Deep brain electrical stimulation of the ventro-medial globus pallidus in Parkinson's disease (PD) is an alternative in the treatment of rigidity in advanced cases which do not response to conventional pharmacological treatment.
ObjectiveTo measure the clinical changes and collateral effects of bilateral electric stimulation of the ventro-medial globus pallidus, in a sample of patients suffering from Parkinson's disease with predominance of rigidity.
MethodologyAn auto-control study of direct assignation was carried out, open to evaluate the changes according to the Unified Parkinson Disease Rating Scale III (UPDRS III) in a basal period, within 6 and 12 months of follow up same as the collateral effect and the medicament doses.
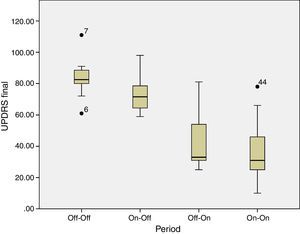
Results12 patients were included (5 women) with ages of 62.4±12.7 years (media±DE), history of PD was14.2±5.3 years, with Hoehn and Yahr stages within II and IV. UPDRS III score in OFF- medication of 73±11.3. Beyond 6 months of follow up, a significant statistical change towards tremor, rigidity, bradykinesia, posture and gait, can be appreciated (p<0.05). The best effect can be observed in the qualification of UPDRS III after implantation (35 to 38 delta points of the scale) and for stimulation particularly for bradykinesia (delta of 11 points). There were no significant changes in levodopa doses. The collateral effects such as phosphenes, contralateral paresthesias, deviation of labial commissure and dysarthria, were avoided with the adjustment of stimulation parameters during the surgery.
ConclusionThis study suggest that bilateral implantation of the ventro-medial globus pallidus is an efficient and safe method in patients PD with rigidity implanted in the Hospital Central Militar.
La estimulación eléctrica cerebral profunda del globo pálido interno en la enfermedad de Parkinson es una alternativa para resolver la rigidez de los casos avanzados que no responden al tratamiento farmacológico convencional.
ObjetivoMedir los cambios clínicos y los efectos colaterales de la estimulación eléctrica bilateral del globo pálido en una muestra de pacientes con enfermedad de Parkinson con predominio de rigidez.
MetodologíaSe aplicó un estudio autocontrolado de asignación directa, abierto para evaluar los cambios en la Unified Parkinson Disease Rating Scale III en el período basal y entre los 6 y 12 meses de seguimiento al igual que los efectos colaterales y en la dosis de medicamento.
ResultadosSe incluyeron 12 pacientes (5 mujeres) con edad de 62.4±12.7 años (media±DE), tiempo de evolución de 14.2±5.3 años, con estadio de Höhen y Yahr entre II y IV. Con una calificación en OFF- medicación 83.6±11.08 y en ON – medicación de 73±11.3. A más de 6 meses de seguimiento se observó un cambio estadísticamente significativo hacia la mejoría del temblor, la rigidez, la bradicinesia, la postura y la marcha (p<0.05). El mejor efecto se observó en la calificación del UPDRS III posterior a la implantación (delta de 35 a 38 puntos de la escala) y para estimulación particularmente para la bradicinesia (delta de 11 puntos). No hubo un cambio significativo en la dosis de levodopa. Los efectos colaterales como fosfenos, parestesias contralaterales, desviación de la comisura labial y disartria fueron evitados con el ajuste de parámetros de estimulación durante la cirugía.
ConclusiónEste estudio sugiere que la implantación bilateral del globo pálido interno es un método eficiente y seguro en pacientes con EP rígidos implantados en el Hospital Central Militar.
Parkinson's disease (PD) presents a different prevalence depending of the ethnical group or the geographic region studied. Indeed, PD is considered that it affects between 1 and 2% of the population older than 60 years 1,2. Unfortunately, the medical treatment presents important secondary effect in more than 50% of the cases after 5 years of treatment, due to the motor fluctuations occasioned by pharmacokinetic and pharmacodynamics of chronic use medicaments, collateral effects of the same (dyskinesia) and by the answer absence of some signs such as bradykinesia, posture disorders and gait 3,4.
Bilateral electric stimulation of the ventro-medial globus pallidus has been an alternative treatment since the original works of Siegfried and Lipptiz in 1994 5 and is considered a useful option in the treatment of patients with Parkinson's disease, mainly with predominant rigidity and bradykinesia. 5–11.
