Visually induced self-motion (vection) affects the speed at which actions are performed.However, it has been unclear whether this speedy action induced by vection is based on the modulation of mental tempo. To clarify this issue, we tested whether the speed of vection influenced an observer's cyclic action related to mental tempo. Observers viewed fast and slow moving optic flow stimuli and dynamic random dots, whilst handclapping at their preferred tempo. The results revealed that the clapping rate was the fastest in the fastest optic flow condition. This effect vanished when optic flow stimuli moved fast but did not induce vection. Fast optic flow stimuli also induced larger pupil dilation, suggesting that it increased the observer's arousal level. These results suggest that illusory self-motion increased arousal levels, thereby modulating mental tempo.
El automovimiento inducido visualmente (vección) afecta a la velocidad con la cual se llevan a cabo las acciones. Sin embargo, no se ha establecido con claridad si esta rápida acción inducida por la vección se basa en la modulación del tempo mental. Con el fin de clarificar este tema, llevamos a cabo pruebas que apuntaban a determinar si la velocidad de la vección afecta a las acciones cíclicas de un observador, en relación con el tempo mental. Los observadores se enfrentaron a estímulos de flujo óptico de movimiento rápido y lento, así como puntos aleatorios dinámicos, a medida que aplaudían al ritmo que prefirieran. Los resultados revelan que la frecuencia de los aplausos era la más rápida bajo la condición de flujo óptico más rápido. Dicho efecto desapareció cuando los estímulos de flujo óptico se movían rápidamente, pero no indujo vección. Los estímulos de flujo óptico rápido también indujeron dilatación de la pupila; esto sugiere que se aumentó el nivel de alerta, incrementando a su vez el tempo mental.
When we view a large visual motion field that simulates the retinal flow in a real situation, we often perceive self-motion, even though we do not actually move (vection: Fischer & Kornmuller, 1930). In everyday life, passengers in a waiting train often experience the movement of their train when they observe another train pulling out from the station. Self-motion in a real environment entails motion information in visual, auditory, and haptic modalities (Gibson, 1966). Moreover, during a uniform motion of the self the vestibular system cannot receive gravitational information; in such a situation, self-motion perception relies on cues from the other modalities. Thus, perception of self-motion appears to be shaped by incorporating the signals from such modalities (Riecke, Väljamäe, & Schulte-Pelkum, 2009; Seno, Hasuo, Ito, & Nakajima, 2012; Seno, Ogawa, Ito, & Sunaga, 2011; Väljamäe, Larsson, Västfjäll, & Kleiner, 2008). Although in the case of visual vection the self-motion perception is induced mainly by visual stimulation, the perceptual system of self-motion uses sensory motion signals from modalities other than vision; hence, visual vection is susceptible to such signals (Seno, Ito, & Sunaga, 2011). For example, a previous study suggested that airflow against an observer's face increased visual vection strength in the direction of self-motion opposite to the airflow (Seno, Ogawa et al., 2011). On the other hand, vection also affects other forms of sensory (e.g., vestibular) inputs, stimulus meaning, gravitoinertial force, and perceived room temperature,etc.(e.g., Durgin, Gigone, & Scott, 2005; Fukuda & Seno, 2011, 2012; Seno, Ito, Sunaga, & Palmisano, 2013; Seno & Van Doorn, 2013). For example, several previous studies showed that visual vection modulates human postural controls (Bringoux, Lepecq, & Danion, 2012; Guerraz & Bronstein, 2008; Thurrell & Bronstein, 2002; Wei, Stevenson, & Kording, 2010). Thus, visual vection and other sensory processes interact with each other.
Vection can affect the speed at which actions are performed. A previous study reported that fast biological motion reduced reaction times to a visual change in a fixation cross (Watanabe, 2008). The study demonstrated that the speed of observed stimuli could increase the speed of an observer's action. Considering the functional connection between action and perception (James, 1890; Jeannerod, 1994; Prinz, 1997), it is possible that visually induced self-motion – vection – can also influence the speed of self-action. Indeed, we reported elsewhere that fast vection increased an observer's speech speed (Seno, Ihaya, & Yamada, 2013).
