Abnormality in Histone-Protamine replacements has been indicated to cause sperm DNA damage and infertility. The aim of the present study was to investigate the relationships between sperm parameters in oligospermia, asthenospermia, and teratospermia with protamine deficiency in infertile men.
Material and methodIn this case–control study, we had three experimental groups including oligospermia (n=100), asthenospermia (n=100), and teratospermia (n=100) as well as normospermia (n=100) as controls. Sperm analyses were performed according to the recommendations of the World Health Organization (WHO, 2010) and sperm chromatin quality was assessed using Chromomycin A3 (CMA3) staining for each sample.
ResultsThe comparison of the data between groups indicated that the percentage of spermatozoa with protamine deficiency was significantly different in patients with oligospermia, asthenospermia, and teratospermia when compared with control ones. However, there was no significant correlation between sperm nuclear protamine deficiency and their parameters of the men with teratospermia using CMA3 test. Regarding the oligospermia and asthenospermia semen samples, the findings showed the negative correlations between the sperm nuclear protamine deficiency and progressive motility as well as immobility (p<0.001).
ConclusionThe higher proportion of spermatozoa with abnormal chromatin packaging was observed in asthenospermic samples than those from other experimental groups as well as controls. It seems that normal morphology cannot have a valuable predictive value for good chromatin quality of spermatozoa, as much as normal motility characteristics, since samples with high mobility rates often have lower protamine deficiencies. The findings may provide a supportable promoting the future wider clinical application of chromatin/DNA integrity testing along with the semen analysis in male infertility.
Se ha indicado que la irregularidad en los reemplazos de histona-protamina provoca daño en el ADN del esperma e infertilidad. El objetivo del presente estudio fue investigar las relaciones entre los parámetros espermáticos en oligospermia, astenospermia y teratospermia con deficiencia de protamina en varones infértiles.
Material y métodoEn este estudio de casos y controles, hubo 3 grupos experimentales que incluían oligospermia (n=100), astenospermia (n=100) y teratospermia (n=100), así como normospermia (n=100) como controles. Los análisis de esperma se realizaron de acuerdo con las recomendaciones de la Organización Mundial de la Salud (OMS, 2010), y se evaluó la calidad de la cromatina de los espermatozoides utilizando la tinción con Chromomycin A3 (CMA3) para cada muestra.
ResultadosLa comparación de los datos entre los grupos indicó que el porcentaje de espermatozoides con deficiencia de protamina fue considerablemente diferente en pacientes con oligospermia, astenospermia y teratospermia en comparación con la de los controles. Sin embargo, no hubo una correlación importante entre la deficiencia de protamina nuclear de esperma y sus parámetros de los varones con teratospermia cuando se utilizaba la prueba de CMA3. En cuanto a las muestras de semen de oligospermia y astenospermia, los hallazgos mostraron las correlaciones negativas entre la deficiencia de protamina nuclear de esperma y la movilidad progresiva, así como la inmovilidad (p<0,001).
ConclusiónLa mayor proporción de espermatozoides con un empaquetado de cromatina anómalo se observó en las muestras astenospérmicas que en las de otros grupos experimentales, así como en los controles. Parece que la morfología normal no puede tener un valor diagnóstico valioso de la buena calidad de la cromatina de los espermatozoides, tanto como las características normales de movilidad, ya que las muestras con altas tasas de movilidad a menudo tienen menores deficiencias de protamina. Los hallazgos pueden ofrecer un soporte que promueva la futura aplicación clínica más amplia de las pruebas de integridad de la cromatina/ADN junto con el análisis del semen en la infertilidad masculina.
