Impairments in olfactory function can be potential indicators of the onset or progression of neurological disorders. Nevertheless, olfaction is barely explored in routine clinical examination. This review provides a general idea of how olfaction is related to normal ageing and neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease and epilepsy, and an overview of recent studies in this field.
Las alteraciones en la función olfatoria pueden ser indicadores potenciales del curso o inicio de enfermedades neurológicas. Sin embargo, no se le ha dado la importancia debida a la exploración de este sentido en la evaluación clínica cotidiana. Este artículo de revisión pretende proporcionar una idea general de la relevancia e impacto que tiene el olfato en las enfermedades neurodegenerativas más comunes como son la enfermedad de Alzheimer, la enfermedad de Parkinson y la epilepsia, así como investigaciones recientes en este campo.
The sense of smell is of great importance in everyday life because it continuously informs us about our surroundings: it assesses and warns us about potential air hazards, thus making it a primary warning mechanism; it is the only sense that remains active during sleep; and, along with the sense of taste, it determines the taste and smell of food. There is a strong connection between smells and memory, with olfaction having a great influence on a person's emotional state.
Deterioration in olfactory function has a detrimental impact on quality of life. Impairments in olfaction have been described in preclinical stages in certain neurodegenerative diseases and may be correlated with disease progression. As a result, olfaction can be considered as a valid potential indicator of ageing brain.
Several hypotheses have been proposed to explain the relationship between olfactory function and neurodegenerative diseases, highlighting the olfactory vector hypothesis, among others.
MethodologyA review of the literature from October 2016 to February 2017 was carried out using the TRIP (Turning Research Into Practice) meta-search engine and databases such as the Cochrane Library, PubMed, Wiley Online Library and Medigraphic; sites like ScienceDirect and JoVE (Journal of Visualised Experiments) were also consulted. Search terms such as “olfaction”, “neurodegenerative diseases”, “epilepsy”, “ageing” and “olfactory dysfunction” were used. Systematic reviews mainly from 2004 to 2016 were included, as were original articles, clinical trials and meta-analyses on the subject-matter, the methodology of which was clearly described from 1993 to 2016.
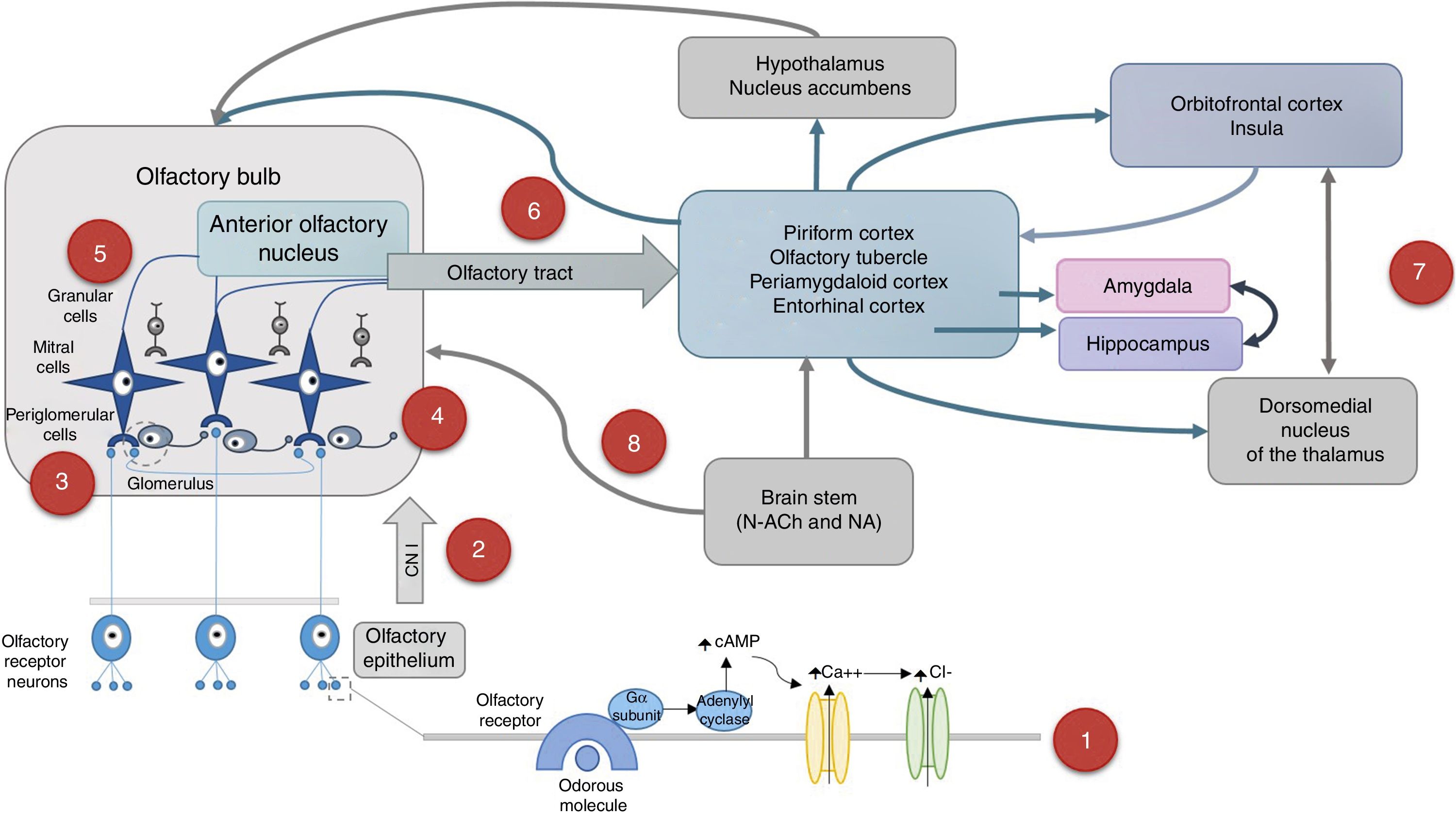
