We previously postulated that orgasmic sensation may occur through recently discovered genital taste bud-like structures. The interaction between the pudendal nerve and Onuf's nucleus may be important for developing orgasmic information. The study aims to investigate whether ischemic damage to Onuf's nucleus-pudendal network following spinal subarachnoid hemorrhage (SAH) causes taste bud degeneration or not.
MethodsThe study was conducted on 22 fertile male rabbits who were divided into three groups: control (GI; n=5), SHAM (GII; n=5) and study (GIII; n=12). Isotonic solution, .7cm3, for the SHAM, and .7cm3 homologous blood was injected into spinal subarachnoid spaces at S2 level of the study group. Two weeks later, Onuf's nucleus, pudendal ganglia and the taste bud-like structures of the penile urethra were examined histopathologically. Degenerated neuron densities of Onuf's nucleus, pudendal ganglia and atrophic taste bud-like structures were estimated per mm3 and the results analyzed statistically.
ResultsThe mean degenerated neuron densities of taste bud-like structures, Onuf's nucleus and pudendal ganglia were estimated as 2±1/mm3, 5±1/mm3, 6±2/mm3 in GI; 12±4/mm3, 35±9/mm3, 188±31/mm3, in GII and 41±8/mm3, 215±37/mm3, 1321±78/mm3, in GIII. Spinal SAH induced neurodegeneration in Onuf's nucleus, pudendal ganglia and taste bud atrophy was significantly different between GI/GII (p<.005); GII/GIII (p<.0005) and GI/GIII (p<.0001).
ConclusionIschemic neuronal degenerations of Onuf's nucleus and pudendal ganglia following spinal SAH lead to genital taste bud-like structure atrophy. This mechanism may be responsible for sexual anhedonia and sterility in cases with spinal cord injury, which has not been documented so far. More studies are needed.
Hemos postulado previamente que la sensación orgásmica puede producirse a través de las recientemente descubiertas estructuras de tipo papila gustativa de los genitales. La interacción entre el nervio pudendo y el núcleo de Onuf puede ser importante para desarrollar información orgásmica. El objetivo del estudio fue estudiar si el daño isquémico a la red núcleo-ganglios pudendos de Onuf tras una hemorragia subaracnoidea espinal (HSA) puede causar o no una degeneración de las estructuras de tipo papila gustativa.
MétodosEl estudio fue realizado en 22 conejos fértiles que se dividieron en 3 grupos: control (GI; n=5), placebo (GII; n=5) y de etudio (GIII; n=12). Se inyectaron 0,7cc de solución isotónica a los miembros del grupo placebo, y 0,7cc de sangre homóloga en los espacios subaracnoideos espinales a nivel de S2, al grupo de estudio. Al cabo de 2 semanas se examinaron histopatológicamente el núcleo de Onuf, los ganglios pudendos y las estructuras de tipo papila gustativa de la uretra. Se calcularon por mm3 las densidades de las neuronas degeneradas del núcleo de Onuf, los ganglios pudendos y las estructuras atróficas de tipo papila gustativa, analizándose estadísticamente los resultados.
ResultadosLas densidades medias de las neuronas degeneradas de las estructuras de tipo papila gustativa, el núcleo de Onuf y los ganglios pudendos se calcularon como 2±1, 5±1, 6±2/mm3 en GI; 12±4, 35±9, 188±31 en GII y 41±8, 215±37, 1321±78/mm3, en GIII. La neurodegeneración inducida de HSA en el núcleo de Onuf, los ganglios pudendos y la atrofia de las papilas gustativas fue significativamente diferente entre los grupos GI/GII (p<0,005); GII/GIII (p<0,0005) y GI/GIII (p<0,0001).
ConclusiónLas degeneraciones neuronales por isquemia del núcleo de Onuf y los ganglios pudendos tras una HSA originaron la atrofia de la estructura de tipo papila gustativa de los genitales. Dicho mecanismo podría ser responsable de anhedonia sexual y esterilidad en casos de lesiones medulares, lo cual no ha sido documentado hasta el momento, por lo que serían necesarios más estudios.
The orgasmic sensation is important for humans. Aydin et al. reported the morphologic mechanism by reporting taste buds like structure in male urethra.1,2 If hedonic sensations are regulated by a network between taste buds-like structure and pudendal nerve, the insults of that disrupt this network may be responsible for sexual dysfunctions and sexual anhedonia. Although anhedonia and impotence are common following spinal pathologies, the mentioned neural network has not been considered as the exact mechanism of orgasm. The pudendal nerves convey sensory information from the genitalia to the brain.3 Dorsal root ganglion lesions may occur following spinal SAH.4,5 Erectile and ejaculatory dysfunctions are the most common disorder of male sexual health following spinal cord injury.6 Yolas et al. previously reported that SAH can also affect the spinal cord and related anatomical structures.7 Adamkiewicz artery vasospasm may cause Onuf's nucleus and sacral spinal nerve complex degeneration following spinal SAH,8 ischemic disruption of that neural network may rely on denervation atrophy in genital taste buds. To explain how spinal cord injuries, cause anorgasmia, we aimed to investigate if spinal SAH could cause taste bud-like structure changes.
Materials and methodsStudy design: experimental observational studySettingAnimal protocols were “approved” by a competent Ethics Committee (Ataturk University's ethics committee). Twenty-two adult male rabbits were used (2.5 years old, 4.5±0.5kg). Five animals were used as the control (GI; n=5), five as the SHAM (GII; n=5) and twelve as the study (GIII; n=12) groups histological changes of the Onuf's nucleus, pudendal nerve and urethral taste bud-like structure. The remaining animals were anesthetized with a subcutaneous mixture of ketamine hydrochloride (25mg=kg), lidocaine hydrochloride (15mg=kg) and acepromazine (1mg=kg). Thoracolumbar regions were cleaned with antiseptics and shaved. After the cutaneal preparation, S1–S3 laminectomy was done, 0.7cm3 physiologic serum saline applied into spinal subarachnoid spaces at the S2 level of SHAM group; 0.7 autologous blood injected into to spinal subarachnoid space of the study group. Animals were followed, and sacrificed under general anesthesia after one week.
Surgical proceduresA posterior approach was performed under an operating microscope in the sacrococcygeal region, a lumbosacral laminectomy was performed. The dura mater was opened and Onuf's nucleus was found. Onuf's nucleus, pudendal nerve ganglia and spinal cord were removed and preserved in 10% formalin solution for light microscopic analysis. Tissues embedded in paraffin blocks were sectioned via Leica RM2125RT microtome (Leica Microsystems, Wetzlar, Germany), and preparations stained with hematoxylin–eosin, gustducin, S-100 and tunel method.