Human sperm abnormalities including oligospermia, asthenospermia, and teratospermia characterized in semen analysis1 are considered as a reliable index for estimating the spermatogenesis quality and infertility.2 Also, it is known that human spermatozoa with abnormal morphology show higher percentages of DNA strand breaks and abnormal chromatin structures.3 In fact, there is a significant relationship between sperm morphological abnormalities, especially head abnormalities e.g. amorphous heads, DNA fragmentation and fertilization rate in intracytoplasmic sperm injection (ICSI)4,5 as well as admissible chromatin packaging by both protamine 1 and protamine 2 in order to affect the normal function of spermatozoa during spermatogenesis.6
A recent review reported that protamine deficiency, as well as protamine ratio measurements, can be applied as a validated biomarker for sperm quality control due to its key role in male infertility.7
Sperms with abnormal chromatin can be induced by apoptosis and finally presented in the ejaculation fluids.8 Moreover, the chromatin with abnormal density can be more sensitive to external attacks such as oxidative stress, resulting in DNA fragmentation.8 Abnormal chromatin structure or DNA strand breaks can cause pregnancy and delivery loss in both natural and assisted conceptions.9
Therefore, the evaluation of chromatin structure status and sperm DNA in male factor patients has been one of the primary works. There are not insufficient data with the aim of a comparison of the sperm protamine deficiency between different categories of human abnormal spermatozoa with normozoospermic control samples. Therefore, we conducted a prospective study for investigating the relationships between human semen parameters and protamine deficiency of infertile patients with oligospermia, asthenospermia and teratospermia in comparison to control samples of normospermic men.
MethodsParticipants, sample collection, and sperm analysisThe semen samples were obtained from 400 men, having been referred to our institute. Semen collection was conducted by masturbation after 3–4 days of sexual abstinence. After liquefaction, routine semen analysis, such as sperm motility, concentration, and morphology, was carried out using light microscopy according to the recommendations of the World Health Organization (WHO, 2010)10 and then, each sample was categorized into 4 experimental groups such as normospermia (n=100), oligospermia (n=100), asthenospermia (n=100), and