The olfactory system and its ageingOlfaction is a chemical sense specialised in detecting odorous molecules that must be volatile so that when vaporised, they reach the nostrils and dissolve in the nasal mucosa, thus initiating the process of olfaction (Fig. 1).
- (1)
Odorous molecules activate specific receptors located in the cilia of bipolar neurons in the olfactory mucosa. Olfactory signal→Olfactory receptor→G protein activationα→↑Adenylyl cyclase 3→↑cyclic AMP→Ca++entry→Cl−exit→depolarisation.7,16,28
- (2)
The axons of receptor neurons form the cranial nerve I (olfactory), cross the lamina cribrosa and synapse with mitral or tufted cells in the olfactory bulb, forming a glomerulus.
- (3)
Each glomerulus receives stimuli from olfactory receptor neurons that express the same type of receptor for a specific scent13; however, there is no chemotropic map in the olfactory bulb.68 Glomerular activity is crucial for detecting odorous stimuli.
- (4)
These axons also synapse with periglomerular cells, which are interneurons that establish a local reciprocal inhibition with other mitral cells and connect glomeruli with each other.
- (5)
Granular cells form dendrodendritic synapses with mitral cells to reinforce the response to a particular scent.
- (6)
The axons of mitral cells project to the ipsilateral olfactory cortex through the lateral olfactory tract. These axons project to primary olfactory areas: anterior olfactory nucleus, piriform cortex, anterior cortical amygdaloid nucleus, periamygdaloid cortex and entorhinal cortex.
- (7)
The piriform cortex connects directly and indirectly to the posterior orbitofrontal cortex via the dorsomedial nucleus of the thalamus, as well as to the entorhinal cortex and the amygdala. The entorhinal cortex sends information to the amygdala and hippocampus. The piriform cortex and the amygdala project to the hypothalamus, the nucleus accumbens and, via the ventral pallidum, the medial portion of the dorsomedial nucleus of the thalamus. The posterior orbital cortex and the adjacent anterior insula have reciprocal connections with all primary olfactory areas and interact with other cortical areas to integrate the information with other sensory stimuli.
- (8)
Both the olfactory bulb and the primary olfactory cortex receive afferents from the hypothalamus and brain stem. Cholinergic stimulation acts on M2 receptors by reducing the release of gamma-amino butyric acid (GABA) in periglomerular and granular cells for modulation. Noradrenergic stimulation of the locus coeruleus acts on adrenergic receptors α1 by increasing the mitral cell response to weak olfactory stimuli.
