Our aim is to compare the serum SIRT1 levels between patients with hypogonadotropic hypogonadism and healthy subjects.
Material and methodTwenty-five male patients diagnosed with isolated hypogonadotropic hypogonadism (IHH) and thirty healthy male as a control group were included in the study. Serum SIRT1, hormone levels and biochemical parameters, age, body mass index and insulin resistance were compared in both groups.
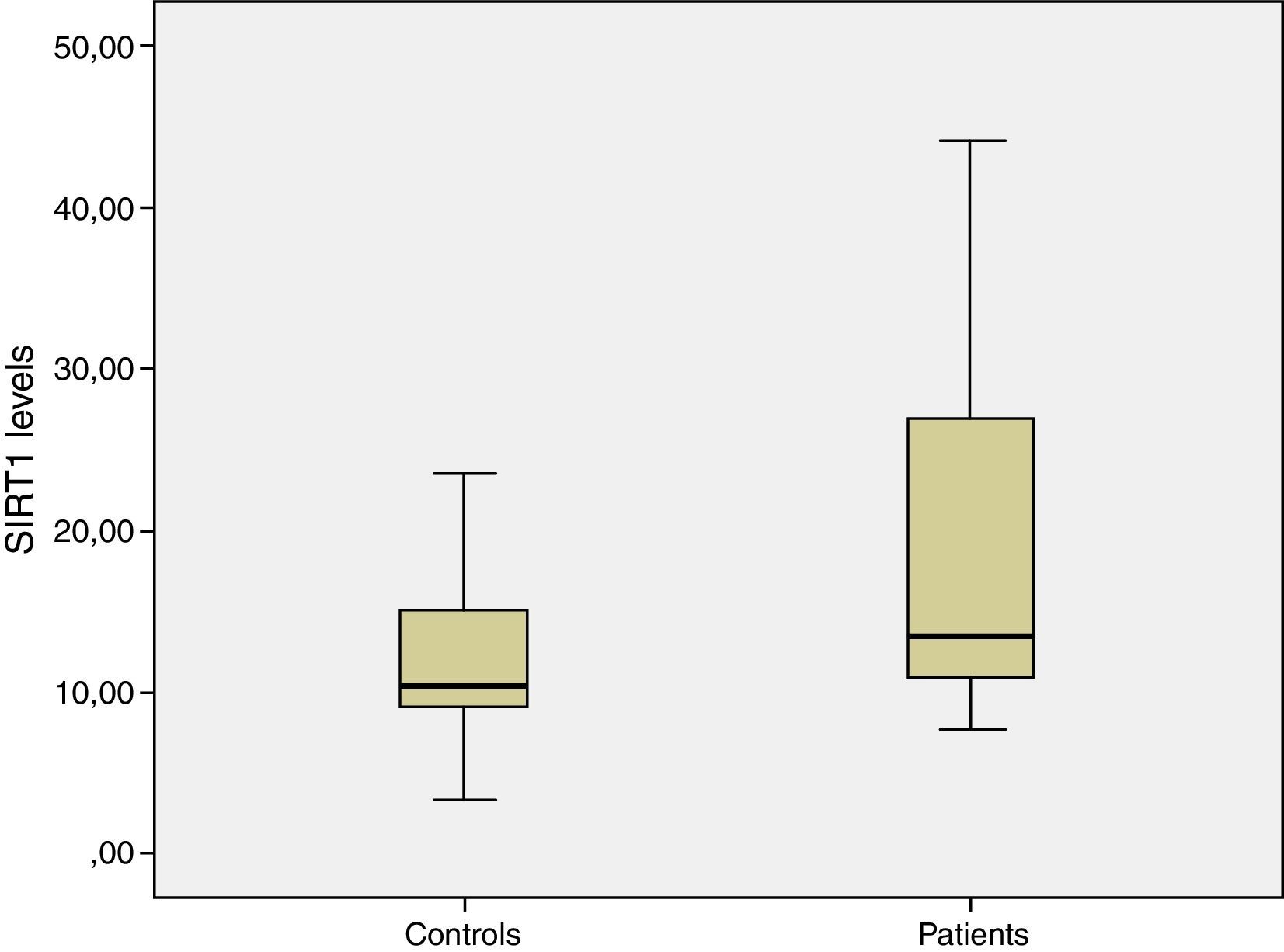
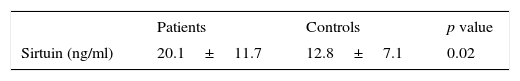
ResultsMean serum SIRT1 levels in patient and control group were 20.1±11.7ng/ml and 12.8±7.1ng/ml, respectively. Difference between both groups was statistically significant (p=0.02). The difference of Homeostasis Model Assessment-Insulin Resistance index (HOMA-IR) between two groups was also statistically significant. Patient group had more insulin resistance than the control group and it was statistically significant (mean HOMA-IR 2.66±1.57 vs. 1.38±0.43; p=0.002).