teratospermia (n=100). For evaluating morphological abnormalities, at least 200 sperm were examined per slide (10). Sperm concentration was assessed by a Makler counting chamber. For evaluating the sperm viability, eosin–nigrosin staining technique was carried out. The amount of 10μl of prepared sperm was mixed with 10μl of eosin–nigrosin stain on a glass slide and assayed using a light microscope to determine the percentage of live sperm. All analyses were performed by an experienced laboratory technician blinded to the study. This prospective study was approved by the ethical and scientific committee of …. Reproductive Sciences Institute….
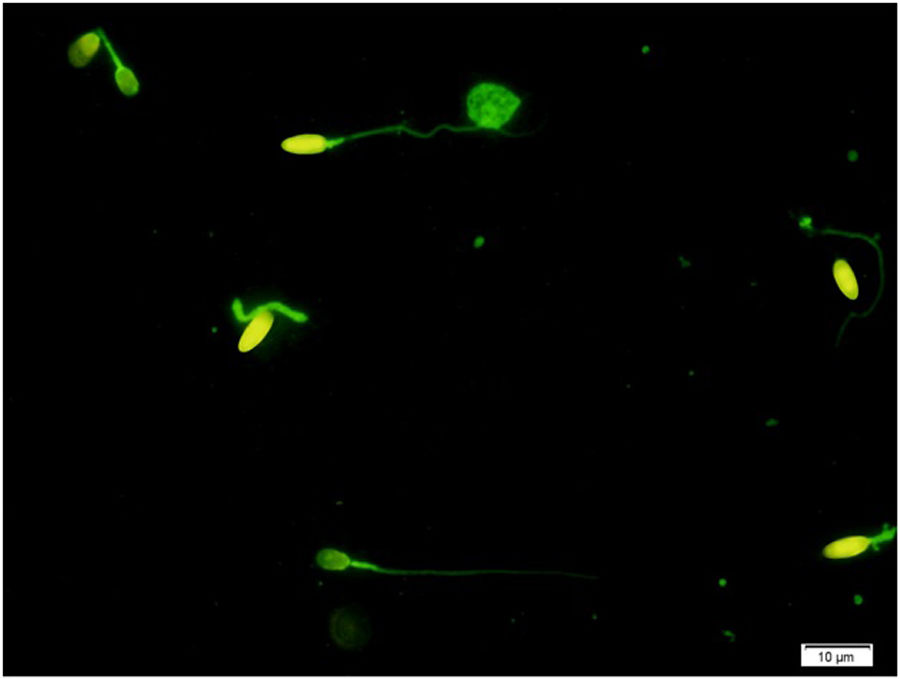
Assessment of protamine deficiencyChromomycin A3 (CMA3) was applied as one of the verified sperm DNA integrity assessments to assess sperm protamine deficiency. Briefly, all semen samples were washed in phosphate-buffered saline (PBS) free of Ca2+ and Mg2+ and fixed in Carnoy's solution (methanol/glacial acetic acid 3:1 (Merck, Darmstadt, Germany)) at 4°C for 5min. Following the preparation of smears, each slide was treated with 100μl of CMA3 solution (0.25mg/ml in McIlvaine buffer (7ml citric acid 0.1M+32.9ml Na2HPO47H2O 0.2M, pH 7.0, containing 10mmol/l MgCl2)) (Sigma, St Louis, MO, USA) for 20min. The slides were then rinsed in PBS buffer and mounted with buffered glycerol (1:1). Microscopic analysis was performed by fluorescent Nikon Eclipse 600 microscope (Tokyo, Japan), with the appropriate filters (460–470nm). In each slide, 200 spermatozoa were assessed and scored as positive (CMA3+ with bright yellow) or negative (CMA3− with dull yellow) for CMA3, according to their brightness (Fig. 1).11
Statistical analysisThe results were analyzed using the SPSS software version 20 for Windows (SPSS Inc., Chicago, IL, USA). ANOVA and Student's t-test were applied to compare the groups, and the term ‘statistically significant’ was used to denote a two-sided p value<0.05 for sperm parameters and the protamine deficiency test. The correlation between CMA3+ and each experimental group was performed using Pearson coefficient of correlation.
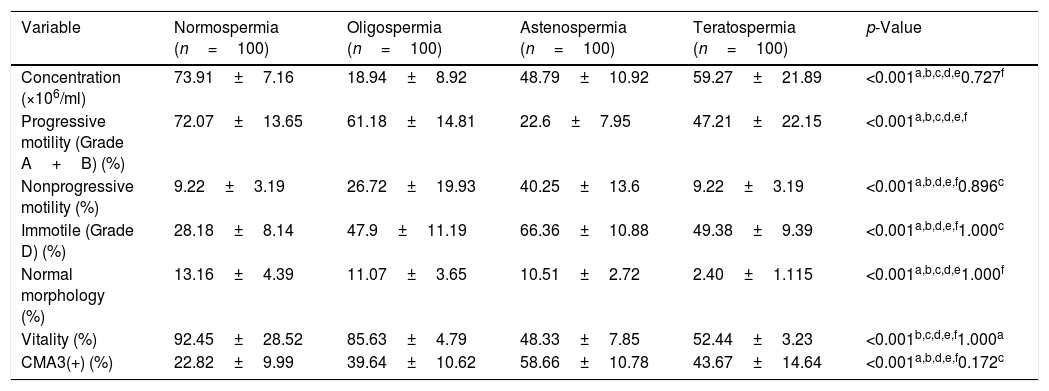
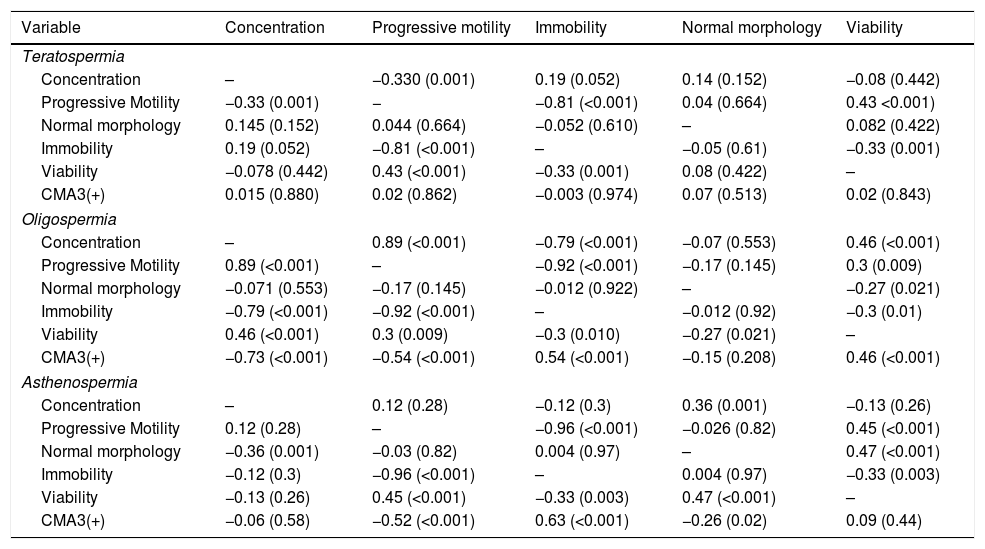