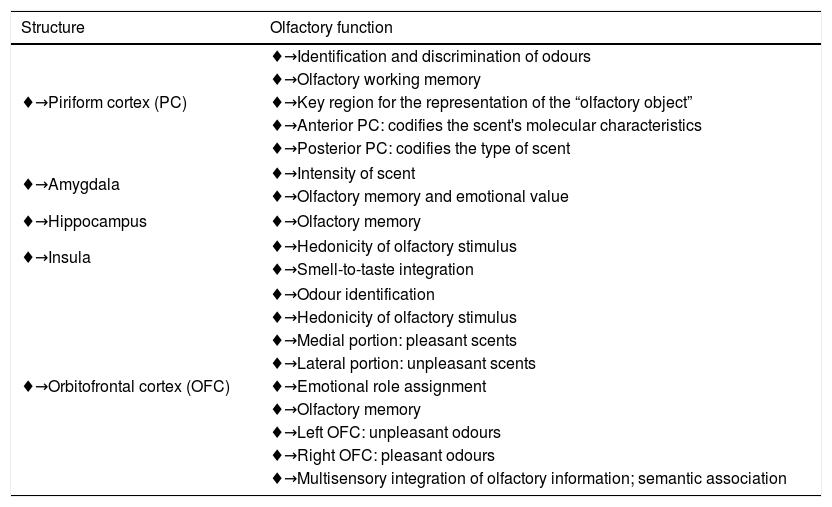
Outlining the olfactory processing,7,26,59,68 which involves the detection, perception, discrimination and identification of scents, in a hierarchical way is not easy because several structures participate simultaneously. Surgical, neurophysiological and neuroimaging studies, particularly functional magnetic resonance imaging and positron emission tomography, have enabled the identification of the activation of regions at different olfactory processing stages6,8,14,24,29,40,51,54,58,61,62 (Table 1).
Brain areas activated during olfactory processing.
| Structure | Olfactory function |
|---|---|
| ♦→Piriform cortex (PC) | ♦→Identification and discrimination of odours |
| ♦→Olfactory working memory | |
| ♦→Key region for the representation of the “olfactory object” | |
| ♦→Anterior PC: codifies the scent's molecular characteristics | |
| ♦→Posterior PC: codifies the type of scent | |
| ♦→Amygdala | ♦→Intensity of scent |
| ♦→Olfactory memory and emotional value | |
| ♦→Hippocampus | ♦→Olfactory memory |
| ♦→Insula | ♦→Hedonicity of olfactory stimulus |
| ♦→Smell-to-taste integration | |
| ♦→Orbitofrontal cortex (OFC) | ♦→Odour identification |
| ♦→Hedonicity of olfactory stimulus | |
| ♦→Medial portion: pleasant scents | |
| ♦→Lateral portion: unpleasant scents | |
| ♦→Emotional role assignment | |
| ♦→Olfactory memory | |
| ♦→Left OFC: unpleasant odours | |
| ♦→Right OFC: pleasant odours | |
| ♦→Multisensory integration of olfactory information; semantic association | |
The olfactory epithelium, which is located in the upper part of the nasal cavity and measures approximately 4–6cm2 in each nostril, is composed of 10–20 million olfactory receptor neurons,21,44 basal cells, supporting cells and olfactory glands. Each bipolar neuron contains 3–50 stationary cilia that project to the mucus layer. The olfactory receptors are located on the cilia and are coded by about 1000 different genes28; there are about 400 types of receptor, and an olfactory neuron can respond to more than one type of smell.7,68
Olfactory receptor neurons are derived embryologically from the olfactory placode and neural crest cells. They have the ability to regenerate from basal cells,42 but their number decreases with age,45,48 particularly from the age of 65 years,19 as a result of an increase in the death of receptor cells36 and a decrease in neuronal proliferation.15
The olfactory bulb and the hippocampus are exceptional structures in terms of neurogenesis in the adult brain.23 Subpopulations of interneurons in the olfactory bulb (periglomerular and granular cells) are constantly replaced by neurons derived from astrocytes originating in the subventricular zone of lateral ventricles and migrating via the rostral migratory stream to become part of the existing network. It is believed that the aim is to maintain adequate transduction of olfactory stimuli to the brain. Inhibitory periglomerular cells using GABA and dopamine are responsible for modulating intra- and interglomerular signalling. Granular cells are primarily inhibitory GABAergic interneurons, and their interaction with mitral cells is associated with odour discrimination. Synaptic loss of olfactory interneurons modifies olfactory perception.1
Neurogenesis in the olfactory bulb adjusts at the level of input stimuli, which plays a crucial role in information processing and olfactory learning.56 Moreover, the hippocampus has been linked to memory formation and pattern discrimination.
