
| Live sperm (%) | 71.0±4.39b | 86.1±2.98a | 68.3±4.49b | 0.005 | 74.6±4.14 | 77.1±4.02 | 74.2±4.93 | 0.9 | 75.7±4.33 | 80.9±4.20 | 69.0±4.13 | 0.1 |
| Sperm abnormality (%) | 5.1±0.55ab | 4.3±0.48b | 6.3±0.81a | 0.05 | 5.2±0.44 | 5.9±0.77 | 4.3±0.52 | 0.2 | 4.9±0.56 | 4.64±0.48 | 6.1±0.81 | 0.2 |
| Sperm concentration (×109cells/ml) | 4.9±0.37b | 6.1±0.36a | 4.6±0.45b | 0.02 | 5.3±0.44 | 4.7±0.35 | 5.6±0.40 | 0.3 | 5.1±0.37 | 5.8±0.36 | 4.7±0.46 | 0.1 |
| Host (%) | 21.0±1.59 | 24.3±2.56 | 20.9±1.54 | 0.4 | 20.9±1.87 | 23.9±1.68 | 21.6±2.41 | 0.5 | 20.9±1.67b | 26.4±2.45a | 18.8±1.24b | 0.02 |
| Plasma testosterone (ng/ml) | 4.4±0.31 | 3.85±0.29 | 4.4±0.24 | 0.3 | 4.3±0.24 | 4.2±0.39 | 4.2±0.22 | 0.9 | 4.35±0.33 | 3.8±0.28 | 4.5±0.22 | 0.2 |
| Plasma glucose (mg/dl) | 144.3±9.31 | 139.3±6.26 | 131.3±4.03 | 0.4 | 142.4±8.20 | 138.4±7.12 | 133.9±4.68 | 0.7 | 146.7±9.55 | 136.2±6.26 | 132.7±4.20 | 0.4 |
T1, T2 and T3 are lowest, intermediate and highest scores of each parameter, respectively.
a,b Within rows, values with different superscripts differ significantly (P≤0.05).
Relationship of blood and sperm parameters with first pace, peck-number, and first-peck in roosters (Mean±SE; n=108).
| Parameters | First pacec | Peck numberd | First pecke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 (1–18) | T2 (20–105) | T3 (115–600) | Pr>F | T1 (0–5) | T2 (6–13) | T3 (14–104) | Pr>F | T1 (12–95) | T2 (100–260) | T3 (320–600) | Pr>F | |
| H:L (%) | 0.7±0.09 | 0.7±0.12 | 0.6±0.05 | 0.8 | 0.5±0.06b | 0.9±0.13a | 0.6±0.06b | 0.01 | 0.6±0.07 | 0.7±0.08 | 0.7±0.12 | 0.7 |
| Sperm forward motility (%) | 85.4±3.16 a | 73.8±3.84b | 73.1±2.92b | 0.02 | 75.2±3.20 | 80.0±2.56 | 77.8±4.36 | 0.6 | 77.0±4.48 | 80.8±2.40 | 75.87±3.01 | 0.6 |
| Live sperm (%) | 73.7±4.28 | 73.6±4.34 | 78.8±4.35 | 0.6 | 76.0±4.48 | 71.7±4.44 | 78.0±4.06 | 0.6 | 72.8±4.55 | 80.3±3.93 | 73.7±4.23 | 0.4 |
| Sperm abnormality (%) | 5.7±0.55 | 5.6±0.86 | 4.2±0.39 | 0.2 | 5.6±0.82 | 5.0±0.55 | 4.9±0.53 | 0.7 | 5.76±0.50 | 4.5±0.68 | 5.35±0.71 | 0.4 |
| Sperm concentration (×09cells/ml) | 5.1±0.38 | 4.9±0.44 | 5.6±0.41 | 0.6 | 5.1±0.43 | 5.0±0.43 | 5.6±0.37 | 0.4 | 5.1±0.35 | 5.77±0.47 | 4.9±0.40 | 0.3 |
| HOST values (%) | 25.2±2.05 | 19.7±1.49 | 21.0±2.15 | 0.1 | 22.2±2.3 | 21.0±1.44 | 23.0±2.10 | 0.8 | 23.8±2.02 | 19.5±1.41 | 22.5±2.19 | 0.3 |
| Plasma testosterone (ng/ml) | 4.3±0.18 | 4.1±0.32 | 4.1±0.36 | 0.9 | 4.2±0.27 | 4.4±0.34 | 4.0±0.25 | 0.7 | 3.8±0.22 | 4.3±0.33 | 4.58±0.29 | 0.1 |
| Plasma glucose (mg/dl) | 137.2±5.61 | 138.7±5.85 | 140.1±9.70 | 0.9 | 133.0±6.62 | 143.3±9.52 | 139.5±4.93 | 0.6 | 131.8±4.29 | 151.2±9.2 | 135.1±7.32 | 0.1 |
T1, T2 and T3 are lowest, intermediate and highest scores of each parameter, respectively.
