To describe the clinical behavior of human papillomavirus in men.
Materials and methodsCurrent international literature was reviewed to describe the clinical behavior of human papillomavirus in men.
ResultsInternationally, the overall prevalence of HPV DNA is 50.8%, HPV considered high risk are 14 types. Prevalence of HPV DNA in invasive penile cancer ranges from 33.1% to 47%. HPV-16 has been the most frequent (68.3%), followed by HPV-6 (8.1%) and HPV-18 (6.9%). Positive HPV is described as an independent prognostic factor for cancer-specific survival.
ConclusionIt is not clear why HPV infection has a predilection in specific areas of the genital tract. However, it is important to note that there are factors that increase the risk of HPV infection.
Describir el comportamiento clínico del virus del papiloma humano en hombres.
Materiales y métodosSe revisó la literatura internacional actual para describir el comportamiento clínico del virus del papiloma humano en los hombres.
ResultadosEn el ámbito internacional, la prevalencia general del ADN del VPH es del 50,8%. Los VPH considerados de alto riesgo son 14 tipos. La prevalencia del ADN del VPH en el cáncer de pene invasivo oscila entre el 33,1% y el 47%, siendo el VPH-16 el más frecuente (68,3%), seguido del VPH-6 (8,1%) y del VPH-18 (6,9%). El VPH positivo se describe como un factor pronóstico independiente para la supervivencia específica del cáncer.
ConclusiónNo está claro por qué la infección por VPH muestra predilección por áreas específicas del tracto genital. Sin embargo, es importante tener en cuenta que existen factores que aumentan el riesgo de infección por VPH.
Human papillomaviruses (HPV) are small viruses (55nm), it has a circular double strand of deoxyribonucleic acid (DNA), enveloped by a capsid comprising the major and minor proteins (L1 and L2). Encoding eight genes, including two capsular structural late proteins, which form the coat of the virus, and encodes six early proteins (E). The two most important HPV proteins in the pathogenesis of malignant diseases are E6 and E7. At the molecular level, the ability of these proteins to transform cells is related in part to their interaction with two intracellular proteins, p53 and retinoblastoma.1
Two pathways for penile cancer have been described, one related to human papillomavirus infection and the other related to other factors including phimosis, chronic inflammation, and lichen sclerosus.2
HPV infectionAn increased risk of HPV infection of any type is observed in patients with more than 30 sexual intercourse per month and the presence of genital warts. A lower risk is seen in patients who are circumcised, who have a regular sexual partner, and who use condoms regularly.3
- •
In uncircumcised patients, HPV has been reported in 19.6%, while in circumcised patients in 5.5%. In circumcised patients, the possibility of HPV infection is lower. (OR 0.37; 95% confidence interval (95% CI): 0.16–0.85).4 In Mexico, only 14.4% are circumcised.5 Male circumcision has also been associated with a moderate but non-significant decrease in the risk of cervical cancer in their female partners (OR 0.72; 95% CI: 0.49–1.04).4
- •
3 female sexual partners has been associated with an increased risk of HPV infection (OR 3.2; 95% CI: 2.1–4.9).6
- •