In older adults, it is associated with overall cognitive decline and diminished episodic memory,3,53 seriously affecting their daily lives.19 It has been reported that half of the population aged 65–80 years suffers from olfaction disorders, rising to 75% in people older than 80 years,3,10,19 with the olfactory threshold being the most affected.53 Unfortunately, this epidemiological report does not correspond to the Mexican population because it has been given little importance by both patients and the medical community.45,46
Multiple age-related factors contribute to this olfactory loss, including: impaired thickening of nasal structures; increased tendency to develop nasal disease; accumulated damage to the olfactory epithelium caused by external agents; decreased metabolic enzymes in the nasal mucosa35; reduced nasal blood flow; ossification of the lamina cribrosa; loss of receptor cell selectivity for certain scents; changes in the neuromodulation and neurotransmitter systems (cholinergic, serotonergic and noradrenergic); and neuronal expression of aberrant proteins associated with neurodegenerative diseases.19,39
The olfactory vector hypothesis indicates that various viruses, bacteria, prions, toxins and other xenobiotic agents have direct access to the brain via the olfactory pathway, thus impairing the structures involved since the neuro-olfactory epithelium is separated from the outside only by a thin layer of mucus, which makes it vulnerable to any external agent.20 Other mechanisms involve neurites, inducing cascades of molecular processes that lead to oxidative stress, neuroinflammation and alterations in cellular processes, resulting in cell death. Recent studies suggest that in adults neurogenesis is affected by environmental factors that have an impact on neuronal survival, olfactory discrimination or pattern recognition, and olfactory memory.
The olfactory pathway is one of the oldest brain structures phylogenetically linked to the limbic system, so the olfactory information stored in memory has a great emotional component64 and lasts longer. Olfaction plays a hedonic-social function: scents are used to influence our emotions,9 which is why the cosmetics and perfume industries are successful.
Clinical evaluationIt is important to consider factors such as the patient's age, gender, occupation, comorbidities and cultural background when evaluating their olfactory function.63 The following are the tests used to examine various tasks such as detection, discrimination, identification and recognition of scents:
- (1)
Detection test: determines the olfactory sensitivity threshold (lowest concentration of substance in which the stimulus can be detected). It is advisable to use scents that only stimulate the olfactory nerve without irritating the trigeminal nerve endings, which end in the olfactory mucosa.32,57
- (2)
Discrimination test: it is a direct comparison between olfactory stimuli to determine how different they are from each other; this task requires good olfactory acuity and working memory and is not influenced by semantic factors.
- (3)
Identification test: the perceived odour is named with or without visual or graphic aids, for which a semantic association is required.
- (4)
Explicit and implicit memory tests: olfactory memory paradigms are used to decide whether the patient has previously come across a certain odour.
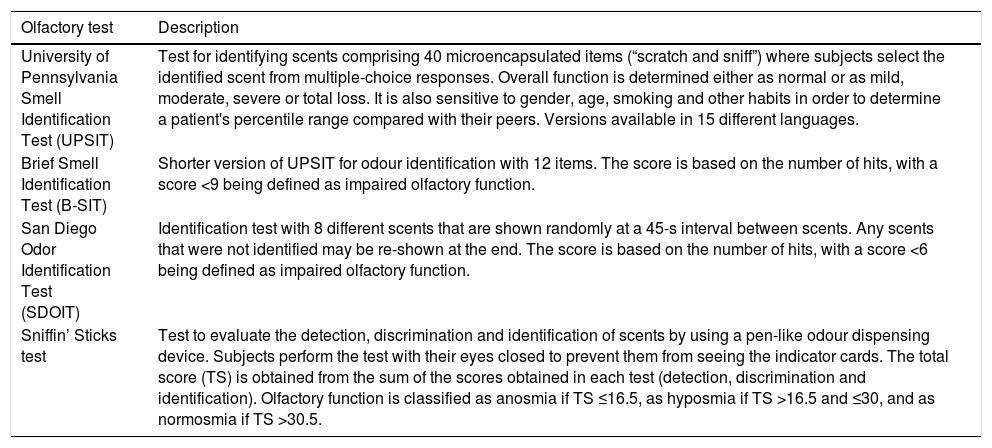
Standardised olfactory tests containing one or more of the tests mentioned above are in use. The most common tests are the University of Pennsylvania Smell Identification Test (UPSIT), the Brief Smell Identification Test (B-SIT), the San Diego Odor Identification Test (SDOIT), the Sniffin’ Sticks test and the Connecticut Chemosensory Clinical Research Center Test19,21,66 (Table 2).