A possible explanation for the effect found in Seno et al. (2013) is the speed up of mental tempo by perceiving fast self-motion. Mental tempo is the preferred rate of events in each person. In earlier research on mental tempo (Stern, 1900), researchers used the pace of spontaneous motor activities like tapping, clapping, and walking as measures of internal timing (Boltz, 1994; Vanneste, Pouthas, & Wearden, 2001). If mental tempo underlies the speech speed (i.e., utterance rate), vection would also affect mental tempo. However, direct evidence for this has not been provided as yet. Hence, this study examined whether mental tempo was influenced by the speed of vection. In Experiment 1 we instructed observers to clap their hands ten times at a tempo that the observers would see fit (not too slow, not too fast), and recorded their handclaps as a measure of mental tempo (Mishima, 1951; McAuley, Jones, Holub, Johnson, & Miller, 2006). If the speed of vection modulates mental tempo, then handclaps would become the fastest in the condition where the fastest vection occurred.
Next, it was unclear what mechanism underlay the change in mental tempo. In previous studies of time perception, the notion of an “internal clock” has been proposed as a kind of pacemaker that governs some temporal aspects of human perception and action (Gibbon, Church, & Meck, 1984; Meck, 2005), although this view is also controversial (e.g., Staddon & Higa, 1999). Moreover, a number of previous studies have reported the effects of arousal on the internal clock (Droit-Volet & Meck, 2007), with increased arousal level speeding up the internal clock. For example, the effects of presenting arousing pictures (Angrilli, Cherubini, Pavese, & Manfredini, 1997), taboo words (Tipples, 2010), and angry faces (Droit-Volet, Brunot, & Niedenthal, 2004), and the effects of body temperature (Wearden & Penton-Voak,1995), visual flickers (Droit-Volet & Wearden, 2002; Ortega & Lopez, 2008) and drug administration (Maricq, Roberts, & Church, 1981) on time perception, have been explained in this framework. Hence, it is possible that mental tempo became faster based on an increased arousal level owing to fast vection. For this reason, in Experiment 2 we recorded the changes in an observer's pupil diameter when the observers freely viewed the stimuli used in Experiment 1. Pupil dilation has been used as a reliable measure of the increase in arousal level (Kampe, Frith, & Frith, 2003). If arousal level increases when observers viewed the stimulus that induced fast vection, pupil diameter would increase the most with fast vection.
Experiment 1Ethics statementExperiments were pre-approved by the Ethics Committee of Kyushu University and an informed consent was obtained from each participant.
MethodsObservers: Seventeen adult volunteers participated in Experiment 1. Observers were either graduate or undergraduate students, with no reported visual or vestibular abnormalities. All observers were naive as to the purpose of the present study.
Apparatus and Stimuli: Stimulus images were generated and controlled by a computer (Apple, MB543J/A). Stimuli were presented on a plasma display (3D VIERA, 50 inches; Panasonic) with a 1,024×768 pixel resolution at a 60-Hz refresh rate. The experiments were conducted in a dark room. Viewing distance was 57cm. A stopwatch (TD-392-BK, TANITA) was used to measure time intervals of observers’ hand clapping.
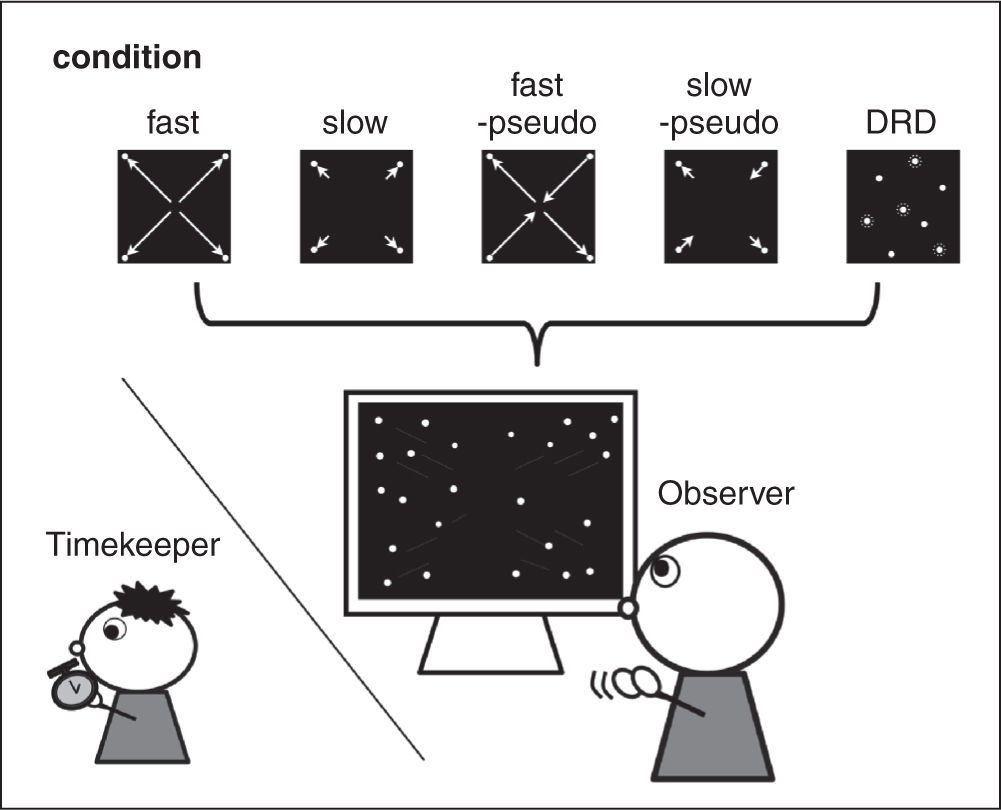
As in Seno et al. (2013), we presented five types of visual stimuli, fast and slow optic flow stimuli, fast and slow pseudo-optic flow stimuli, and dynamic random dots (DRD). The fast and slow optic flow stimuli corresponded to fast and slow vection, respectively. We used optic flow stimuli involving expansion and contraction. Stimuli were created by positioning 16,000 dots at random inside a simulated cube and moving the observer's viewpoint to simulate forward self-motion of 32 m/s or 1m/s (fast and slow optic flow conditions, respectively).Dynamic random dots, corresponding to absence of vection, were presented at 0.1Hz (1,240 dots/frame).