The use of condoms is related to a lower frequency of HPV infection (65.9% vs 71.9%; p=0.005).7
Regarding the age and prevalence of HPV, in Mexico is slightly higher in the group aged 40–44 years (68.8%).5
In patients with phimosis, the prevalence of HPV is 43.3%, of which 53.8% are high risk.8
Internationally, the geographic region with the highest prevalence of HPV DNA has been Africa with 87.5% (75.6–95.8), followed by South America with 59.3% (50.1–68.2); the overall prevalence of HPV DNA is 50.8%.9 In Mexico, the prevalence of HPV infection is 61.9%.5
Non-oncogenic HPV infectionAn increased risk of non-oncogenic HPV infection has been observed in patients with genital warts; in turn, a lower risk has been observed in circumcised patients and the use of condoms.3
The international prevalence of non-oncogenic HPV infection has been described in 22%; in Mexico it has been described in 35%.5,7
The non-oncogenic HPVs described worldwide are 6, 11, 26, 40, 42, 53–55, 61, 62, 64, 67–73, 81–84.7,10
In Mexico, the most frequent non-oncogenic types are HPV-84 (6.6%), HPV-62 (5.5%), HPV-61 (4.4%), HPV-6 (4.1%).5
Oncogenic HPV infectionAn increased risk of oncogenic HPV infection has been observed in patients with more than 30 sexual intercourse per month; Circumcised patients, condom use, and a stable sexual partner are at lower risk.3
HPV considered high risk are (14 types) 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.7,10,11
High-risk HPV DNA has been reported in up to 29%.11,12 In Mexico, a prevalence of 30% has been reported.5
In Mexico, the most frequent oncogenic types are HPV-59 (7.2%), HPV-51 (6.6%), HPV-16 (5.5%), HPV-66 (4.1%), HPV-39 (5%), HPV .52 (3.6%), HPV-56 (1.9%), HPV-58 (1.9%), HPV-18 (1.7%), HPV-31 (1.4%), HPV- 35 (0.8%), HPV-33 (0.3%), HPV. 45 (0.3%).5
DiagnosisDual p16/Ki-67 staining has been shown to have higher sensitivity (in women of all ages) against papanicolau (86.7% vs 68.5%; p<0.001), to detect cervical intraepithelial neoplasia (CIN) 2; with a specificity of 95.2% versus 95.4% (p=0.15). HPV E7 protein disrupts the cell cycle, leading to increased expression of the cellular p16 protein. High-grade CIN contain high levels of p16, and pathologists often immunostain cervical biopsies to distinguish between high-grade CIN and immature squamous metaplasia, which is not associated with HPV and is not precancerous.
DNA testing HPV: has higher sensitivity to the dual staining to detect precursors of cervical cancer, as cervical intraepithelial neoplasia (CIN) 2 and 3 (93.3% vs 84.7%; p=0.03) but less specificity (93.0% vs 96.2%). %; p<0.001).
DNA tests for HPV approved by the Food and Drug Administration (FDA) are:
- •
Hybrid Capture 2 (HC2): detects 13 different types of high-risk HPV oncogenic.
- •
Cobas test identifies types HPV 16 and 18, while the remaining 12 types detected in a probe mixture.
- •
Cervista test indicates positivity for one or more types in the mix of 14 probes. Cervista and Cobas tests detect HPV 66 in addition to the 13 types of HPV detected by HC2.
HPV ribonucleic acid (RNA) test: looks for the expression of E6 and/or E7 RNA, significantly improves the specificity of the detection of cervical intraepithelial neoplasia grade 2 and higher (CIN2+), reducing the number of “false HPV” positives “compared to HPV DNA testing.
Aptima HPV test is an FDA approved RNA-based test.