ConclusionsThis is the first human study that investigates SIRT1 levels and its relation with metabolic and hormonal parameters. We demonstrated that serum SIRT1 levels in patients with IHH were significantly higher than controls, and there is a negative correlation between testosterone and SIRT1 levels. The reason for elevated SIRT1 levels may be a compensation mechanism against both hypogonadotropic hypogonadism and insulin resistance. To understand the relation between SIRT1 and androgen deficiency, further large-scale randomized control studies are needed.
Nuestro objetivo es comparar los niveles séricos de SIRT1 en pacientes con hipogonadismo hipogonadotrópico (IHH) y en sujetos sanos.
Material y métodoVeinticinco pacientes masculinos con IHH y 30 hombres como grupo control fueron incluidos en el estudio. Se determinaron los niveles séricos de SIRT1, los niveles hormonales y los parámetros bioquímicos en ambos grupos y se compararon los niveles séricos de SIRT1, testosterona, gonadrotropinas, la edad, el índice de masa corporal y la resistencia a la insulina.
ResultadosLos niveles promedio de SIRT1 en los pacientes y el grupo control fueron de 20,1±11,7 y 12,8±7,1ng/ml, respectivamente. La diferencia entre los 2 grupos fue estadísticamente significativa (p=0,02). La diferencia del Homeostasis model assessment-insulin resistance index (HOMA-IR) entre los grupos también fue estadísticamente significativa. El grupo de pacientes tuvo mayor resistencia a la insulina que el grupo control y también fue estadísticamente significativa (HOMA-IR promedio 2,66±1,57 vs. 1,38±0,43; p=0,002).
ConclusionesEste es el primer estudio en humanos que mide los niveles séricos de SIRT1 y su relación con parámetros hormonales y metabólicos en pacientes con IHH. Nosotros demostramos que los niveles séricos de SIRT1 en pacientes con IHH fueron significativamente más altos que en el grupo control. También hay una correlación negativa entre los niveles de testosterona y los niveles de SIRT1. Los mecanismos compensatorios del IHH y la resistencia a la insulina pueden ser la principal razón de los niveles elevados de SIRT1. Para entender la relación entre los niveles de SIRT1 y la deficiencia androgénica se necesitan estudios aleatorizados con mayor número de pacientes.
Sirtuins, a family of highly conserved protein modifying enzymes, are Nicotinamide Adenine Dinucleotide (NAD+) dependent histone deacetylases and play important roles at various metabolic pathways.1–3 The first member of sirtuin was isolated from a yeast as examining silencing factors. In studies, using Saccharomyces cerevisiae as a model, calorie restriction extended survival owing to the increase of silent information regulator 2 (SIR2) protein.4–6 Sirtuins are SIR2 orthologous in mammalian.7 Till now, seven sirtuin groups have been identified (SIRT1-7).