Before the experimental session of Experiment 1, we confirmed the existence of vection in eighteen participants using a 60-second stimulus presentation (conducting one trial for each condition). Eighteen observers were asked to press a key as soon as they perceived self-motion and to hold down the key during the self-motion perception. The interval from the stimulus initiation to the first key press was measured as latency and the cumulative period of the key press was measured as duration. The average latencies were 8.8 and 11.6 seconds for the fast and slow optic flow conditions, respectively. Durations were 42.8 and 38.5 seconds for the fast and slow optic flow conditions, respectively. There was a significant difference between fast and slow optic flow conditions in terms of latency (t(17) = 2.45, p< .03). There was also a marginally significant difference between them in terms of duration (t(17) = 1.98, p= .06). These results indicate that vection was significantly stronger in the fast optic flow condition than in the slow one.
Pseudo-optic flow stimuli consisted of optic flows depicting contraction and expansion, with expansion presented in the right upper and left bottom visual fields and contraction presented in the right bottom and left upper visual field. Vection was largely inhibited in this condition, even though observers perceived the same speed of visual motion as in the normal optic flow condition. This is because vection was not determined in a unique direction in pseudo optic flow conditions (Seno et al., 2012). Before the experimental session of Experiment 1, and simultaneous to the confirmation of vection in the fast and slow optic flow conditions, we also confirmed the absence of vection in the fast and slow pseudo-optic flow conditions in fifteen participants using a 60-second stimulus presentation. Average latencies were 28.5 and 32.3 seconds for the fast and slow pseudo-optic flow conditions, respectively. Durations were 7.2 and 5.4 seconds for the fast and slow pseudo-optic flow conditions, respectively. The results revealed significant differences between the fast optic flow and fast pseudo-optic flow conditions (t(27) = 6.93, p< .01) and the slow optic flow and slow pseudo-optic flow conditions (t(27) = 7.44, p< .01). There were no significant differences between the fast and slow pseudo-optic flow conditions (latency: t(14) = 1.04, p> .05; duration: t(14) = 1.22, p> .05). Thus, we successfully confirmed that vection was highly inhibited in those conditions.
Procedure: Observers viewed each of the stimuli throughout the trial. Ten seconds after the stimulus presentation began, observers started to clap their hands according to the verbal instructions from an experimenter (i.e., ‘start!’). Observers were instructed to clap their hands at their preferred tempo (Figure 1). A timekeeper counted the number of claps and asked observers to stop the hand clapping after the number of claps exceeded 10. The clapping sounds were timed using a stopwatch (we did not record the clapping sounds, so time intervals between claps were unclear). It was unlikely that the timekeeper's reaction time or other variables based on the manual measurement affected the tendency of these results, as the timekeeper was naive to the purpose of the present study and he/she did not observe the visual stimuli. Consequently, even though there were no variations in observers’ action itself, said variations should not be biased. The clapping rate was calculated based on the time elapsed from the first clap until the last clap. The order of presentation of visual stimuli was also randomized. One condition was repeated once, therefore there were five trials in total.