Psychophysical olfactory tests.
| Olfactory test | Description |
|---|---|
| University of Pennsylvania Smell Identification Test (UPSIT) | Test for identifying scents comprising 40 microencapsulated items (“scratch and sniff”) where subjects select the identified scent from multiple-choice responses. Overall function is determined either as normal or as mild, moderate, severe or total loss. It is also sensitive to gender, age, smoking and other habits in order to determine a patient's percentile range compared with their peers. Versions available in 15 different languages. |
| Brief Smell Identification Test (B-SIT) | Shorter version of UPSIT for odour identification with 12 items. The score is based on the number of hits, with a score <9 being defined as impaired olfactory function. |
| San Diego Odor Identification Test (SDOIT) | Identification test with 8 different scents that are shown randomly at a 45-s interval between scents. Any scents that were not identified may be re-shown at the end. The score is based on the number of hits, with a score <6 being defined as impaired olfactory function. |
| Sniffin’ Sticks test | Test to evaluate the detection, discrimination and identification of scents by using a pen-like odour dispensing device. Subjects perform the test with their eyes closed to prevent them from seeing the indicator cards. The total score (TS) is obtained from the sum of the scores obtained in each test (detection, discrimination and identification). Olfactory function is classified as anosmia if TS ≤16.5, as hyposmia if TS >16.5 and ≤30, and as normosmia if TS >30.5. |
The olfactory impairments described in various neurodegenerative and psychiatric disorders involve deficits in the detection, discrimination and identification of odours,2,5,6,37,38 and so they are likely to share affected brain anatomical substrates. They are mainly associated with Alzheimer's disease, Parkinson's disease (including other diseases caused by Lewy bodies), mesial temporal lobe epilepsy, schizophrenia, Huntington's disease and multiple sclerosis.9,17,22,33,44 The most common diseases are briefly described below:
Alzheimer's disease (AD)Progressive loss of olfactory function can be considered an objective diagnostic indicator in the earliest stage of AD,49 as it occurs in approximately 90% of patients and correlates with disease progression.60 Immunohistochemical studies have shown β-amyloid deposits and hyperphosphorylated tau filaments in the olfactory epithelium, with the latter being closely related to the olfactory cortex.19 Neurofibrillary tangles have been detected in the olfactory bulb, along with dystrophic neurites with neurofilament proteins.3,4 Magnetic resonance imaging has shown smaller bilateral olfactory bulb volume, which is negatively correlated with the odour discrimination threshold.56,69 Similarly, reduced thickness in the entorhinal cortex is associated with poor ability to identify scents.25,67
Parkinson's disease (PD)Olfactory deficit has been considered one of the primary symptoms of PD,60 appearing years before motor symptoms do. The structures involved correspond mainly to the olfactory bulb and the amygdala, with neuronal loss and presence of Lewy bodies.3,4 The correlation between the onset of PD and olfactory deficit increases the likelihood that olfactory bulb neurogenesis is affected.23 Some MRI scans, however, have shown no alterations in the olfactory bulb volume,56 whereas others have reported a smaller volume compared with controls.27 Patients with PD have a diminished hedonic olfactory range, i.e., they do not perceive smells that are considered pleasant as such,47 a similar finding in patients with depression.
Mesial temporal lobe epilepsy (MTLE)The importance of the temporal lobe in human olfactory function has been recognised since the nineteenth century when Jackson and Stewart described the olfactory auras in detail and coined the term “uncinate crises” for unpleasant scents that are generally perceived by patients.12
The olfactory pathway and the limbic system share structures involved in the MTLE43 such as: amygdala, hippocampus, piriform cortex, entorhinal cortex, orbitofrontal cortex, anterior cingulate gyrus and insula.15,40,61,62 The interaction between the dominant inferior frontal gyrus, the fusiform gyrus and the temporal region is crucial for the interaction between the semantic and olfactory networks. Such structures form a connecting network between them, becoming more susceptible to excitatory damage caused by uncontrolled electrical activity.