There are no clinically available tests to detect HPV infection in the penis.13–15
Multiple infectionsIn Mexico, multiple infections have been reported up to 23.8%.5
Prevalence of multiple infections was higher in penile high-grade squamous intraepithelial lesions (HGSILs) in 17.6%, compared with invasive penile cancer. (9.0%) (p=0.027).16
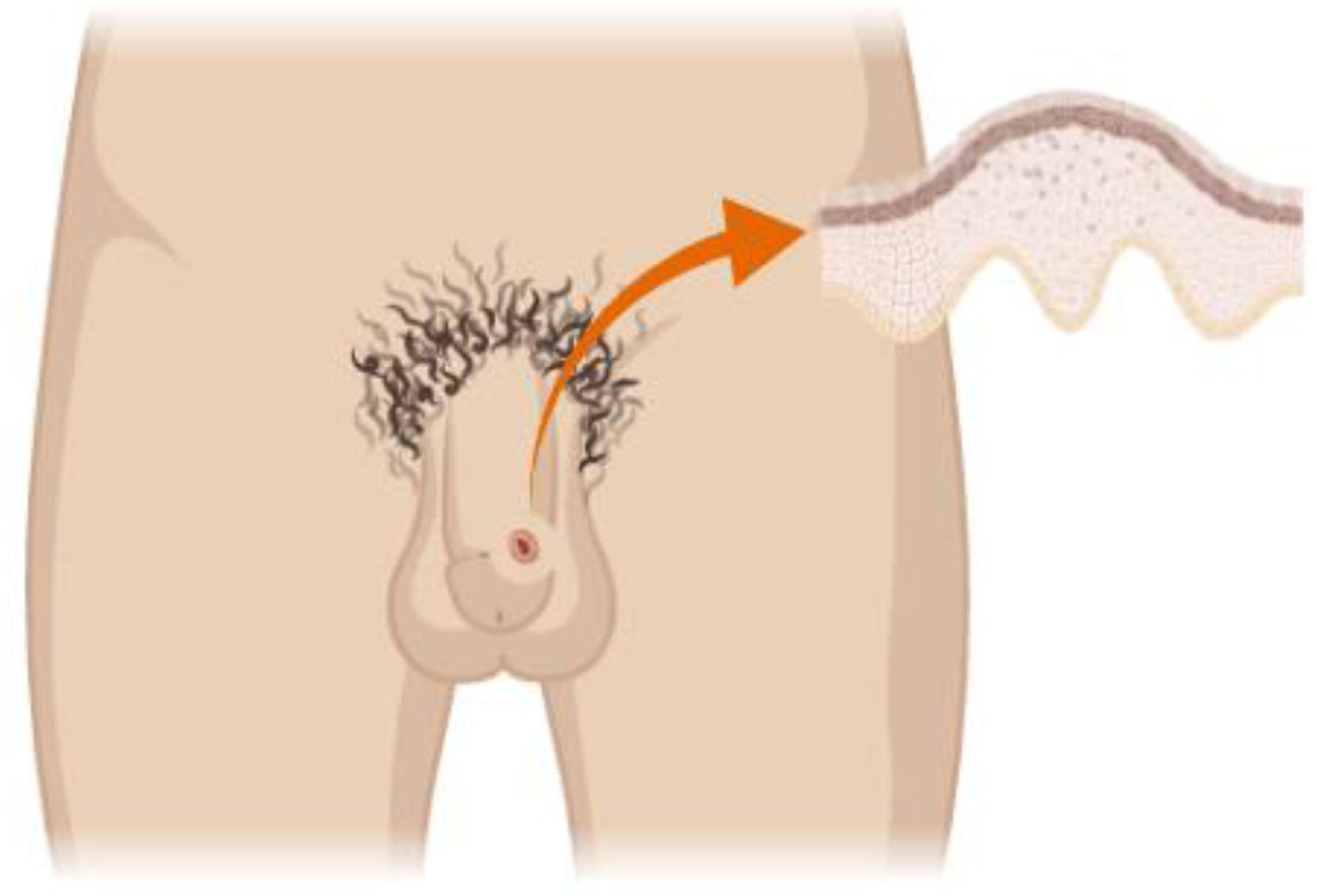
Infection sitesHPV is identified in up to 65.4% of men at 1 or more anatomical sites. The most frequent site is the shaft of the penis (49.9%), followed by the glans/coronal sulcus (35.8%) and scrotum (34.2%), Fig. 1. HPV detection is lower in the perianal area (20.0%) and in the anal canal (17.6%); its lower in urethra (10.1%) and semen (5.3%).17
HPV 6, 11, 16 and/or 18 DNA has a higher prevalence in the penis (7.0%), followed by the scrotum (5.1%) and the perineal/perianal area (2.9%).6
It is not clear why the glans/coronal sulcus in uncircumcised men is particularly vulnerable to oncogenic genotypes. However, it is important to note that the glans penis is the most common subsite of penile carcinomas.18
ClearanceMedian duration of HPV infection in the glans/coronal sulcus is significantly longer in uncircumcised men (154 days) than in circumcised men (91 days) (p=0.04).19
A mean HPV infection of 7.52 months (6.80–8.61) was reported for any HPV and 12.19 months (7.16–18.17) for HPV 16.20
Other sitesMen with a previous genital HPV-16 infection have a 4 times higher risk of getting an anal infection. 45% of these men are under 30 years old. Prevalence of HPV infection in the anal canal in men who have sex with women is 12.2%. HPV is non-oncogenic in 5.4%, oncogenic in 6.8% and multiple infection in 3.2%.21
A higher prevalence of infection has been observed in the perianal region (21.3%) than in the anal canal 16.6%.21
Transport mechanism to the anal canal is unclear. HPV genotypes have been detected in mucosa/genitals, in fingers; and transmission of HPV between hands and anogenital sites has been proposed.10
HPV and intraepithelial lesionsPrevalence of HPV DNA in penile intraepithelial lesions (SILs) is 79.8%; its more frequent in low grade with 98.6% compared to high grade 80.5%.9 Other studies reported a prevalence of HPV DNA in penile high-grade intraepithelial lesions (HGILs) of up to 87.1% (95% CI 78.0–93.4).16
Regarding age, HGILs are diagnosed 10 years earlier than patients with invasive penile cancer (54.8 vs 64.1; p<0.001).16
Morphological characteristics of HGILs are warty-basaloid in 78.8%.16
HPV-16 was the most frequent in HGILs (79.6%).16