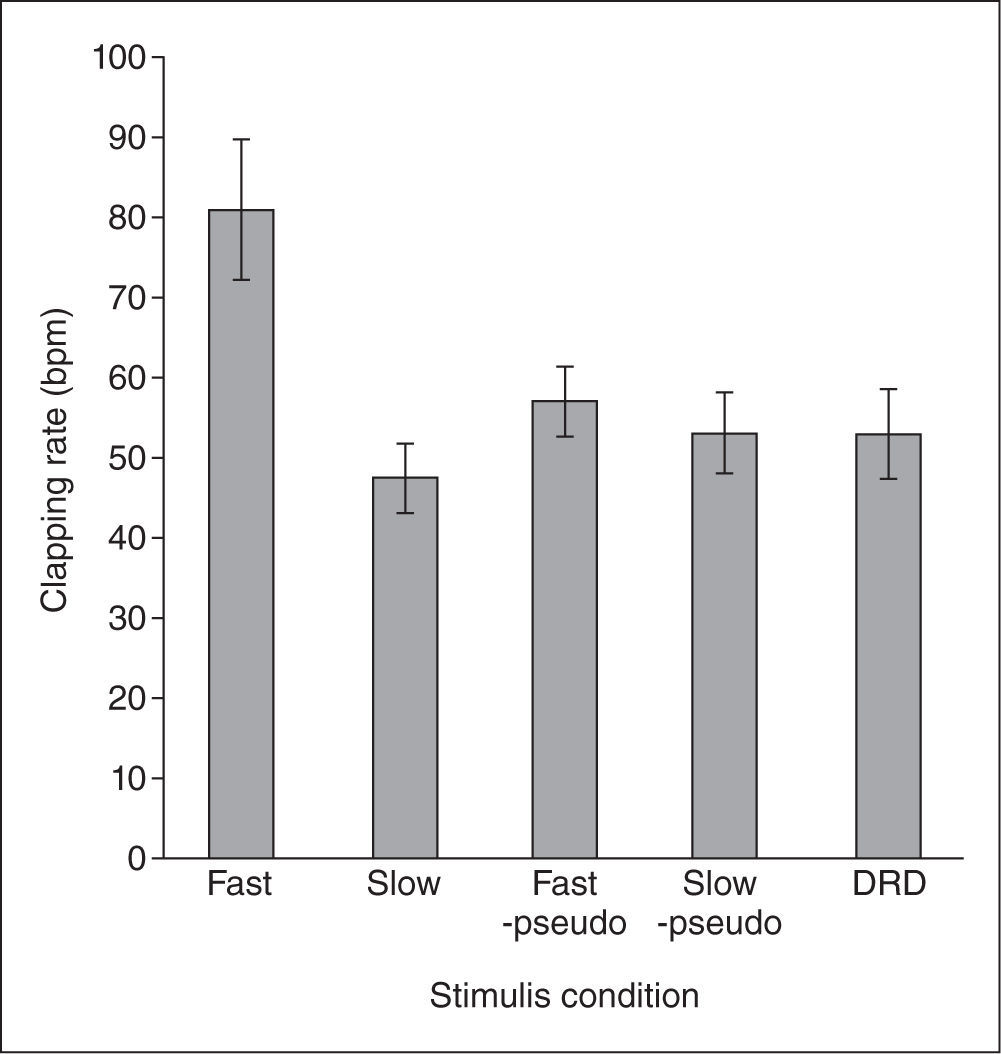
Results and DiscussionTo assess the observer's mental tempo, we calculated the clapping rate expressed as bpm (beats per minute), using the formula [clapping rate = (10/TI)*60], where TI is the temporal interval between the first and last claps: Larger values denote a faster tempo. As shown in Figure 2, the clapping rate in the fast optic flow condition seems to be larger than those in the other four conditions. Statistical analyses supported this view. A one-way analysis of variance (ANOVA) revealed a significant main effect of the five conditions (F(4, 56) = 8.83, p< .0001, prep = .997, ηp2 = 0.39). Multiple comparisons revealed that the clapping rate was significantly greater in the fast optic flow condition than in the other conditions (ps< .0004).
Tapping rate for each stimulus condition. The labels “Fast” “Slow” “Fast pseudo” and “Slow pseudo” represent the results of the fast optic flow, slow optic flow, fast pseudo-optic flow, and slow pseudo-optic flow conditions, respectively. Error bars denote standard errors of the means.