SIRT1 is the best known, most studied and found evolutionarily the closest one to yeast Sir2. It does not only correlates with life span but also has important roles in several energy dependent metabolic pathways in almost all tissues. Favorable effects of SIRT1 have been shown on glucose, lipid and energy metabolism, inflammation, oxidative stress, cell repair, oncogenesis and neurodegeneration.8–10 SIRT1 is a positive regulator of insulin secretion.11,12 At the food deprivation state, SIRT1 stimulates insulin secretion and ATP production by down regulating uncoupling protein 2.13 SIRT 1 gene expression is reduced in relation to insulin resistance.14 SIRT1 has also effects on reproductive system. SIRT1 is expressed in the gonads.15 In SIRT1 knockout mice, oogenesis and spermatogenesis are abnormal failed the cell of maturation.16,17 SIRT1 is crucial for postnatal spermatogenesis and testicular development. It has no effects on male gonads during embryonic and fetal development. The gonadal effects of SIRT1 are arisen mostly by hypothalamic-pituitary-gonad (HPG) axis but the underlying molecular mechanism has not been exactly elucidated.
Idiopathic Hypogonadotropic Hypogonadism (IHH) is a result of an unknown deterioration of HPG axis and characterized by the impairment of gonadotropins at anterior hypohysis and/or gonadotropin-releasing hormone (GnRH) secretion from hypothalamus. This impairment causes deficient androgen synthesis and insufficient spermatogenesis. In addition to inadequate sexual maturation, some metabolic problems like obesity, insulin resistance, and osteoporosis develop in patients with IHH.18–20
So far, data about plasma SIRT1 levels and the effects of SIRT1 on the control of the reproductive system have usually been obtained from animal studies. As far as we know, there is not any human study in the literature that examine relation between SIRT1 level and androgen deficiency in male with IHH. The aim of this human study was to evaluate plasma SIRT1 levels and its relation with several metabolic and biochemical parameters in male patients with IHH.
Materials and methodsStudy designThis cross-sectional study was performed at the Department of Endocrinology and Metabolism and Internal Medicine Department of Haydarpasa Training Hospital, Gulhane School of Medicine, Istanbul, between November 2012 and May 2013. Twenty-five patients with IHH and 30 eugonadal healthy males without any hormonal or metabolic disorders (as the control group) were enrolled to the study. The study protocol was approved by the Ethics Committee of Haydarpaşa Training Hospital, Gulhane School of Medicine, in accordance with the declaration of Helsinki and written informed consent forms were obtained from all participants.
PatientsWe evaluate more IHH patients than patients with primary hypogonadism in endocrine clinic practice. A total of forty-four patients with IHH were evaluated during the study. The IHH defined as the total testosterone levels were less than 3ng/ml and gonadotropin levels were close to lower limit or lower than normal range without any additional pituitary pathology such as mass or other pituitary failure. Patients who were under testosterone treatment in last month (7 patients), formerly responded totally or partially to the treatment (4 patients), who refused to continue to the study (4 patients), having body mass index (BMI) ≥ 25kg/m2 (3 patients) and having comorbid pituitary dysfunction (1 patient) have been excluded from the study.
The control group consisted of 30 volunteers who have applied to the Endocrinology and/or Internal Medicine clinics of our hospital for different reasons and found out that they do not have any hormonal or metabolic disease, whose ages were between 20 and 30 years, and BMI<25kg/m2.
Biochemical assaysAfter an overnight fasting, serum levels of glucose, insulin, follicle stimulating hormone (FSH), luteinizing hormone (LH), total testosterone (TT), sex hormone binding globulin (SHBG) concentrations were measured in all subjects by chemiluminometric immunoassay methods (Architect I2000 SR apparatus, Abbott Laboratories). The insulin resistance was computed by Homeostasis Model Assessment-Insulin Resistance index (HOMA-IR): fasting glucose (mg/dL)×immunoreactive insulin (mU/mL)/405.
Human serum SIRT1 elisa kit has been produced by Eastbiopharm brand with catalog number CK-E90527. The content of the kit, standard 64ng/ml serial, has been prepared to 32, 16, 8, 4, 2ng/ml divisions by dilutions. Fifty μl from the standards, 40μl from the samples has been added to the little wells. In addition, 10μl of anti SIRT1 antibody reactive has also been added to this mixture. Fifty μl of streptavidin – HRP has also been added. This mixture has been incubated for 1h at 37°C. After incubation the plate has been washed 5 times with 300μl washing solution in a Pasteur brand LP35 micro plate washer. Fifty μl Chromogen solution A and 50μl Chromogen solution B has been added to all little wells. The reading has been made by 450nm filter CIOM brand Ca-2000 model micro plate reader. The standard graphic has been prepared with Lin-Lin design and sample results have been obtained from this graphic.