Immunostaining procedureThis procedure was performed according to the study of Ozcan et al.9 and Karadeniz et al.10 Many types of antibodies have been recognized in human cells, so the main goal of the use of the following immunostaining techniques was to compare different antigen retrieval techniques using both enzymatic and non-enzymatic preparations in order to determine the expression and distribution of several neuronal markers in the Onuf's nuclei and pudendal ganglia on formalin-fixed, paraffin-embedded tissues as used in the study of Perez et al.11
Five micro sections of tissue specimens were taken under a positively charged lamp. After placing the sections on a Leica Bon-Max immunohistochemistry device, the following processes were performed using the immunohistochemical staining device. Paraffin was allowed to melt at 60°C for 30min and removed by keeping it in the device solution for 15min, then rehydrated by keeping it in 99% alcohol for 15min. Specimens were washed using a wash buffer solution for 3min, and antigens were retrieved in Epitop 2 solution. Specimens were re-washed using a wash buffer solution for 3min. The specimens were then let in 3% hydrogen peroxidase and washed again using a wash buffer solution for 3min. Corresponding antibodies (Tunnel, Leica S-100) were added and left for 60min; specimens were then washed using a wash buffer solution for 3min. After dropping the post primer solution, the samples were left for 10min and washed using a wash buffer solution for 3min. A polymer solution was added and left for 10min; specimens were then washed using a wash buffer solution for 3min. The specimens were washed in deionized water for 3min. DAB+Choromogen was added and left for 3min. The specimens were then washed using distilled water and stained by the previously mentioned dyes for 5min. After this, specimens were washed with distilled water, alcohol, and xylol, removed from the devices, and covered by a thin lamella for microscope examination.
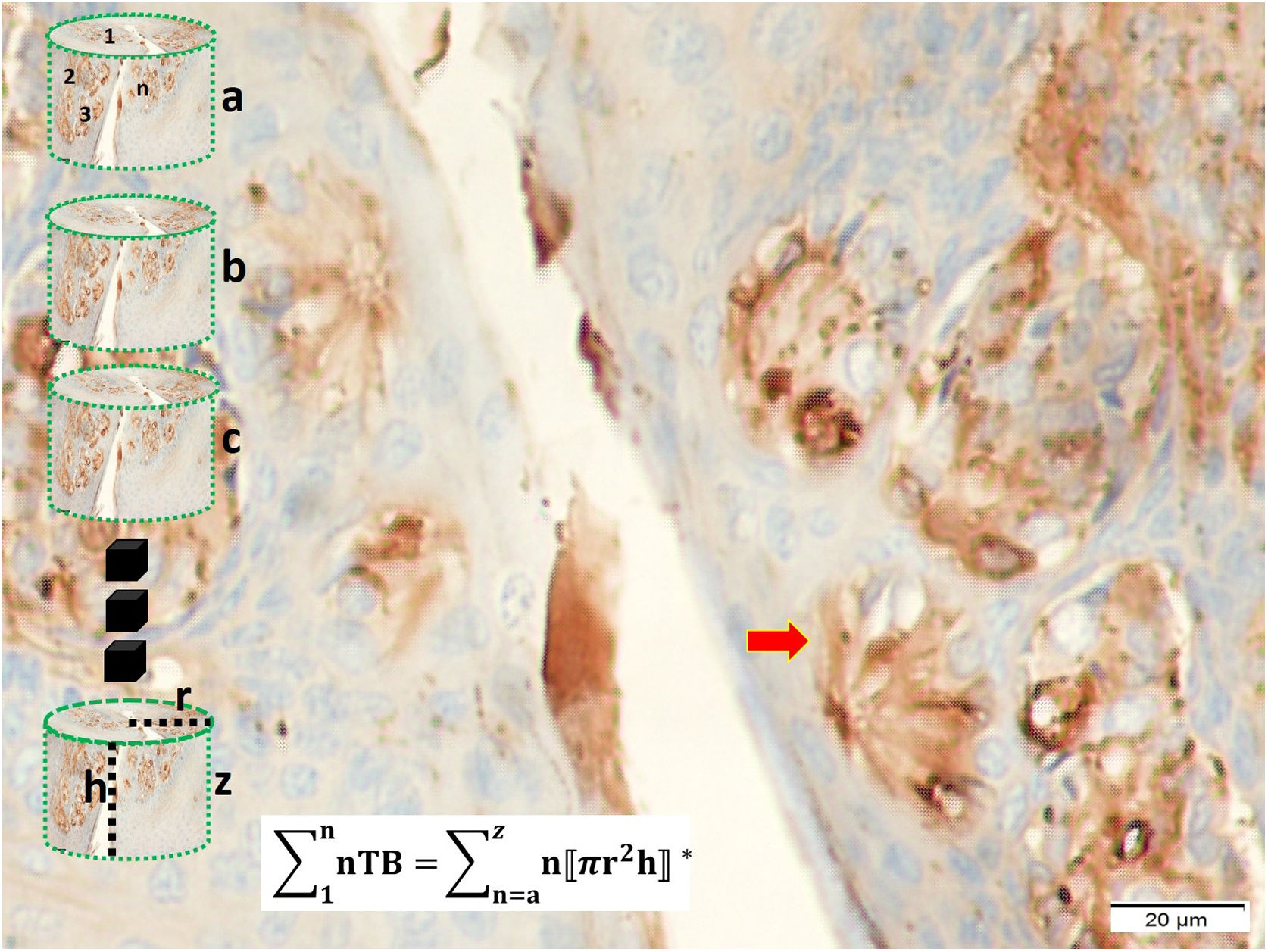
Tissue preparation for taste bud-like structure analysisThe penil specimens were divided to ten longitudinal segments and 5-μm thick and twenty consecutive sections were taken to determine of taste bud-like structure and their distribution in penil tissues. Specimens stained with H&E, gustducin, GFAP, S-100, and tunel method. Cytoplasmic condensation, peri-cytoplasmic halo formation, cellular angulations, nuclear shrinking and nuclear migration to the periphery were regarded as degeneration criteria of neurons and taste bud-like cells. To estimate urethral taste bud numbers, all urethras accepted as cylinder-shaped. To estimate the degenerated neurons numbers of Onuf's nuclei and pudendal ganglia, the physical dissector method was used. Data were analyzed with our previous stereological methods as same as described by Yolas et al.12
The urethral volumes calculated by the following formula: V=ΣΠh(re2−ri2). The taste bud like structure density (d=n/Plicae) and a total number of taste bud/all urethra were estimated the following formula: Σn=dΣΠh(re2−ri2).