Recent research has highlighted the role of the piriform cortex in focal epilepsy.65,70 Considered as the largest olfactory cortical area and as the association cortex, it retains its phylogenetic structure of three layers that are vulnerable to excitotoxic damage, such as the hippocampus. Due to the arrangement and large number of ramifications between pyramidal cells in the piriform cortex, an extensive excitatory neural network is formed that requires strong inhibitory feedback at a local level to prevent the uncontrolled propagation of electrical activity. It is therefore considered a highly epileptogenic area.55
Some recurrent local temporal lobe circuits may provide a favourable substrate for epileptogenic activity, such as projections from the piriform cortex to the amygdala and back to the endopiriform nucleus from the basolateral amygdala, as well as projections to the subunit connecting the hippocampus with the piriform cortex and back to the piriform cortex via the entorhinal cortex. Also, piriform cortex feedback to the olfactory bulb can give rise to a re-entry circuit. This was important in a study of mice with bilateral olfactory bulbectomy (an animal model used to study rodent depression) which investigated the potential role of olfaction in depression and epilepsy, resulting in postoperative damage in the anterior olfactory nucleus and in the piriform cortex.34 This model provided the basis for the investigation of underlying pathophysiological mechanisms in post-traumatic epilepsy, but no conclusive results have been shown. Further, smaller olfactory bulb volume has been reported in patients with MTLE, although it has not been correlated with the laterality of the epileptic focus.31
Olfactory sensitivity has varied in reports of epileptic patients: some studies report a normal olfactory threshold,18 whereas others have reported alterations in screening tests compared with healthy subjects.31
By contrast, abnormalities in specific olfactory tasks—e.g., discrimination, matching, short- and long-term recognition of scents, and difficulty naming or identifying them—are a consistent finding in several studies.11,18,30,41,42,52 Nevertheless, due to differences in the methodology used, it has not been possible to determine olfactory deficit patterns in terms of the location and laterality of the epileptogenic focus, or the duration or frequency of the seizures. It should be noted that it is anatomically desirable that primary olfactory projections be predominantly ipsilateral, so the lateralisation of hemispheric deficits would be more likely in olfactory processing.
At the Epilepsy Clinic of Hospital General de México there is a line of research to describe the olfactory function in patients with MTLE and its correlation with functional magnetic resonance imaging (fMRI) and recording with hippocampal depth electrodes in order to determine its usefulness as a potential complementary diagnosis tool. This research aims at avoiding diagnostic surgery, which patients with MTLE refractory to medical treatment who are eligible for surgery must undergo. Preliminary fMRI results have shown an overall olfactory dysfunction with little or no bilateral activation in areas corresponding to the olfactory system. As a result, it has not been possible to correlate the laterality of the epileptic focus with the lower activation of these structures. On the other hand, the study sample still needs to be completed, and the correlation between fMRI images and olfactory potentials analysed, as these potentials show the direct neurophysiological response of the most epileptogenic areas.
SchizophreniaIt is interesting that olfactory deficits similar to those of patients with mesial temporal lobe epilepsy, such as reduced ability to identify, recognise and discriminate odours, have been reported in patients with schizophrenia,50 without significant changes in the olfactory sensitivity threshold.2
Discussion and conclusionsSense of smell changes over time through a process of physiological ageing that affects the ability to detect, identify and discriminate odours to varying degrees. In addition, some neurodegenerative diseases tend to present greater deficit in certain specific olfactory tasks, besides directing disease progression over time.
The use of olfactory tests in routine clinical practice allows for the detection of subtle cognitive and sensory impairments, especially during ageing and in neurodegenerative diseases. Assessing the olfactory system may alert doctors to a probable diagnosis, resulting in the early detection and timely treatment of a neurodegenerative disease. Clinical evaluation should be focused on the identification and recognition of scents in order to detect subclinical cases of AD, whereas for PD it is preferable to focus on detection or olfactory threshold tests.53
Olfactory function may be a valid indicator of brain-ageing integrity. Some research studies aim at determining its usefulness as a biomarker or marker of genetic vulnerability in order to provide the possibility of early neuroprotective treatment.
Unanswered questions remain, however, regarding the correlation between olfactory deficit and neurodegenerative diseases—e.g., their causes; whether the deficit is a product of physiological ageing or a pathological process; the degree of involvement of genetic factors; whether damage to the olfactory system per se can trigger the neurodegenerative process in genetically susceptible individuals; and whether damage to the olfactory system is capable of triggering an inflammatory process that induces potentially harmful mediators of the immune system or producers of aberrant proteins, among others.
Using a standardised methodology to carry out future research protocols for olfactory impairment in neurodegenerative diseases is desirable, because of the difficulties in analysing and correlating the results obtained in both basic and clinical research.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank the entire multidisciplinary team of the Epilepsy Clinic of Hospital General de México, in particular, neuropsychologists Diego Alberto Manjarrez Garduño, Hector Becerril Montes and David Trejo Martínez, neuropharmacologist Dr Manola Cuéllar Herrera and neurosurgeon Dr Daruni Vázquez Barrón, as well as Erika Lucero Flores Ramírez, for their support.