HPV and invasive cancerAccording to Globocan 2018, penile cancer ranks 32nd in incidence and 33rd in mortality worldwide. In Mexico, penile cancer ranks 27th in incidence and 30th in mortality.22,23
Prevalence of HPV DNA in invasive penile cancer ranges from 33.1% to 47%.2,16,24 In invasive penile cancer, basaloid/warty (66.3%) and warty squamous cell carcinoma (22.4%) are seen more frequently.9,16,24
HPV-16 has been the most frequent (68.3%), followed by HPV-6 (8.1%) and HPV-18 (6.9%).2,9,11,16,24,25
HPV 16 and HPV 18 accounted for 70% of all invasive cancers.16
HPV 35, 39, 45, 51, 52, 54, and 59 each accounted for less than 1.5% of cases.4
In Mexico, the most frequent oncogenic types are HPV-59 (7.2%), HPV-51 (6.6%), HPV-16 (5.5%), HPV-66 (4.1%), HPV-39 (5%), HPV-52 (3.6%), HPV-56 (1.9%), HPV-58 (1.9%), HPV-18 (1.7%), HPV-31 (1.4%), HPV-35 (0.8%), HPV-33 (0.3%), HPV-45 (0.3%).5
Survival of specific diseases5-Year disease-specific survival in patients with positive HPV is 96% and 82% for negative HPV (log rank p=0.016).12
5-Year disease-specific survival among high-risk positive HPV patients versus the negative group is 92% versus 78% (log range p=0.03).11
Cancer-specific survival rates at 2 and 5 years for patients with p16 INK expression are 95% and 85% versus 73% and 57% for those who do not express it (p=0.011).26
Prognostic factorsThe most important disease-specific survival factors are positive lymph nodes (HR 7.0; 95% CI: 2.8–17.6; p≤0.001), positive HPV (HR 0.21; 95% CI: 0.06–0.76; p=0.017), T2, T3 (HR 4.0; 95% CI: 1.1–14.0; p=0.03) and vascular invasion (HR 4.5; 95% CI: 1.4–14.6; p=0.014).11
Another study reported koilocytosis (HR 0.24; 95% CI: 0.07–0.83; p=0.024), expression of p16 INK4a (HR 0.44; 95% CI: 0.23–0.84; p=0.013), histological stage (HR 3.54; 95% CI: 1.88–6.67; p<0.001) and grade (HR 2.47; 95% CI: 1.00–6.09; p=0.049) as independent prognostic factors for cancer-specific survival.26
HPV vaccine in menProphylactic HPV vaccines are formulations of the L1 capsid protein, made from the natural HPV particle. Virus-like particles (VLP) are recombinant proteins, which have no oncogenic potential, making them ideal candidates for use as vaccines. VLP administration generates neutralizing antibodies that bind to HPV virions preventing their entry into cells.27
Considering HPV-16 and HPV-18 are the cause of approximately 70% of cancers and HPV-6 and HPV-11 are the cause of approximately 90% of genital warts, for vaccination in men it can be used:
- •
4v-HPV (6, 11, 16, and 18), approved by the Food and Drug Administration in 2009.