ResultsThe mean age of participants in different groups was 35.40±2.2 for normospermia (normal sperm parameters), 35.32±4.1 for moderate oligospermia, (less than 10 million spermatozoa/mL), 34.63±2.8 for asthenospermia (fewer than 32% of the sperm cells with progressive motility) and 33.78±4.53 for teratospermia (sperm morphology fewer than 5%) based on WHO 2010 criteria. The results of sperm parameters from experimental groups are summarized in Table 1. As expected, sperm concentration and normal morphology were significantly different between all experimental groups as well as controls (p<0.05) except those between oligospermia and asthenospermia semen samples. The comparison of the data between groups indicated that the percentage of spermatozoa with protamine deficiency was significantly different in patients with oligospermia, asthenospermia, and teratospermia when compared with control ones. However, There was no significant difference between sperm nuclear protamine deficiency of the men with teratospermia and oligospermia using CMA3 test (p-value=0.172). Regarding the teratospermia sperm samples, CMA3 (+) spermatozoa were not correlated with sperm parameters. In contrast, significant correlations were found between sperm protamine deficiency and sperm immobility as well as progressive motility in asthenospermic samples. However, we observed significant correlations between all sperm parameters (especially concentration and motility status) and protamine deficiency, except the normal morphology in men with oligospermia (Table 2).
Comparisons of human sperm characteristics and protamine deficiency between the experimental groups.
| Variable | Normospermia (n=100) | Oligospermia (n=100) | Astenospermia (n=100) | Teratospermia (n=100) | p-Value |
|---|---|---|---|---|---|
| Concentration (×106/ml) | 73.91±7.16 | 18.94±8.92 | 48.79±10.92 | 59.27±21.89 | <0.001a,b,c,d,e0.727f |
| Progressive motility (Grade A+B) (%) | 72.07±13.65 | 61.18±14.81 | 22.6±7.95 | 47.21±22.15 | <0.001a,b,c,d,e,f |
| Nonprogressive motility (%) | 9.22±3.19 | 26.72±19.93 | 40.25±13.6 | 9.22±3.19 | <0.001a,b,d,e,f0.896c |
| Immotile (Grade D) (%) | 28.18±8.14 | 47.9±11.19 | 66.36±10.88 | 49.38±9.39 | <0.001a,b,d,e,f1.000c |
| Normal morphology (%) | 13.16±4.39 | 11.07±3.65 | 10.51±2.72 | 2.40±1.115 | <0.001a,b,c,d,e1.000f |
| Vitality (%) | 92.45±28.52 | 85.63±4.79 | 48.33±7.85 | 52.44±3.23 | <0.001b,c,d,e,f1.000a |
| CMA3(+) (%) | 22.82±9.99 | 39.64±10.62 | 58.66±10.78 | 43.67±14.64 | <0.001a,b,d,e,f0.172c |
Evaluating the sperm parameters was according to World Health Organization (WHO, 2010).
Data are presented as Mean±SD; p-value<0.05 represented the significant data. CMA3, chromomycin A3.
Correlations between semen parameters and protamine deficiency.