Statistical analysisNeuron densities of Onuf's nucleus, pudendal nerve ganglia and taste buds-like structure were compared. Since the data showed a normal distribution, intergroup differences were assessed using a one-way ANOVA/SPSS-21.0 version was used. p<0.05 was accepted as statistically significant.
ResultsIntestinal and bladder distension, paraparesis, spastic or flask gait disturbances an limitations of tail movements were observed in some animals (n=4) of study group. Histopathologicaly, notified severe vasospasm of Adamkiewicz artery branches and arteria nervorums, neuronal degeneration and neuronal apoptosis were observed in the Onuf's nucleus and dorsal root ganglia of pudendal nerves in some animals of SAH group (n=5).
Histopathological observationsThese observations were shown SAH induced spinal cord edema, clot formation in subarachnoid spaces and nerve roots. Bloody material collection in pudendal nerve roots, constructed radicular arteries were observed in the study group Apoptotic neurodegeneration was detected in Onuf's nucleus, pudendal ganglia and taste buds in study group.
Numerical/statistical analysisThe mean degenerated neuron densities of taste buds-like structure, Onuf's nucleus and pudendal ganglia were estimated as 2±1/mm3, 5±1/mm3, 6±2/mm3 in normal; 12±4/mm3, 35±9/mm3, 188±31/mm3, in the SHAM