As we predicted, the results showed that the clapping rate in the fast optic flow condition was larger than that in the slow optic flow condition, suggesting that faster vection made the observers’ mental tempo faster. Moreover, this effect was not observed in the pseudo optic flow conditions or in the DRD condition. These results suggest that it is not the speed of optic flow stimuli, or the continuous change of dot stimuli, but rather the speed of perceived self-motion that affected the observer's mental tempo.
In Experiment 2 we conducted an observation with five naive volunteers, in which we recorded the pupil diameter while observers viewed the five types of visual stimuli used in Experiment 1. This was done in order to examine whether fast vection increased an observer's arousal level.
Experiment 2MethodsObservers: Five naive graduate students participated in Experiment 2. The observers reported no visual or vestibular abnormalities.
Apparatus, Stimuli, and Procedure: The stimuli were the same as those used in Experiment 1. The stimuli were controlled by a Macbook Pro (Apple) computer, presented on a 46-inch plasma display. Observers first adapted to the darkened room for about ten minutes. We recorded the right pupil diameter using an eye tracker (EMR-8B, NAC Image Technology, Japan)at 60Hz during the experiment. Viewing distance was 45cm. The five conditions (fast and slow optic flow conditions, fast and slow pseudo-optic flow conditions, and DRD condition) were randomly presented for 30 sec. Each condition was repeated five times.
Results and DiscussionAfter discarding data which contained eye blinks and recording errors, pupil data were binned into 1-sec segments. Because of a recording error, the last 1 sec of data was not recorded in all observers. Average pupil diameter of 1 sec before the stimulus presentation on each trial was used as the baseline. Changes in pupil diameter from the baseline during the presentation period were subjected to analysis.
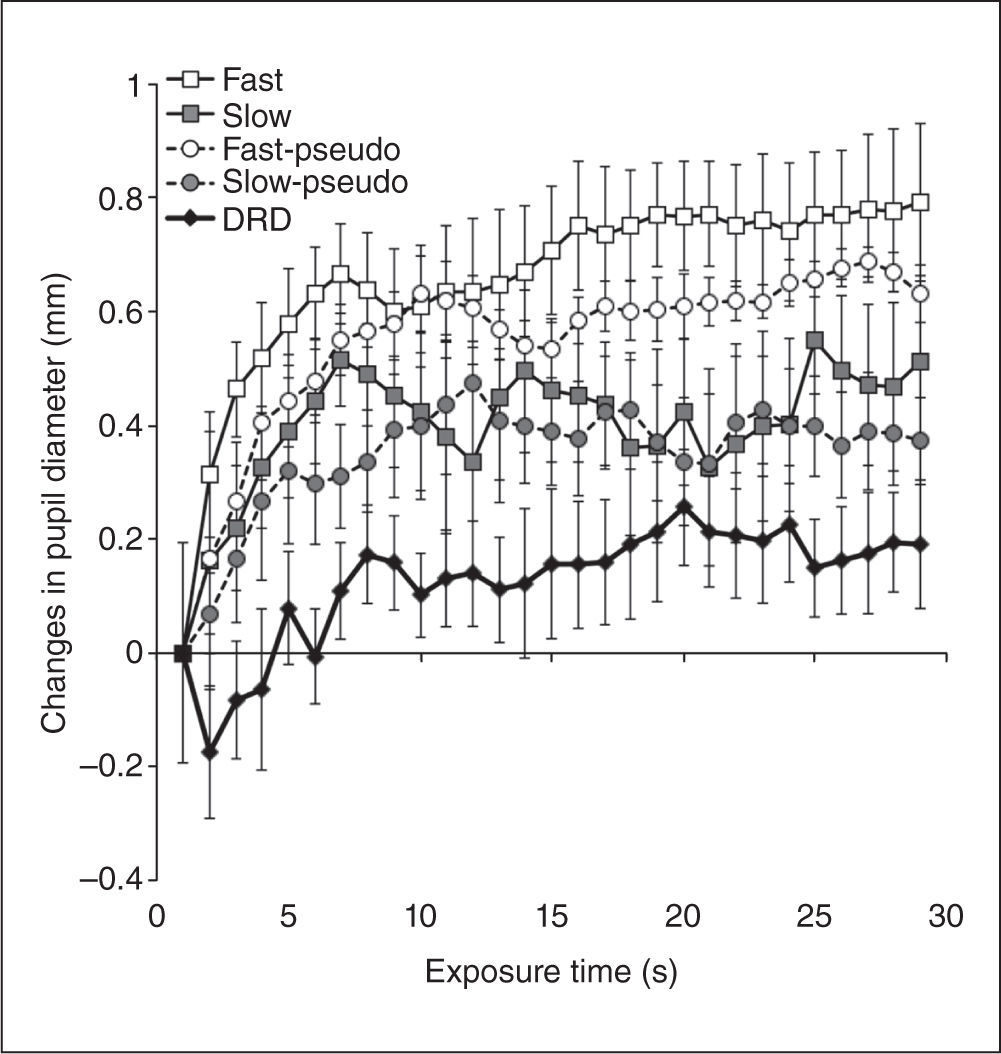
As shown in Figure 3, average changes in pupil diameter were largest in the fast optic flow condition as compared to the other four conditions. A one-way ANOVA revealed a significant main effect in all five conditions (F(4, 16) = 3.91, p< .03, prep = .949, ηp2 = 0.49). Multiple comparisons revealed that change in pupil diameter was significantly larger in the fast optic flow condition than in the DRD condition (p< .003). Differences with the other conditions were not significant. Thus, as we predicted, the largest pupil dilation occurred in the fast optic-flow condition. These results support the notion that the speed of perceived self-motion can modulate arousal level.