a,b Within rows, values with different superscripts differ significantly (P≤0.05).
Relationship of blood and sperm parameters with crow-number and coming-forward in roosters (Mean±SE; n=108).
| Parameters | Crow numberc | Coming forwardd | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 (0–9) | T2 (10–17) | T3 (18–32) | Pr>F | T1 (1–45) | T2 (60–360) | T3 (425–600) | Pr>F | |
| H:L (%) | 0.9±011a | 0.6±0.06b | 0.4±0.05b | 0.0004 | 0.8±0.09 | 0.5±0.06 | 0.7±0.11 | 0.2 |
| Sperm forward motility (%) | 72.0±4.03 | 81.6±2.62 | 81.0±2.90 | 0.08 | 83.8±2.26 | 75.8±4.82 | 74.1±2.95 | 0.1 |
| Live sperm (%) | 66.4±3.77b | 81.3±4.26a | 80.5±4.24a | 0.02 | 67.6±4.28b | 75.8±4.55ab | 81.7±3.74a | 0.05 |
| Sperm abnormality (%) | 5.4±0.71 | 4.05±0.46 | 5.9±0.58 | 0.1 | 5.5±0.55ab | 6.3±0.90a | 4.0±0.34b | 0.04 |
| Sperm concentration (×109cells/ml) | 4.57±0.36 | 5.59±0.45 | 5.6±0.40 | 0.1 | 4.8±0.40 | 5.0±0.46 | 5.7±0.36 | 0.2 |
| HOST (%) | 19.2±1.44b | 19.4±1.63b | 28.0±2.26a | 0.001 | 21.0±1.77 | 21.9±1.76 | 23.2±2.27 | 0.7 |
| Plasma testosterone (ng/ml) | 4.78±0.31a | 3.9±0.20b | 3.8±0.27b | 0.02 | 4.7±0.34a | 4.3±0.25ab | 3.7±0.23b | 0.03 |
| Plasma glucose (mg/dl) | 130.7±4.51 | 148.0±8.42 | 139.6±8.48a | 0.2 | 140.2±5.88 | 135.4±5.9 | 139.9±8.73 | 0.9 |
T1, T2 and T3 are lowest, intermediate and highest scores of each parameter, respectively.
a,b Within rows, values with different superscripts differ significantly (P≤0.05).