Statistical analysisStatistical analyses were performed through SPSS 15.0 (SPSS Inc., Chicago, IL, USA) software package. All data were reported as the mean values±SD. Distributions of variations were assessed using Kolmogorov and Shapiro–Wilk tests. Comparisons between parameters were analyzed using Student's t-test and Mann Whitney U test. The relationship between variables was tested using correlation analysis. Statistical significance was set at p<0.05.
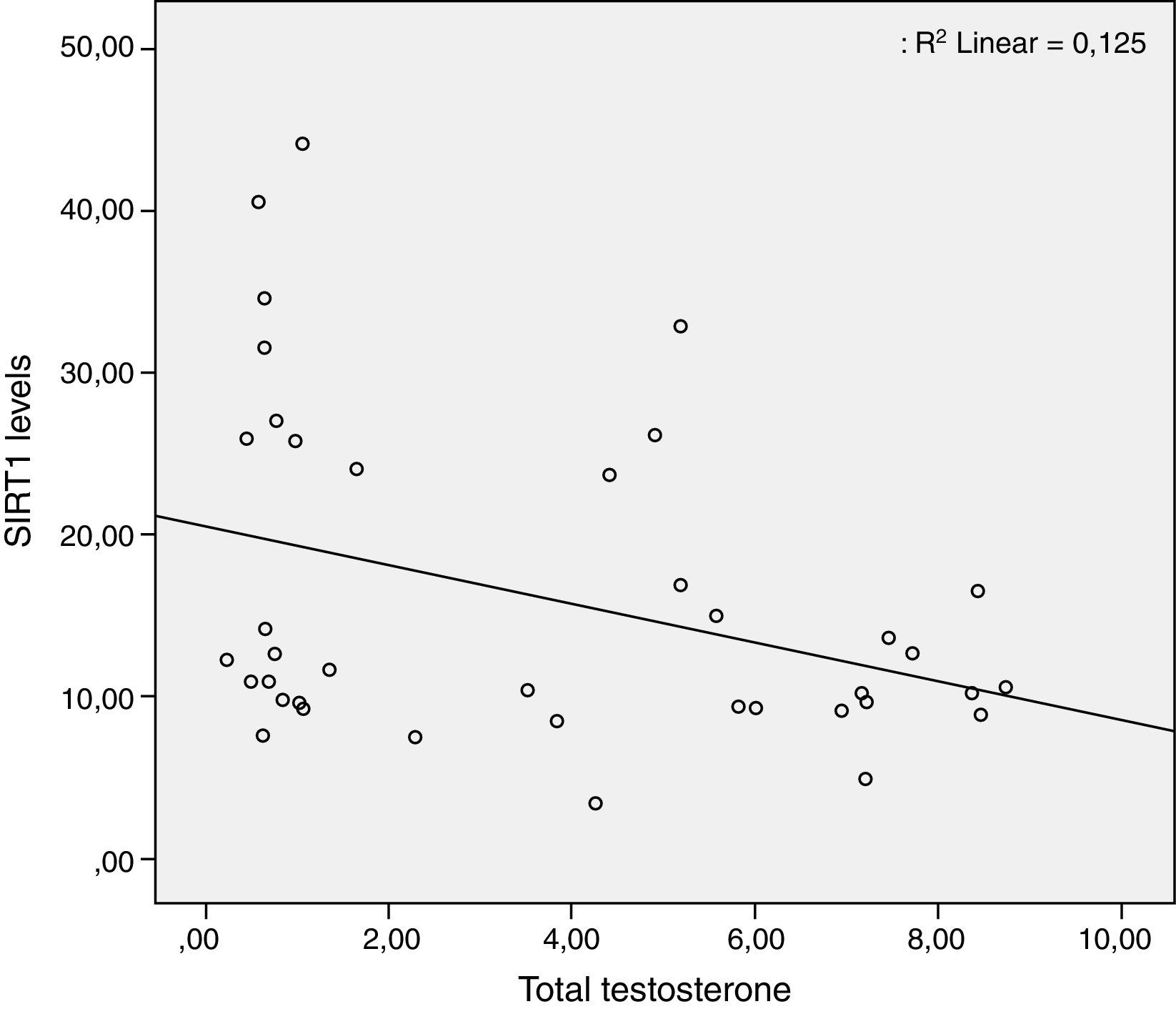
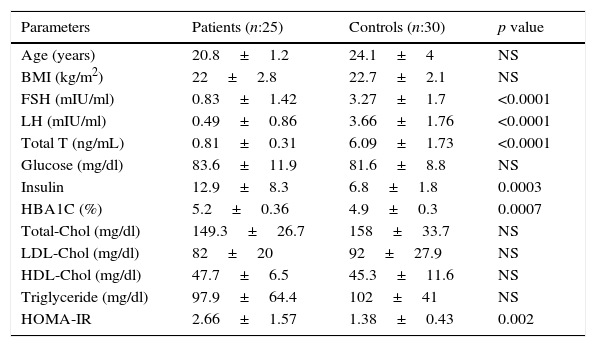
ResultsTwenty-five patients with IHH and thirty eugonadal males without any metabolic disorder as the control group were enrolled into this study. Mean age and BMI of patient group and control group were 20.8±1.2 vs. 24.1±4.0 (p>0.05), and 22±2.8 vs. 22.7±2.1 (p>0.05) respectively, and the differences were not statistically significant (p>0.05) (Table 1). The patient group had lower FSH, LH, total testosterone levels and higher insulin, HbA1C, HOMA-IR levels than control group (Table 1). Median value of serum SIRT1 levels in patient and control groups were 20.1±11.7ng/ml and 12.8±7.1ng/ml, respectively, and were significantly higher in patient group when compared with controls (Table 2, Fig. 1). While there was a negative correlation between SIRT1 levels and testosterone levels (r: −0.35; p: 0.027) (Fig. 2) even after elimination of the confounding factors including HOMA-IR, insulin, HbA1C (r: −0.40 p: 0.037), no correlation was found between SIRT1 levels and age, BMI, HOMA-IR, FSH and LH levels.