Time lapses of changes in pupil diameter. Error bars denote within-subject standard errors (Cousineau, 2005).
In Experiment 1 we examined whether cyclic action (clapping) related to mental tempo was modulated by the speed of illusory self-motion, and confirmed that this was the case. In addition, the results confirmed that this effect was not due to the speed of optic flow or the continuous change in visual patterns. Moreover, the results of Experiment 2 revealed that fast vection induced a larger change in pupil diameter than did slow vection. The results support the hypothesis that fast vection increased the arousal level of observers. Vection observed here was much stronger in the fast optic flow condition, and thus this might be a proof of higher arousals level in the aforementioned condition.
Our findings were consistent with the view of the internal clock (Gibbon et al., 1984; Meck, 2005). This view has been widely applied to explain interval timing perception, duration perception, and musical rhythmic performance (Keele, Nicoletti, Ivry, & Pokomy, 1989). The internal clock consists of oscillator, accumulator, and comparator processes. Here, the oscillator appears to be related to the mental tempo in that the oscillator continuously emits pulses, and this pulse rate regulates the speed of mental tempo (Vanneste et al., 2001). Present results suggest that the arousal-based speeding up of the oscillator in the internal clock system (Droit-Volet & Meck, 2007) induces an increase in mental tempo. This is consistent with the findings of Boltz (1994), who showed that the arousal level modulates the preferred tempo. However, it is still unclear how vection increases the arousal level. Because vection is a kind of self-motion, and the vection conditions in the present study evoked a relatively high arousal level, further studies are required so as to clarify the relationship between self-related processing and arousal level. Moreover, this interpretation of the results presented is based on the involvements of many hypothetical processes such as arousal, clock, and mental tempo. More specific evidence is needed for clarifying whether each process of the cascade is actually affected by fast vection. As of now, we certainly obtained the fact that vection was stronger in the fast optic flow condition. It is likely that the stronger vection could modify the arousal level more strongly.
As another explanation, one could argue that the speed up of the cyclic action observed in Experiment 1 was a product of cognitive semantic processing. In the present study, the semantic representation of “fast” was consistent between the speed of cyclic action and vection. This semantic similarity may establish a link between the two, producing the current results. Our previous studies have shown that upward vection induces positive memories (Seno, Kawabe, Ito, & Sunaga, 2013), and inversely that positive sounds enhance upward vection (Sasaki, Seno, Yamada, & Miura, 2012). Similarly, a semantic representation of “fast” formed from vection, through semantic processing, could induce an effect of vection on other mental activities - in the present case, a hand-clapping action. Future research needs to test whether an inverse effect occurs; that is, whether a fast cyclic action induces fast vection.
Otherwise, the present results can be explained by a simpler mechanism; that is, a preferred tempo for the task was modulated by vection. Specifically, it is possible that temporal information in the fast optic flow condition directly biased the rhythm of a cyclic action (i.e., hand clapping). This hypothesis also calls for evidence in further research.
Present findings suggest that the speed up of utterance rate found in Seno et al. (2013) might be related to a speed up of mental tempo induced by fast vection. This has important implications for future research on vection and action. Speed of vection is likely to influence various mental tempo-based phenomena as such as time perception, biological motion perception, musical performance, and the social interaction of people with different tempos.
The second author was supported by the Japan Society for Promotion of Science.