group and 41±8/mm3, 215±37/mm3, 1321±78/mm3, in the study group. Spinal SAH induced neurodegeneration in Onuf's nucleus, pudendal nerve ganglia and taste buds like structure atrophy was significantly different between GI/GII (p<0.005); GII/GIII (p<0.0005) and GI/GIII (p<0.0001).
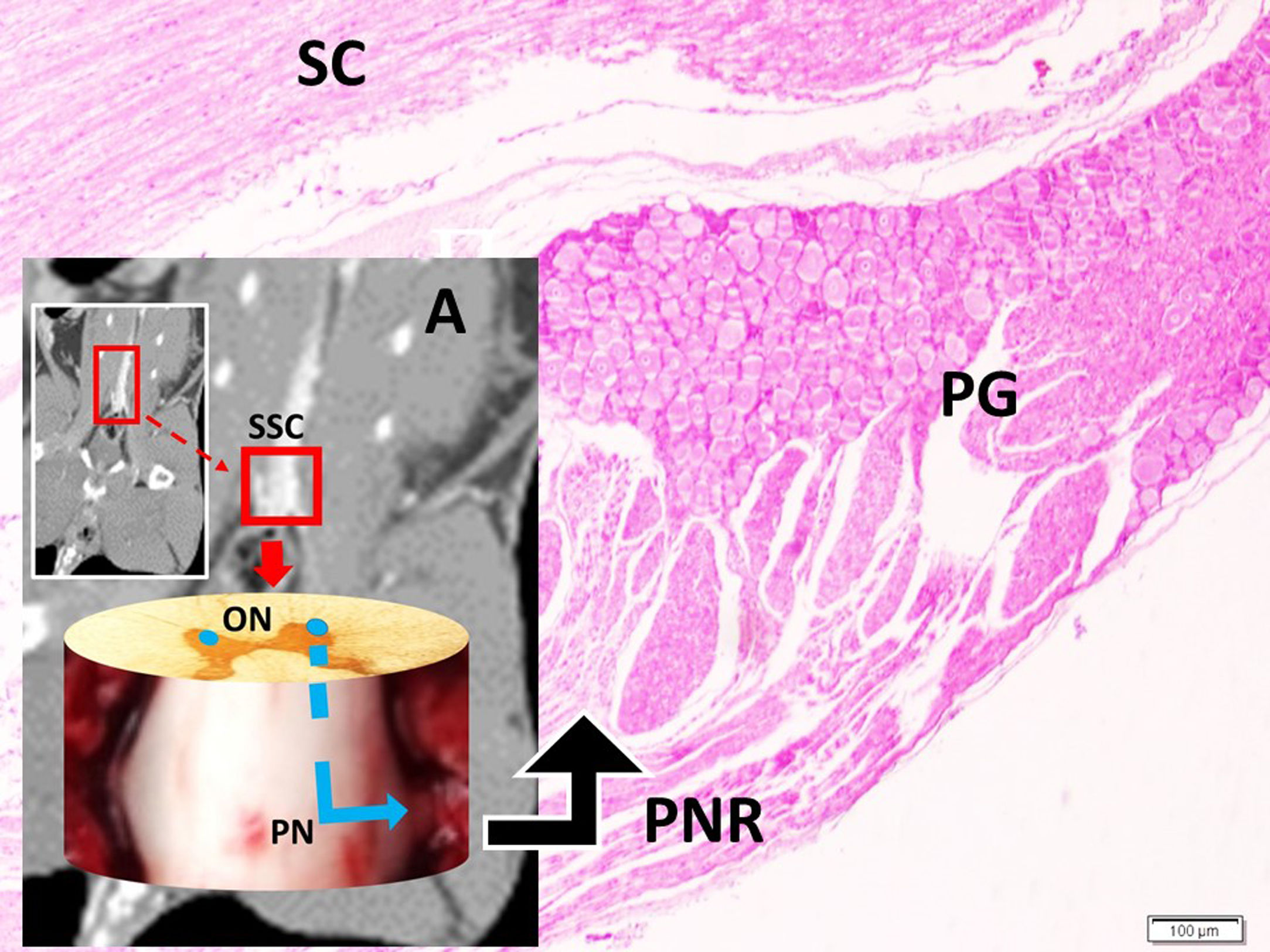
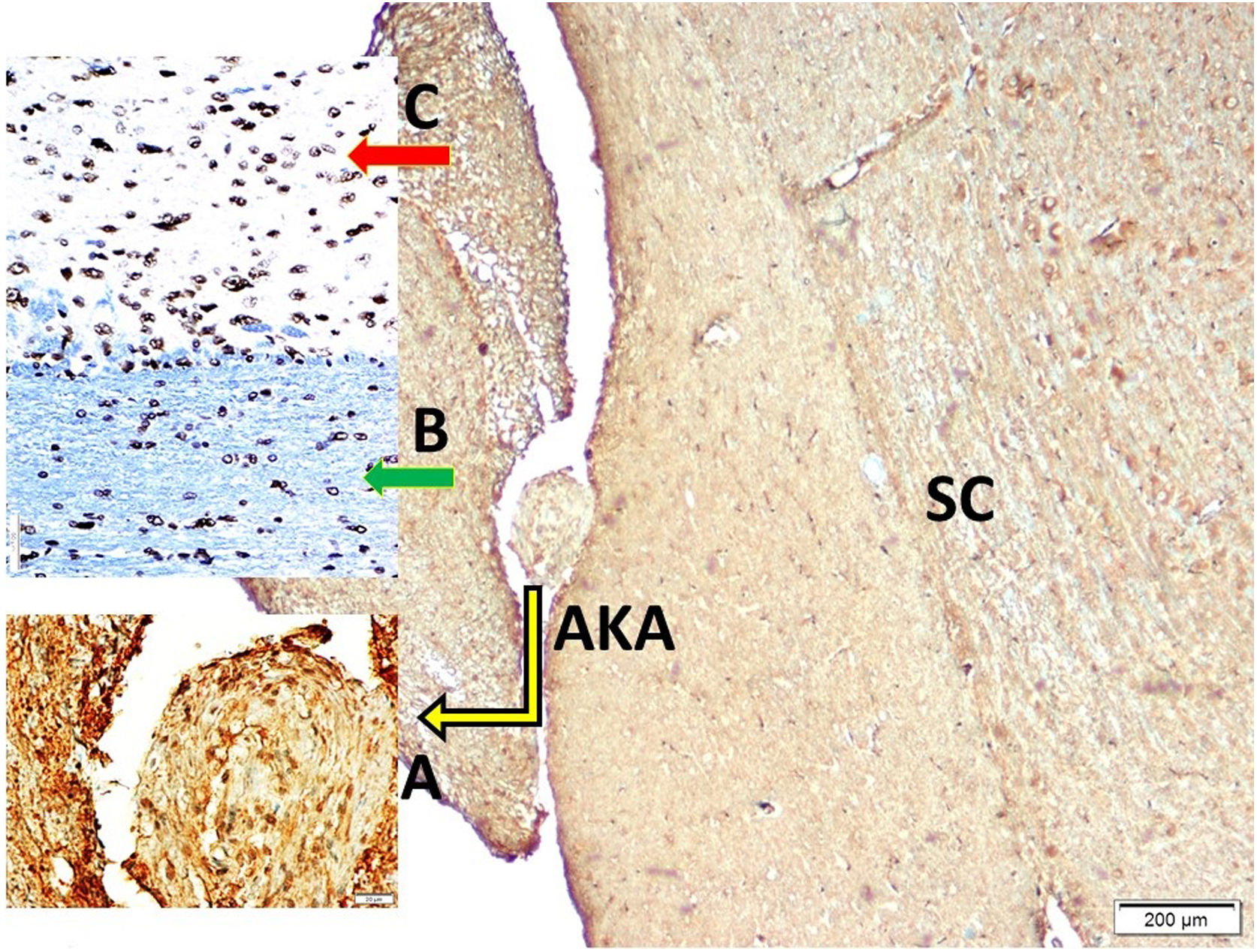
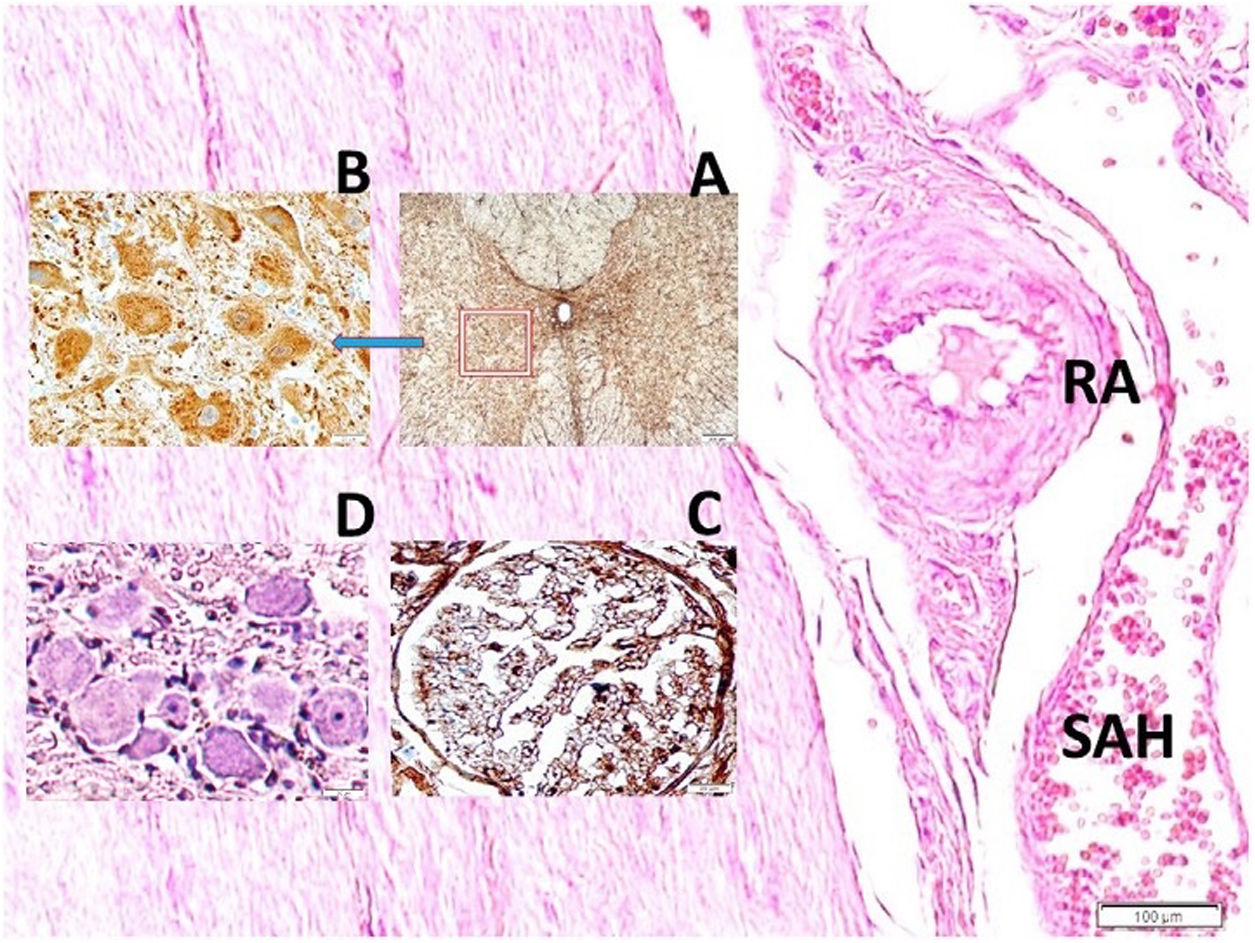
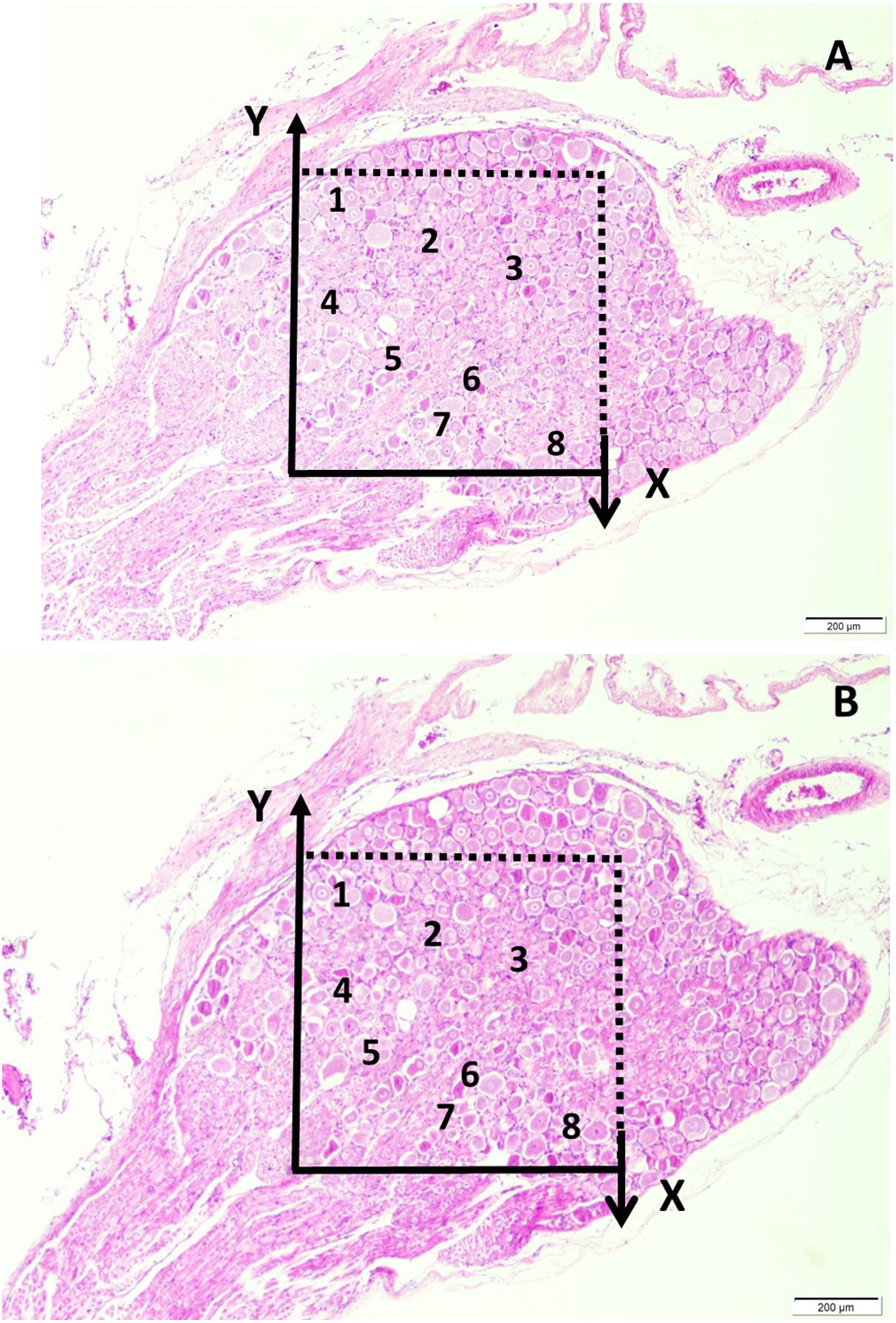
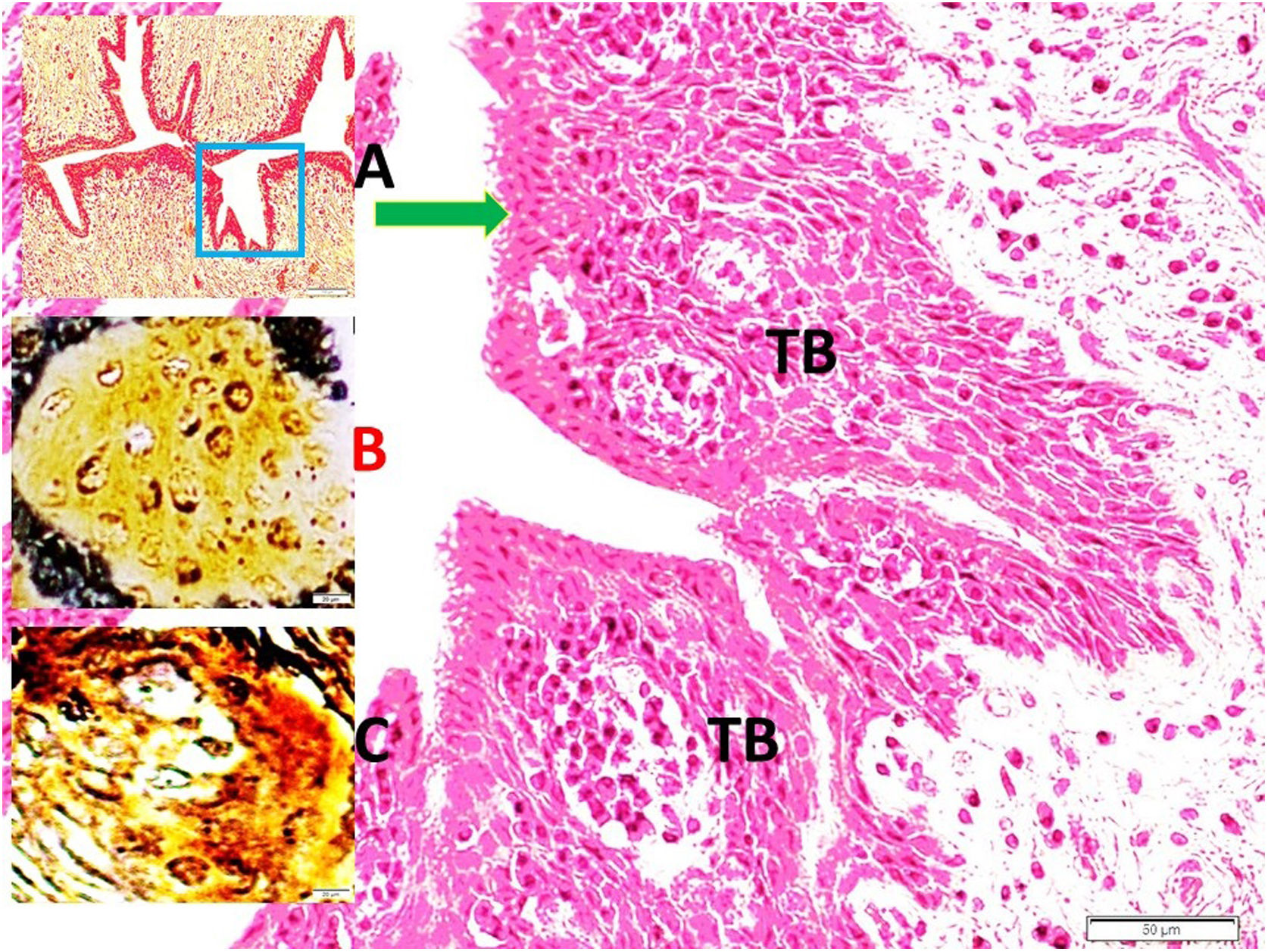
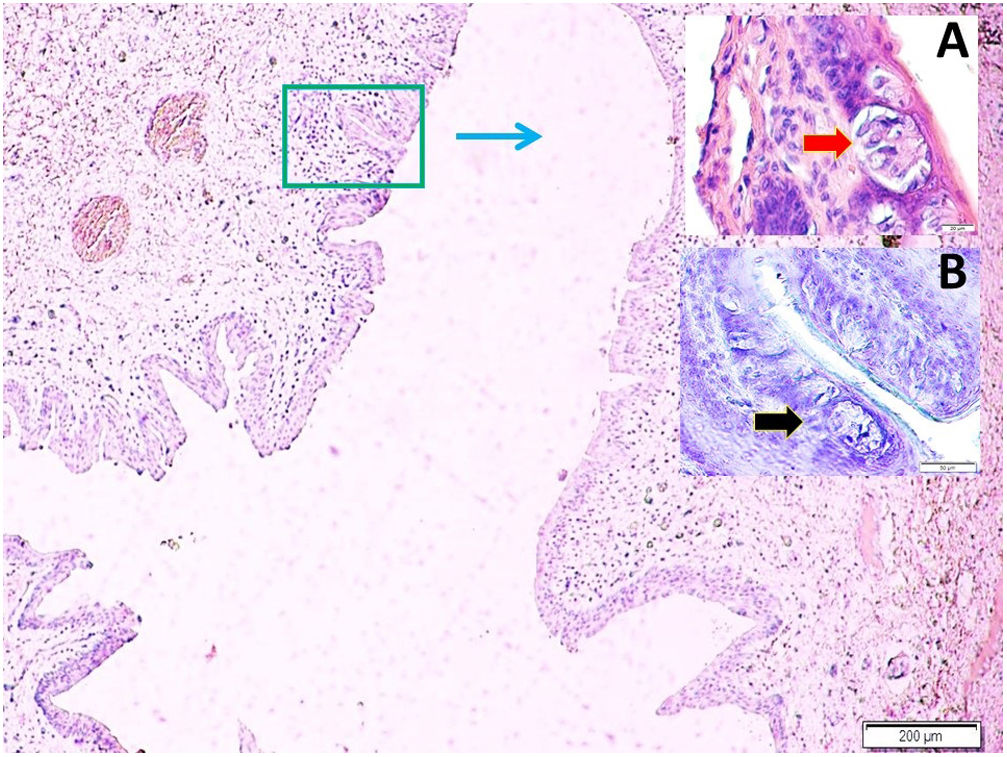
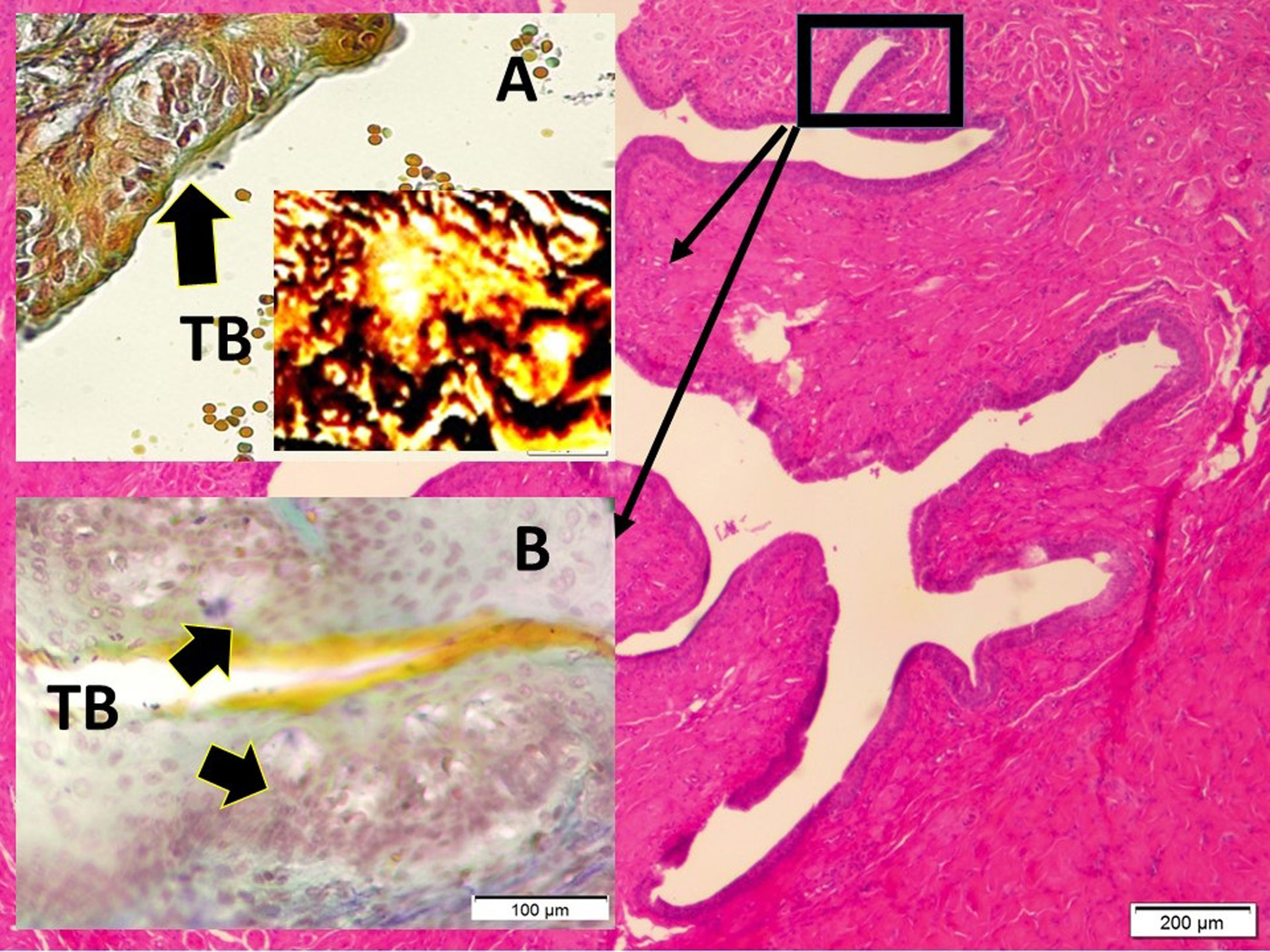
Histopathological analysisClot formation, pia-arachnoid adhesions, thickening, vascular contraction with micro-embolism, neuronal and cellular degeneration criterias were detected in Onuf's nucleus, pudendal ganglia and taste bud including tissues. Fig. 1 shows the CT images of the spinal colon and S3 nerve root including sacral spinal cord, vertical section of spinal cord with Onuf's nucleus in the anterior horn, pudendal nerve exiting level; pudendal nerve root and pudendal ganglia and the spinal cord is seen in a rabbit. In Fig. 2, S3 pudendal nerve root and root complex included in autonomous and somatosensitive fibers and supplying constructed Adamkiewicz artery between spinal cord and S3 root and magnified form are seen in a rabbit with SAH; the less somatic