| Variable | Concentration | Progressive motility | Immobility | Normal morphology | Viability |
|---|---|---|---|---|---|
| Teratospermia | |||||
| Concentration | – | −0.330 (0.001) | 0.19 (0.052) | 0.14 (0.152) | −0.08 (0.442) |
| Progressive Motility | −0.33 (0.001) | − | −0.81 (<0.001) | 0.04 (0.664) | 0.43 <0.001) |
| Normal morphology | 0.145 (0.152) | 0.044 (0.664) | −0.052 (0.610) | – | 0.082 (0.422) |
| Immobility | 0.19 (0.052) | −0.81 (<0.001) | – | −0.05 (0.61) | −0.33 (0.001) |
| Viability | −0.078 (0.442) | 0.43 (<0.001) | −0.33 (0.001) | 0.08 (0.422) | – |
| CMA3(+) | 0.015 (0.880) | 0.02 (0.862) | −0.003 (0.974) | 0.07 (0.513) | 0.02 (0.843) |
| Oligospermia | |||||
| Concentration | – | 0.89 (<0.001) | −0.79 (<0.001) | −0.07 (0.553) | 0.46 (<0.001) |
| Progressive Motility | 0.89 (<0.001) | – | −0.92 (<0.001) | −0.17 (0.145) | 0.3 (0.009) |
| Normal morphology | −0.071 (0.553) | −0.17 (0.145) | −0.012 (0.922) | – | −0.27 (0.021) |
| Immobility | −0.79 (<0.001) | −0.92 (<0.001) | – | −0.012 (0.92) | −0.3 (0.01) |
| Viability | 0.46 (<0.001) | 0.3 (0.009) | −0.3 (0.010) | −0.27 (0.021) | – |
| CMA3(+) | −0.73 (<0.001) | −0.54 (<0.001) | 0.54 (<0.001) | −0.15 (0.208) | 0.46 (<0.001) |
| Asthenospermia | |||||
| Concentration | – | 0.12 (0.28) | −0.12 (0.3) | 0.36 (0.001) | −0.13 (0.26) |
| Progressive Motility | 0.12 (0.28) | – | −0.96 (<0.001) | −0.026 (0.82) | 0.45 (<0.001) |
| Normal morphology | −0.36 (0.001) | −0.03 (0.82) | 0.004 (0.97) | – | 0.47 (<0.001) |
| Immobility | −0.12 (0.3) | −0.96 (<0.001) | – | 0.004 (0.97) | −0.33 (0.003) |
| Viability | −0.13 (0.26) | 0.45 (<0.001) | −0.33 (0.003) | 0.47 (<0.001) | – |
| CMA3(+) | −0.06 (0.58) | −0.52 (<0.001) | 0.63 (<0.001) | −0.26 (0.02) | 0.09 (0.44) |
The Spearman correlation test was used. In each cell, the top value is r and the bottom is the p-value. The p-values<0.05 were considered to indicate statistical significance. CMA3: chromomycin A3.
Our experiences on previous studies have shown that infertile men with different sperm abnormalities such as globozoospermia, teratospermia as well as oligoasthenoteratozoospermia (OAT) have high percentage of spermatozoa with the abnormal chromatin/DNA integrity, but, there was still a lack of prospective comparative study on a variety of sperm abnormalities in subfertile than those with normospermia.12,13 Regarding this absence, it is worth mentioning this point that a considerable number of researches are in the form of case report studies, evaluating a limited number of cases.3,13 However, the present study evaluated the sperm parameters and chromatin condensation in large groups of patients by using standard assays to demonstrate sperm chromatin quality in three types of human sperm abnormalities.
Sperm parametersAlmost all of our findings in sperm analyses corresponded to WHO 2010 criteria as expected in the current study. For instance, the sperm progressive motility and the sperm count showed a significant decrease in asthenospermia and oligospermia than others respectively (Table 1). In accordance with our results, Rahiminia et al. (2018) assessed the semen from 32 OAT and 32 normal fertile men. They demonstrated significantly lower sperm concentration, progressive motility, and normal morphology in OAT patients than in the normozoospermic controls.14 One study by Dehghanpour et al. reported the similar observation of significantly lower percentages of sperm concentration, motility, and normal morphology in the tapered-head spermatozoa when they were compared to normozoospermic samples.15 Moreover, the data showed significant correlations between viability of abnormal spermatozoa and their motility status, as an indicator of spermatozoa vitality in the groups with defined sperm abnormalities. In contrast, we did not observe considerable correlations between normal morphology and other sperm characteristics as well. The study aimed to compare sperm characteristics among patients with male factor infertility (MFI) etiology (n=166), patients undergoing infertility evaluation (n=406), and fertile men (Proven fertile men or normal donors (n=147)), and showed similarities between normal morphology rates (%) in most of the patients and almost half of the fertile men with abnormal morphology.16 Therefore, they concluded that sperm motility and concentration can prepare more valued data than morphology during an infertility evaluation.