Baseline characteristics of both groups.
| Parameters | Patients (n:25) | Controls (n:30) | p value |
|---|---|---|---|
| Age (years) | 20.8±1.2 | 24.1±4 | NS |
| BMI (kg/m2) | 22±2.8 | 22.7±2.1 | NS |
| FSH (mIU/ml) | 0.83±1.42 | 3.27±1.7 | <0.0001 |
| LH (mIU/ml) | 0.49±0.86 | 3.66±1.76 | <0.0001 |
| Total T (ng/mL) | 0.81±0.31 | 6.09±1.73 | <0.0001 |
| Glucose (mg/dl) | 83.6±11.9 | 81.6±8.8 | NS |
| Insulin | 12.9±8.3 | 6.8±1.8 | 0.0003 |
| HBA1C (%) | 5.2±0.36 | 4.9±0.3 | 0.0007 |
| Total-Chol (mg/dl) | 149.3±26.7 | 158±33.7 | NS |
| LDL-Chol (mg/dl) | 82±20 | 92±27.9 | NS |
| HDL-Chol (mg/dl) | 47.7±6.5 | 45.3±11.6 | NS |
| Triglyceride (mg/dl) | 97.9±64.4 | 102±41 | NS |
| HOMA-IR | 2.66±1.57 | 1.38±0.43 | 0.002 |
BMI, body mass index; FSH, follicle stimulating hormone; LH, luteinizing hormone; Chol, cholesterol; T, testosterone; SHBG, sex hormone binding globulin; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; NS, nonsignificant. Data are shown as mean values±SD.
This is the first human study in patients with IHH investigating serum SIRT1 levels and its relation with metabolic and hormonal parameters. In this study, we found that serum SIRT1 levels were significantly higher in patients with IHH than controls and there was negative correlation between testosterone and SIRT1 levels.