and the more apoptotic parasympathetic nerve fibers in S3 root section were also noted in this animal. Fig. 3 shows S3 pudendal nerve root and root complex supplying constructed radicular artery in bloody subarachnoid space; spinal cord section included Onuf's nucleus in gray matter; deformed neurons, thinned-ghosted axons and apoptotic darkened neuron in S3 nerve root ganglion in a rabbit with SAH (study group). Fig. 4A and B shows the stereologic cell counting method of the pudendal ganglia in rabbit. The used stereological method as the same as the study Aydin et al.13 and Yolas et al.8 Location of taste buds like structure in penil urethra, magnified forms of taste buds special identification of a normal and degenerated taste buds with gustducine is shown in Fig. 5. Location and identification of taste buds like structure in penil urethral sulcus and apoptotic-degenerated taste buds are seen in Fig. 6. Fig. 7 shows number of taste bud like structure s estimation method at the distal urethra covered by penil glandulary tissues. In the SHAM group we also noted some changes, whichs can be seen in Fig. 8. This figure shows the taste buds like structure in the penil urethral sulcus, magnified form of taste buds, apoptotic-degenerated taste buds.
The CT images of spinal colon and S3 nerve root including sacral spinal cord (SSC), vertical section of spinal cord with Onuf's nucleus (ON) in anterior horn, pudendal nerve (PN) exiting level (A); pudendal nerve root (PNR) and pudendal ganglia (PG) and spinal cord (SC) is seen in a rabbit (LM, H&E, ×10/Base).
S3 pudendal nerve root and root complex included in autonomous and somatosensitive fibers and supplying constructed Adamkiewicz artery (AKA/magnified form of AKA, ×20/A) between spinal cord (SC); and S3 root (LM, S-100, ×4/Base) and magnified form with normal somatosensitive (Part B) and apoptotic parasympathetic axons (Part C) (LM, Tunel, ×4/B) are seen in a rabbit with SAH; the less somatic and the more apoptotic parasympathetic nerve fibers in S3 root section with were also noted in this animal.
S3 pudendal nerve root and root complex supplying constructed radicular artery (RA) in bloody subarachnoid space (SAH) (LM, H&E, ×10/Base); spinal cord section included Onuf's nucleus in gray matter (LM, GFAP, ×4/A); deformed neurons (DN) (LM, GFAP, ×20/B), thinned-ghosted axons (LM, S-100, ×40/C) and apoptotic darkened neuron in S3 nerve root ganglion (LM, Tunel, ×20/D) in a SAH created rabbit.