Chromatin quality assessmentRegardless of the asthenospermia group having a large proportion of sperm cells with excessive histones and protamine deficiency, we predicted a significantly elevated sperm DNA fragmentation and the data showed no significant correlations between CMA3 (+) spermatozoa of abnormal spermatozoa and its normal morphology in experimental groups with defined sperm abnormalities.
Having a methodology like that of ours, we found one study (2014) that evaluated sperm DNA damage in a large cohort of infertile men with isolated sperm defects grouped as oligozoospermia, asthenozoospermia, and teratozoospermia.16 However, they focused on evaluating their % sperm DNA fragmentation (%SDF) by using flow cytometry-based Terminal deoxynucleotidyl transferase-mediated dUTP Nick End-Labeling assay.17 They stated that the proportion of men with high %SDF was remarkably superior in the asthenospheric samples compared to the oligospermia and teratospermia groups (31% vs. 18% and 19%, respectively, p<0.0001).17
There was a significant decrease in the proportion of spermatozoa with normal chromatin condensation in men with OAT as well as teratospermic patients, as has been previously reported.14,15,18 Moreover, it should be considered that the abnormal replacement of histones by protamine can increase sperm DNA fragmentation, and there is a clear relation between sperm chromatin quality and DNA damage.19
However, the nature of the relationship between sperm DNA damage and sperm motility has not been fully characterized; our data, in line with those of Belloc et al. (2014) indicated that poor motility is the sperm parameter abnormality most closely related to both sperm DNA and chromatin deficiency.17 A possible explanation for this relationship is that the formation of a mature, compacted sperm nucleus and the development of the sperm flagellum, both originate during spermiogenesis.20,21 Furthermore, experimental research studies reported that disruption of nuclear chromatin compaction is associated with the development of an abnormal flagellum and defective motility.7,21 By working on both total and partial globozoospermic men two recent studies with similar results stated that the sperm chromatin/DNA anomalies may be considered as the main etiology of ART failure in globozoospermic patients as well.22,23
Also, we observed significant negative correlations between sperm concentration and progressive motility and spermatozoa with positive CMA3, in men with oligospermia, representing high proportion of abnormal chromatin packaging in ejaculated sperm of oligospermic men. Staining by CMA3 has shown to be one of the SDF testing methods with vigorous correlation with other SDF assays. It demonstrates a DNA protamination status associated with quality of sperm chromatin packaging.24 Regarding promising outcomes in the use of testicular sperm for ICSI as a treatment plan for high SDF, it has been suggested that the use of testicular sperm rather than ejaculated sperm may be beneficial in men with oligozoospermia due to the three- to fivefold lower SDF detected in testicular sperm rather than ejaculated spermatozoa.25,26
In spite of as evaluating the sperm parameters and chromatin condensation in a large group of patients with different types of sperm abnormalities, we could not perform several standard assays to demonstrate different disorders of sperm chromatin and DNA integrity among these groups. This is the main weakness of the current study.
ConclusionThe higher proportion of spermatozoa with abnormal chromatin packaging was observed in asthenospermic samples than those from other experimental groups as well as controls. It seems that normal morphology cannot have a valuable predictive value for good chromatin quality of spermatozoa, as much as normal motility characteristics, since samples with high mobility rates often have lower protamine deficiencies. Despite the inexpressive evidence for routine chromatin quality assessments for the evaluation of infertile men, present study in companion with recent promising evidence may provide a supportable promoting the wider clinical application of chromatin/DNA integrity testing along with the semen analysis in male infertility.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Ethical statementThis study was approved by the ethical and scientific committee of Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Financial discloseThis study was supported by a grant of Yazd Reproductive Sciences Institute and Research and Deputy of Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of interestThere is no conflict of interest in this article.
The authors would like to thank Prof. Mohammad Hossein Fallahzadeh for her skillful statistical assistance during this study. All authors contributed to the manuscript including study design, drafting of the manuscript and contribution in sample collection, sperm analysis, banking, slide preparations and CMA3 staining.