SIRT1 is an NAD+ dependent deacetylase and playing a modulator role in miscellaneous metabolic pathways regulating the energy homeostasis of body in response to nutrient availability.3 So far, the researchers have shown that SIRT1 is expressed in various tissues such as pancreas, liver, skeletal muscle, adipose tissue and brain.21 SIRT1 is important for both insulin sensitivity and glucose metabolism through the insulin secretion, stimulation of lipolysis and glucose utilization in muscle tissue; thus, the development of insulin resistance (IR) is prevented by SIRT1 activation.22,23 Among other sirtuins, SIRT1 is the only one that has relation with IR. SIRT2, SIRT3, SIRT4 and SIRT5, levels do not change in human metabolic syndrome, IR or pre-diabetes. SIRT6 and SIRT7 show nonsignificant reduction in metabolic syndrome.14 Studies in patients with IHH have shown an increase in body fat and raised IR.18–20 IR can be ameliorated by overexpression of SIRT1.24 SIRT1 can be a useful target in the treatment of IR.25 In our study, we detected that the patients having high serum SIRT1 levels also had significantly higher insulin and HOMA-IR levels. Nevertheless, glucose levels were found similar in both groups. The insulin resistance was significant in patients with IHH as expected. The higher SIRT1 levels in the patient group may be due to compensation mechanism against insulin resistance because sirtuin ameliorates insulin and glucose mechanism and improves insulin sensitivity in humans. Although we could not find significant correlation between SIRT1 levels and insulin resistance markers, unexpectedly increased SIRT1 levels in IHH patients, in contrast to animal studies,16 imply a compensation mechanism against insulin resistance.
Gonadal function and sexual development are controlled by the HPG axis, secreting hypothalamic GnRH, which directs the pituitary to produce and release LH and FSH.26 IHH results from a deterioration of HPG axis with low testosterone levels and infertility. SIRT1 knockout mice have been shown to be infertile,15 but the physiopathological background of this finding has not been totally explained. Kolthur-Seetharam et al. had shown that SIRT1 is not required for normal fetal testis development and SIRT1 regulates spermatogenesis at postnatal stages by controlling HPG signaling in mice. According to that study, SIRT1 inactivation causes significant reduction in hypothalamic GnRH expression and FSH levels, but also causes almost total loss of LH levels.16 However, data about SIRT1 expression and function in the hypothalamus and how it could regulate GnRH expression are not clear. In a mice study, hypothalamic SIRT1 expression is increased with fasting. Deletion of neuronal SIRT1 increases hypothalamic insulin action and improves systemic insulin sensitivity.27 In our study, we have found significantly higher serum SIRT1 levels in contrast with animal studies. Because of the lack of similar human studies, different suggestions can be made upon this finding. In patients with IHH, with unknown mechanisms, because of deterioration of GnRH impulses FSH and LH levels start to decrease. So Kolthur's SIRT1 null mouse model and IHH patients carrying SIRT1 gene cannot be considered as identical. Already, we have found significantly higher SIRT1 levels in patients with IHH. The elevation in SIRT1 levels can be due to a mechanism to compensate the deterioration in HPG axis like it is in insulin resistance.
ConclusionsIn this study we evaluated the serum SIRT1 levels in patients with IHH. Low number of participants in patient and control groups and lack of explanation of molecular mechanism are the main limitations of this study. Other limitation of this study is lack of primary hypogonadotropic group. Determinations of SIRT1 levels in primary Hypogonadism would probably add information that can help to clarify its relation to metabolic syndrome or to a hypothalamic defect. On the other hand this is the first human study on this topic. Although we have found serum SIRT1 levels significantly high in patients with IHH, SIRT1 cannot be accepted as a specific marker for diagnosis of IHH according to only this study. This finding may be attributed both to compensate IHH and insulin resistance. To investigate the reasons and the effects of high serum SIRT1 levels in patients with IHH, studies with larger patient groups are needed. There is also a need for more detailed human studies on measuring SIRT1 levels after testosterone therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.