- •
9v-HPV (6, 11, 16, 18, 31, 33, 45, 52 and 58), approved by the Food and Drug Administration in 2014.28,29
Routine HPV vaccination is recommended at 11 and 12 years, but can be given from 9 years of age. Seroconversion has been observed in 99%. Geometric mean titers (GMTs) are significantly higher in adolescents aged 9–15 years, compared to those aged 16–26 years.29,30
13–26 yearsVaccination is recommended for unvaccinated and for those who have not completed the vaccination schedule.
Before 15 years, 2 doses are recommended (0, 6–12 months).
After 15 years, 3 doses are recommended (0, 1–2, 6 months).28,30
Seroconversion rates in the first month after the third dose range from 97% to 99%; at 36 months the positivity for HPV-6: 88.9%; HPV-11: 94.0%; HPV-16: 97.9%; and HPV-18: 57.0%.31
GMT for HPV-18 is significantly different between 15 and 20 year olds (473.5mMU/ml [95% confidence interval (CI) 427.5–524.6]) and 21–27 year olds (339.1mMU/ml [95% CI, 304.7–377.3], respectively).31
The efficacy in the prevention of genital warts in men related to HPV-6, HPV-11, HPV-16 and HPV-18 is 89.4%.28
27–45 yearsIt is not recommended, but in some circumstances such as having multiple sexual partners, a history of HPV infection, and immunosuppressed diseases can be evaluated.28,30
The number needed to vaccinate, men through age 26 year, to prevent anogenital warts is 40; high-grade lesions 450; and cancer 3260.30
More than 45 yearsIt is not recommended. The number needed to vaccinate, men through age 45 year, to prevent anogenital warts is 120; high-grade lesions 800; and cancer 6500.30
Vaccination is not recommended for men already infected with HPV.28
Seroconversion rates are higher in heterosexual men compared to men who have sex with men.32
In men with HIV, the vaccine is immunogenic and well tolerated.28
HPV vaccine in both men and womenBy vaccinating 12-year-old women, the risk of HPV-16 and HPV-18 infection in heterosexual men is reduced. With 60% of women vaccinated, the lifetime infection risk reduction for heterosexual men is estimated to be 53% for HPV-16 and 62% for HPV-18.
Observing a 54% reduction in the risk of penile cancer.
If women were not vaccinated, 466 men would be required to get vaccinated to avoid one case of cancer.
If women were vaccinated, 795 men would need to be vaccinated to avoid one case of cancer; to prevent a case of penile cancer, the number to be vaccinated would be 3486 men.33
By vaccinating against a specific type of HPV, both men and women, a decrease of up to 44% in the prevalence of this type of infection would be obtained, while vaccinating only women would result in a reduction of 30%.34
Regarding the cost effectiveness of HPV vaccination, it is lower in men compared to the female vaccination strategy.35
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo funding received.
Conflict of interestThe author declares no conflict of interest.