(A and B) Stereologic cell counting of the pudendal ganglia in a rabbit. Application of the physical dissector method in which micrographs in the same fields of view (A and B) were taken from two parallel, adjacent thin sections separated by a distance of 5μm. The upper and right lines in the unbiased counting frames represent the inclusion lines, and the lower and left lines, including the extensions, are exclusion lines. The neuronal nucleoli touching the inclusion lines were excluded, and the nucleoli profiles touching the inclusion lines and located inside the frame were counted as dissector particles unless their profile extended up to the reference section. The number of neurons from the two dissectors occurs in a volume given by the product of the counting frame area and the distance between the sections. The numerical density of the neurons is calculated as NvGN=ΣQ−GN/txA. In this application, the nucleoli marked with ‘1, 3-5, 7’ are dissector particles in (A). Section B shows them as they disappeared. The nucleoli marked with ‘2, 6, 8’ are not a dissector particle in (A). Section B shows they are disappeared (H&E, LM, ×20) in a normal rabbit.
Taste bud numbers estimation method is seen at the distal urethra covered by penil urethral tissues. Each urethra considered as a sylender and their volumes were calculated as sylender volume calculation method. Taste bud number (nTB) for each urethral part (a, b, c, …, z) estimated as in figure a. Total TB numbers was estimated as the algebraic formula (LM, GFAP, ×40).