Environmental factors, whether chemical, physical or emotional, might adversely affect testicular function.30,31 Rostami et al. showed that injection of 125μg testosterone decreased fear behavior in Wistar rats which was probably due to the inhibitory effect of testosterone on the hypothalamic–hypophyseal–adrenal (HPA) axis.32 Prenatally-stressed rats were found to be more fearful than the control group. This effect was because of the changes in the sexually dimorphic nucleus of the preoptic area (POA) and anteroventral periventricular nucleus (AVPV) as well as an increase in corticotropin-releasing hormone (CRF).33 Rostami et al. also concluded that prenatal immobilization affected the fear behavior which was decreased by testosterone treatment in a dose-dependent manner.32 Plasma testosterone concentration can be a marker to assess semen quality and sperm concentration.34 In the current investigation we found that sperm motility was higher in roosters that had the lowest delay to their first pace (T1; Table 3); this delay has been shown to be higher in fearful animals.4
Fertility in the roosters is a function of the mating behavior, semen quality and blood parameters.28,35,36 We found that the open field behavior was correlated with several sperm characteristics (Table 1). In one study in rabbits, subjected to eight generations of divergent selection for high and low locomotor activity in the open field, fertility of the female rabbits showing high open field activity decreased in the 3rd and 4th generations.9 To the best of our knowledge, this is the first study on the association between ethological traits and reproductive parameters. An important point to be mentioned here is that crow number in the open field may interfere with activity as roosters stop walking while crowing; the negative correlation between crow number and pace number (r=−0.21) reflects this point. This interference is not easily surmountable as one cannot correct each of these variables for the other. It is not clear that a rooster would walk or not while not crowing. Therefore, the relationship between crow numbers and activity needs further investigations. Besides, simple correlation coefficients imply some kind of association but not a cause and effect relation. Our result is in agreement with Galeotti et al. who showed the singing frequency in male barn swallows was negatively correlated to testosterone concentration.37
Collectively, our data indicate that semen characteristics were correlated with open field behavior; however plasma glucose was more correlated with semen characteristics. Roosters with the lowest delay to their first pace had the highest sperm forward motility and lower sperm abnormality. The lowest level of H:L was found when pace bout and pace number were 20 and 35, respectively. The negative correlation between H:L ratio and semen characteristics (live sperm percentage, sperm concentration, and HOST values) may be an indication of poor reproductive performance in fearful roosters with higher H:L ratio. Further investigations are needed to reveal the potential application of our findings. Moreover, the association between semen characteristics and animal behavioral indices might be applicable in other animal models.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestsThe authors declare no conflicts of interests.
The authors would like to thank Ehsan Ommati for English proof-reading of the current manuscript and also Fatemeh Saemi, Farid Pazhoohi and also Elnaz Fadaee, Shiraz university, Shiraz, Iran; Dr. F. J. del Río Olvera, Instituto Andaluz de Sexología y Psicología, Spain; Dr. M. R. Kumar, Shanxi Agricultural University, China; for their help during the conduct of the experiment.
Ingredients and chemical composition of the basal diet (DM basis).
| Ingredient | (%) |
|---|---|
| Corn | 78.67 |
| Soybean meal | 16.40 |
| Dicalcium phosphate | 1.60 |
| Fish meal | 1.20 |
| Oyster shell | 1.00 |
| Sodium chloride | 0.28 |
| Vitamin premixa | 0.40 |
| Trace- mineral premixb | 0.40 |
| DL-Methionine | 0.05 |
| Compositions | |
| Metabolizable energy (kcal/kg) | 2793 |
| Crude protein (%) | 14.62 |
| Ca (%) | 0.90 |
| P (%) | 0.50 |
| l-Lysine HCl (%) | 0.52 |
| dl-Methionine (%) | 0.23 |
| Total sulfur amino acids (%) | 0.46 |
| l-Threonine (%) | 0.42 |
(ZnO), 67.3mg; Cu (CuSO4·5H2O), 10.9mg, and Se (Na2 SeO3), 0.18mg.
Behavioral ethogram.
| Behavior | Description |
|---|---|
| Pace number | Number of paces (Lifting one foot and putting it forward) |
| Pace bout | Bouts of pacing with a threshold of 5 seconds lack of pacing |
| First pace | Time (S) from putting rooster in the open field box to first pace |
| Peck number | Number of pecks at floor or walls of the open field |
| Peck bout | Bouts of pecking with a threshold of 5 seconds lack of pecking |
| First peck | Time (S) from putting rooster in the open field box to first peck |
| Crow number | Number of usual rooster crows |
| Coming forward | Time of reaching the front of cage as an indicator of exploratory behavior and fear fullness |
The multivariate regression of open field test with blood and seminal characteristics.
| Y | X | a | b1 | b2 | CV (%) | R2% | Differentiation |
|---|---|---|---|---|---|---|---|
| H:L (%) | Pace bout | 0.85** | −0.04* | 0.001* | 63.33 | 7 | 20 (Number) |
| H:L (%) | Pace number | 0.74** | −0.007* | 0.0001** | 60.61 | 15 | 35 (Number) |
| Plasma testosterone (ng/mL) | Crow number | 4.90** | −0.14* | 0.004* | 30.05 | 12 | 17.5 (Number) |
| Sperm forward motility (%) | Coming forward | 87.46** | −0.12* | 0.0002* | 19.70 | 14 | 300 (Second) |
Asterisks indicate significant difference (* P < 0.05, ** P < 0.01).