Some alternatives like stimulation of the subthalamic nucleus or pallidotomy are widespread and are currently used. However, These procedures show collateral effects which can be considered daunting 11–15. The ventro-medial globus pallidus can be an area of brain stimulation with a good clinical effect over the illness and with a shorter amount of complications.
The objective of this work was to quantify the clinical change in a sample of patients with PD from the Hospital Central Militar, with predominance of rigidity, subdued to bilateral electric stimulation of the ventro-medial globus pallidus.
Patients and methodsThe target population was defined as: those patients with diagnosed PD, that were beneficiaries of the Hospital Central Militar, with definitive diagnosis of this condition according to the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria 16, with a minimal age of 50 years, with a minimal evolution time of 5 years, in Hoehn and Yahr stage within II and IV 17, with predominance of rigidity, posture and gait alterations, without any important cognitive impairment, with partial response to levodopa, without any important surgical risk according to the qualification of the American Society of Anaesthesiology.
The clinimetric evaluations were effectuated by a neurologist specialized in movement disorders. Two neurosurgeons evaluated every case and decided the pertinence of the surgical target. The follow up was effectuated within 6 and 12 months through the design of a self-controlled study, open and of direct assignation. Surgical procedure was performed with Zamorano Dujovny stereotactic frame and IPS system for implantation (Inomed, Frieburg Germany). The sites of implantation were corroborated through an axial computed tomography or by postoperative magnetic resonance. A register of the stimulation parameters and the collateral effects was performed. The design included descriptive statistic for demographic variables and the range proofs of Wilcoxon for the outcome variables.
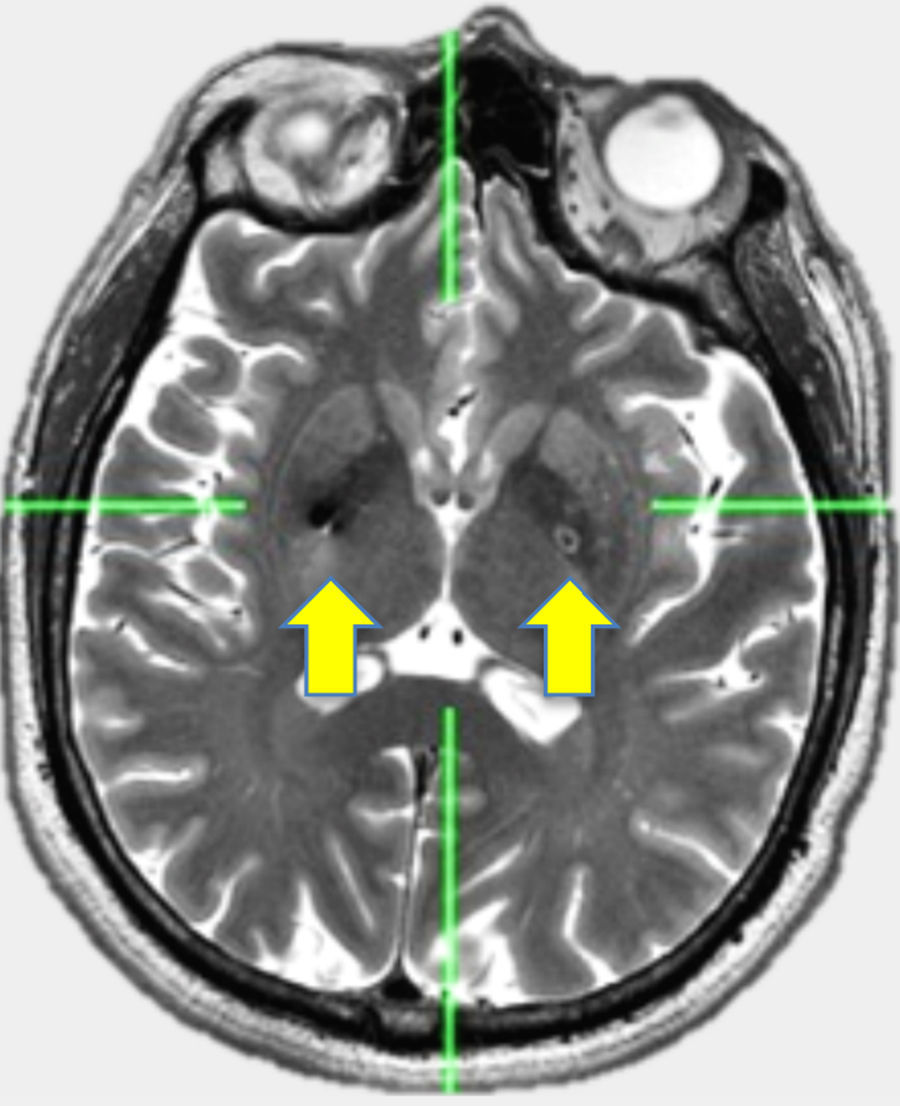
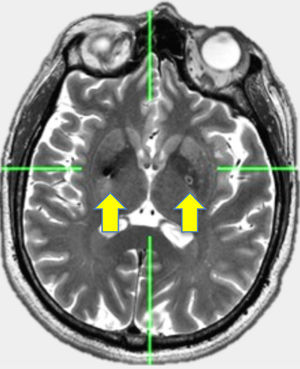