Although many different physiological, histological and neurobiological theorems have been proposed, the discovery of genital taste buds like structure. these genital structures are similar to that of tongue.1 Aydin et al. suggested that taste bud-like structure which was stimulated by fructose may cause male erection.2 Although sexual dysfunction is very common after spinal trauma, there is no convincing rational explanation. Here, we investigated whether there is a relationship between the taste buds like structure-pudendal nerve-Onuf's nucleus complex network or not. Orgasm may be related to integrity of network. Spinal ischemic events, trauma, and hemorrhage such as SAH may disrupt the integrity of network. Sexual function is important for human.
In mammals, taste sensation helps to chose and digestion of nutrients. Various taste sensitive cells or receptors found in digestive systems. They have been found in the tongue, digestive system, respiratory system, brain, testis, and spermatozoa. Li et al. suggested that further research is required for detection if there are taste buds-like in genitalia to provide for male reproduction,14 and some investigators have discovered taste bud like structures in genitalia,14 and the present study provides, as in the study Aydin et al.,1, new finding about about Onuf's nucleus. Pudendal nerve degeneration could cause taste bud-like structure atrophia in genitalia. It is easily hypothesized that this network injury have potential interest on the collapse of hedonia owing to degenerated taste bud like structure secondary to pudendal Onuf's nucleus network dysruption. The sacral parasympathetic nucleus (SPN) is localized in the lateral horn of the L6–S1 spinal cord segments.2,8 This cell group was first described in 1899 by Onufrowicz and became as known as Onuf's nucleus.2,8 Sexual pleasure conveying pudendal nerve fibers arising from Onuf's nucleus and dorsal root ganglia of S1-3 sacral parasympathetic nerves.15 The S2–S4 somatic motor fibers, play a contributory role in penile erection.16 Erectile and ejaculatory dysfunctions occur following spinal cord injury,6 transverse17 myelitis, anterior spinal cord syndrome.18 Vasospasm following SAH is an important complication.19 Ganglionary neuron degeneration following SAH was investigated by various authors.4,20–22 Spinal SAH may also lead to neuro-degeneration in dorsal root ganglia,4,22 Adamkiewicz artery supply terminal spinal cord and lumbosacral plexus.22,23 Adamkiewicz artery may be damaged following SAH.22,23 Adamkiewicz artery vasospasm related Onuf's nucleus degeneration8 can be possible cause of orgasmic disorders. The compression of the dorsal root ganglion3 may induce anorgasmia.2 The proper functioning of neuron cells relies on an abundant and continuous supply of oxygen,24 as occurs in the heart. The neuron density of ganglion cells after SAH is an important issue.7 The balance between cell proliferation and cell death is crucial in all tissues, particularly in the nervous system.8 In the present study, cell death or apoptotic degeneration of the Onuf's nucleus, pudendal ganglia and taste buds-like structure were noted which can be determined by hematoxylin–eosin, gustducin, S-100 and TUNEL staining. These changes may be related with vasospasm of the Adamkiewicz artery. This artery may have enormous clinical importance on the regulation of sexual functions. Adamkiewicz artery vasospasm secondary to spinal SAH may lead to ischemic degeneration in the Onuf's nucleus and disrupted sacral parasympathetic network. We suggested that if sexual functions and hedonic sensations are regulated by Onuf's nucleus/pudendal web/taste buds like structure network, the insults of that network may cause to r sexual dysfunctions and anhedonia.
Pudendal motoneurons are distributed within Onuf's nucleus. The majority of afferent axons of pudendal and pelvic nerves ended in the sacral parasympathetic nucleus. This network is essential for the normal regulation of micturition, defecation, and sexual function.15 This study shows that ischemic injuries of pudendal nerves nerve complex secondary to Adamkiewicz vasospasm following spinal SAH is a possible cause of taste bud like structure degeneration because pudendal nerve convey somatosensory informations of genital organs to the spinal cord and higher brain centers.15 Although some authors advised using spinal cord25 and hypogastric plexus stimulators in the treatment of anorgasmia following spinal injuries26; the present study will add a new dimension on the understanding the underlying mechanism of spinal cord injury induced sexual disfunctions. The investigator will recognize the role of genital taste bud network functions in the pathogenesis of sexual disfunctions.
LimitationThis is an experimental animal study. Only histopathological observations were performed without any functional approach Our experimental model may not mimic human spinal disorder, so why did we concentrate on experimental studies?27 The histopathologic changes can be seen in only experimental animal studies,28 such histopathologic changes between pudendal nerve, Onuf's nucleus and taste bud-like structure. Absolutely, the low sample size of the present study is another limitation. As the authors, we aware that the sample size is one of the important components of a study.29 Low sample size may lead to missing significant differences even if it exists in the population.29 On another hand, a greater number of animals may lead to unnecessary wastage of animals,29 so this study was conducted on the twenty-two adult male rabbits. Given the limited access to the vital human spinal cord cells, the findings of the present are valuable. In this study, the mean degenerated neuron densities of taste buds like structure, Onuf's nucleus, and pudendal ganglia were estimated as 2±1/mm3, 5±1/mm3, 6±2/mm3 in normal; 12±4/mm3, 35±9/mm3, 188±31/mm3 in the SHAM group. As it can be seen in these results, we also noted some changes in the SHAM group (Fig. 8), so saline injection in the SHAM group can be harmful.30,31
ConclusionThis study shows that SAH-induced pudendal nerve and Onuf's nucleus network affection leads to develop taste bud atrophy and likely anorgasmia following spinal SAH. Our finding in the present study, ischemia of pudendal network secondary to Adamkiewicz artery vasospasm may be considered a possible cause of anorgasmia owing to hedonia network disruption induced by spinal SAH. Because the pudendal network conveys somatosensory information from genitalia to the brain.32 If Onuf's nucleus and the pudendal nerves-taste bud-like network-like structure is essential for sexual functions and orgasmic pleasure, we easily announced that the ischemic lesions of mentioned network degeneration may result in both sexual dysfunctions, anorgasmia and even infertility which has not been mentioned in the literature so far.
Future insightsNewly described neuropathological mechanism of Onuf's nucleus-pudendal nerve complex-genital taste bud circuitry degeneration would be accused of an important cause of lossed orgasmic sensation. We provide new information that taste rosea atrophies could be prevented by neurostimulation devices in the future.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo funding was received for this article.
Conflict of interestThe authors declare that they have no conflict of interest to disclose concerning the topic of the present paper.