ResultsDemographic data is showing in Table 1. Twelve patients with PD were included (5 women and 7 men) with ages of 62.4±12.7 (Media±standard deviation), history of PD of 14.2±5.3 years, with Hoehn and Yahr stage within II and IV. They showed predominance of rigidity. UPDRS III score OFF – medication was 83.6±11.08 and in ON - medication of 73±11.3. Patients reported doses of levodopa of 789±792 mg/day. They were underwent to a bilateral stereotactic implantation of electrodes in the ventro-medial globus pallidus through Zamorano Dujovny stereotactic frame, CT scan slides were fused with MRI slices thought out IPS system and indirect and direct calculation of coordinates allowed lead location. Bilateral implantation was made in awareness condition using local anaesthesia. Entry points were performed just over coronal suture and 35mm lateral from midline. Micro-recording system was not used. RMI or CT scan was performed immediately after implantation in order to confirm right location and absence of bleeding. Internalization of Activa system by Medtronic was made in the same day. Results of mean of coordinates (±SD) in mm were for right side (x=20.5±1.5, y=1.9±1.7, z=-3.5±0.5) and for left side (x=19.7±1.1, y=2.6±1.8, z=-3.3±0.6).(Figs. 1 and 2)
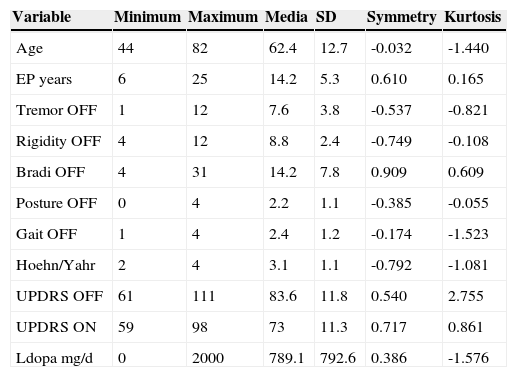
This table shows demographic data of 12 patients enrolled in this study. Initial scores of every important sign were recorded in OFF and ON medical condition. Also It is showing is UPDRS III score were recorded. Symmetry and kurtosis are showed in order to review normal distribution of sample.
| Variable | Minimum | Maximum | Media | SD | Symmetry | Kurtosis |
|---|---|---|---|---|---|---|
| Age | 44 | 82 | 62.4 | 12.7 | -0.032 | -1.440 |
| EP years | 6 | 25 | 14.2 | 5.3 | 0.610 | 0.165 |
| Tremor OFF | 1 | 12 | 7.6 | 3.8 | -0.537 | -0.821 |
| Rigidity OFF | 4 | 12 | 8.8 | 2.4 | -0.749 | -0.108 |
| Bradi OFF | 4 | 31 | 14.2 | 7.8 | 0.909 | 0.609 |
| Posture OFF | 0 | 4 | 2.2 | 1.1 | -0.385 | -0.055 |
| Gait OFF | 1 | 4 | 2.4 | 1.2 | -0.174 | -1.523 |
| Hoehn/Yahr | 2 | 4 | 3.1 | 1.1 | -0.792 | -1.081 |
| UPDRS OFF | 61 | 111 | 83.6 | 11.8 | 0.540 | 2.755 |
| UPDRS ON | 59 | 98 | 73 | 11.3 | 0.717 | 0.861 |
| Ldopa mg/d | 0 | 2000 | 789.1 | 792.6 | 0.386 | -1.576 |
SD=standar deviation.
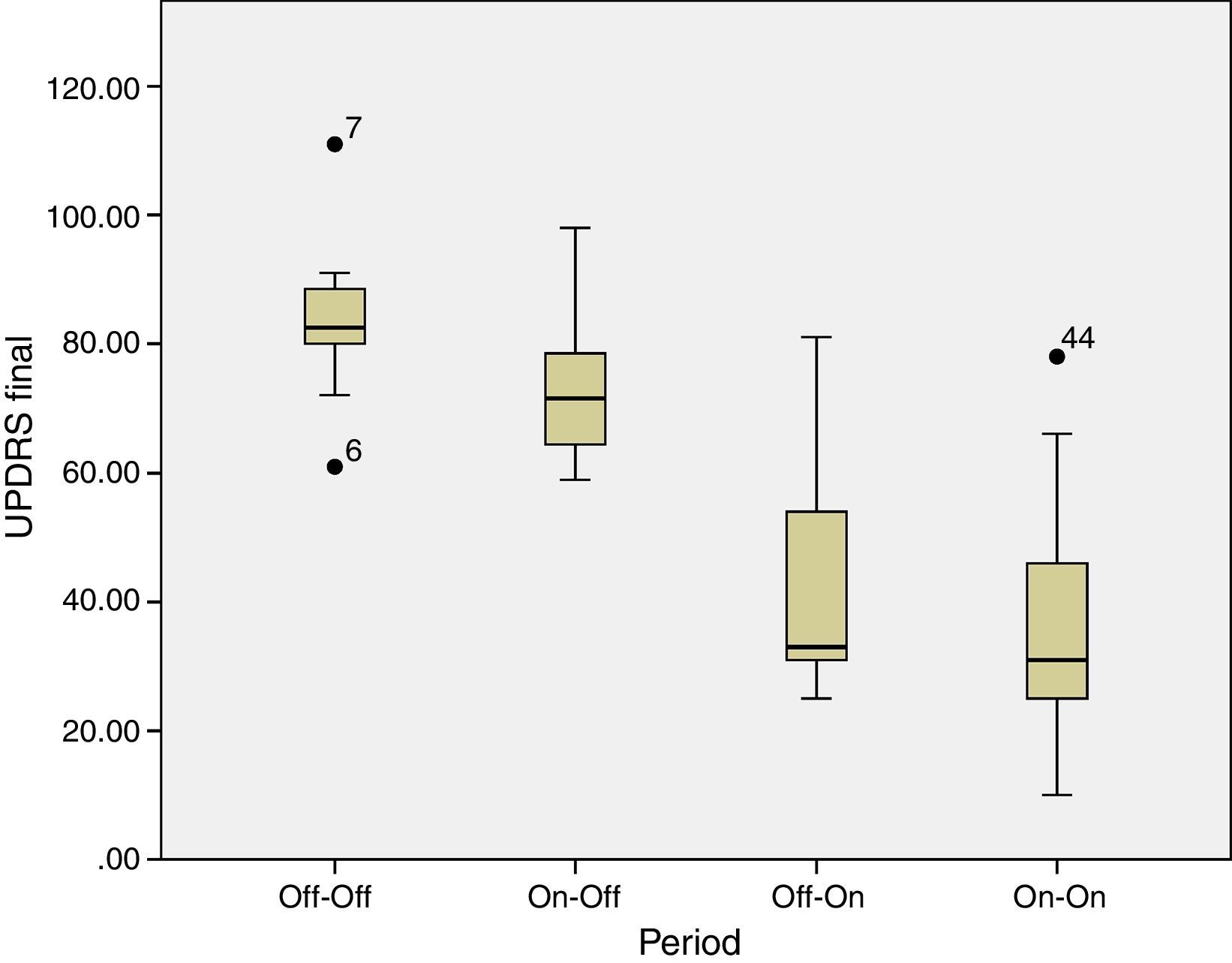
Box plot graphic shows statistical results through Wilcoxon test. There is significant delta (p=0.005) between the period without levodopa previous and after electrical stimulation (OFF-OFF versus OFF-ON). Similarly, during levodopa period there was significant differences (p=0.013) (ON-OFF contra ON-ON). By other side, there was not effect by levodopa both pre or postoperative period (OFF-OFF) (ON-OFF) (p=0.213).
The ranges in the stimulation parameters applied for amplitude, have been of 1.3 to 2.5 volts, for the width of 60 to 120 microseconds, for a frequency of 120 to 145 hertz, for impedance of 992 to 3450 ohms, with stimulation mono or bipolar depending on the best reaction (Lead 3389 and Activa RC by Medtronic Minneapolis MN). A evaluation with the UPDRS III scale was made in order to measure their clinic performance, and was followed up for 6 to 12 months.(Table 2)
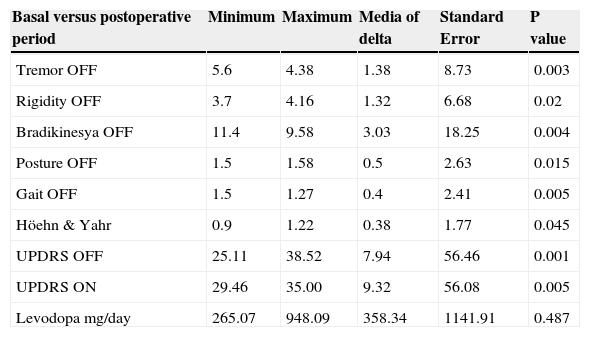
In this table is showing minimal, maximal differences and media of delta changes of cardinal signs in the sample of patients. Significant changes were evaluated through t-paired test. The only variable without statistical changes was levodopa doses.
| Basal versus postoperative period | Minimum | Maximum | Media of delta | Standard Error | P value |
|---|---|---|---|---|---|
| Tremor OFF | 5.6 | 4.38 | 1.38 | 8.73 | 0.003 |
| Rigidity OFF | 3.7 | 4.16 | 1.32 | 6.68 | 0.02 |
| Bradikinesya OFF | 11.4 | 9.58 | 3.03 | 18.25 | 0.004 |
| Posture OFF | 1.5 | 1.58 | 0.5 | 2.63 | 0.015 |
| Gait OFF | 1.5 | 1.27 | 0.4 | 2.41 | 0.005 |
| Höehn & Yahr | 0.9 | 1.22 | 0.38 | 1.77 | 0.045 |
| UPDRS OFF | 25.11 | 38.52 | 7.94 | 56.46 | 0.001 |
| UPDRS ON | 29.46 | 35.00 | 9.32 | 56.08 | 0.005 |
| Levodopa mg/day | 265.07 | 948.09 | 358.34 | 1141.91 | 0.487 |
Beyond 6 months of follow up, a significant statistical change could be appreciated in the improvement of the tremor, the rigidity, bradykinesia, posture and gait (p<0.05) according to a paired t-Student test. The best effect was observed in the UPDRS III score after implantation (maximum delta of 35 to 38) and for stimulation particularly for bradykinesia (delta 11 points) according to a range of Wilcoxon test. There were no significant changes in the doses of levodopa. No permanent secondary effects were found. Collateral effects over sight were present in a case as well as the stimulation of the internal capsule (contralateral paraesthesia of the face or hemibody, and deviation of the labial commissure) or speech was avoided with the adjustment of stimulation parameters during the surgery.
DiscussionIn this study the electric stimulation of the ventro-medial globus pallidus improved the performance of patients with PD in advanced phase, considering the diminishment in the qualification of the UPDRS III scale. This change translates to the diminishment in the intensity of cardinal signs of the disease, in particular the qualification of bradykinesia with an average reduction of 3 points. This data is similar to the reported for electric stimulation of subthalamic nucleus and of the ventro-medial globus pallidus by other groups 11,18. Up to this moment, the target selection to obtain the best effect out of the stimulation depends on the probability to improve the more incapacitating signs, the frequency with which these collateral effects can complicate the clinical picture and especially by the ability to implant the electrodes with precision. In consequence, the electric stimulation of the ventro-medial globus pallidus remains as one of the major alternatives to improve rigid-akinetic patients 10,18. As far as we know, the action mechanisms of electric stimulation of the ventro-medial globus pallidus and of the subthalamic nucleus are similar whether in function of the spontaneous somatic activity inhibition of neurones close to the electrode, to the depolarizing effect of these nucleus exit paths or of the important interconnectivity and interaction that exists between these two anatomic structures 18. It is probable that electric stimulation of the ventro-medial globus pallidus could produce a propagation effect within the neuronal net and thus facilitate the motor answer of a bradykinetic patient, in a similar way to the subthalamic nucleus.
There were various limitations in this study, such as the open design and the direct manoeuvre assignation, also the tracking and the number of patients could be relative limitations. However, this studyis an approximation as a pragmatic study within a recently installed stereotactic neurosurgery unit to perform electric brain stimulation into adequate clinimetric assays.
ConclusionsThis study suggests that bilateral implantation of the ventro-medial globus pallidus is an efficient and safe method in patients with severe PD implanted in the Hospital Central Militar.
FundingThis study was a collaboration of the Neurosurgery Service of Hospital central
Militar and the Mexico General Hospital. Financial support was exclusively from
Hospital central Militar, and this work has been presented at XVIII Meeting of
Neurosurgey Society of Occident in July 2014.