T cells constantly recognise and eliminate circulating tumour cells. They require two signals in order to be activated: antigen presentation via TCR, and costimulatory interaction with different surface molecules of the B7 family, which may either enhance or attenuate immune response. In several cancers these molecules are deregulated, thereby promoting immune escape and the settlement or migration of cancer cells. In multiple myeloma, the expression of B7 co-inhibitory molecules and their relationship to disease progression have been identified. The MM1.R cell line is a useful cellular model for studying the progression of this disease, and therefore an analysis of the pattern of expression in this cell line helps to cast light on the immune status of this disease. Using a real-time PCR (quantitative RT-PCR) assay, we found that the main molecules expressed were those with an inhibitory function (B7-H1, B7-H3 and B7-H4), which suggests a high level of immune inhibition by these cells.
El reconocimiento y eliminación constante de células tumorales circulantes se logra principalmente mediante la acción de los linfocitos T, células cuya activación requiere de dos señales para poder llevarse a cabo: la presentación del antígeno a través del TCR y una interacción co-estimuladora liderada por distintas moléculas de superficie de la familia B7 que pueden potenciar o atenuar la respuesta. En varios tipos de cáncer, estas moléculas se encuentran desreguladas, favoreciendo el escape inmunológico y el asentamiento y/o migración de las células neoplásicas. En mieloma múltiple se ha identificado la expresión de moléculas B7 co-inhibitorias y su relación con la progresión de la enfermedad. La línea celular MM.1R es un modelo celular útil para estudiar la progresión de esta enfermedad, por lo que el análisis del patrón de expresión en esta línea celular contribuye a entender el estado inmunológico en la enfermedad. Mediante un ensayo de RT-PCR en tiempo real (qRT-PCR) encontramos que las moléculas mayormente expresadas fueron aquellas con función inhibitoria (B7H1, B7H3 y B7H4), lo cual indica una alta inhibición inmunológica por parte de estas células.
One of the main features of the immune system is immune surveillance, which is characterised by the system recognising and eliminating circulating tumour cells, thus preventing cancer from developing.1–4 In the immune system, T cells are primarily responsible for immune surveillance, activating apoptosis in neoplastic, senescent or undifferentiated cells.5,6 For T cells to be activated, two major signals are needed: recognition of an antigen bound to MHC molecules via their TCR, and a second costimulatory signal transmitted by the interaction of B7-CD28 in T cells.7 However, some neoplastic cells (bladder, colon, liver, lung, ovary, thyroid, kidney, sarcoma and melanoma) escape the immune system because they express surface molecules with inhibitory functions, thus causing T cell clonal anergy and evading elimination by the immune system.8–12 Negative costimulatory molecules in the B7 family are overexpressed in various cancers, and this overexpression is related to immune tolerance and disease progression.13–15 In multiple myeloma, the expression of these negative costimulatory molecules and their relationship to disease progression have been reported.16–18 The use of cellular models enables us to analyse gene expression in a more efficient and controlled manner. Therefore, in order to evaluate the pattern of expression of several members of the family of costimulatory molecules in this disease, we decided to use a cell line derived from the MM1.R multiple myeloma cell line (IgA multiple myeloma resistant to dexamethasone). The results showed a greater negative differential expression of costimulatory molecules and higher expression levels of B7-H3 and B7-H4.
Material and methodsCell cultureAn MM1.R myeloma (IgA) cell line in RPMI/10% FBS medium was cultured in CO2 atmosphere (5%). Once expanded, a volume amount equivalent to a cell concentration of 106 cells was taken, and they were placed in culture plates (6 wells) with a total volume of 3ml. After one day, the cells were collected and stored until use at −80°C.
Total RNA extractionTotal RNA from the MM1.R cell line was isolated by TRIzol® (Invitrogen, Life Technologies) according to the manufacturer's instructions. The concentration and purity of the total RNA was measured by ultraviolet-visible spectrophotometry (Thermo Scientific, GENESYS™ 10S UV-Vis). The integrity of genetic material was confirmed via agarose gel electrophoresis (1.5%) for 40′ at 70 v. The RNA was stored at −80°C until it was needed.
cDNA synthesis and real-time PCRcDNA synthesis was performed using 4μg of RNA, previously quantified by UV–Vis (Thermo Scientific, GENESYS™ 10S UV-Vis), 1μg of Oligo(dT) (Promega, Madison, WI, USA) and 200U of M-MLV (Promega, Madison, WI, USA).
The PCR assays were performed using a MultiGene™ OptiMax thermal cycler (Labnet). The constitutive genes GAPDH and β2 microglobulin were used as an internal reaction control. Included for the characterisation were genes belonging to the following costimulatory molecules: B7-1, B7-2, B7-H1, B7-H2, B7-H3, B7-H4 and B7-H6. Table 1 shows the primers and product length of each gene and the amplification conditions.
Amplification sequences and conditions used.
| Primer | Sequence | Product length | Amplification conditions |
|---|---|---|---|
| GAPDH forward | CGGGAAGCTTGTCATCAATGG | 221pb | 96°C – 45s 55°C – 45s 72°C – 45s 30 cycles |
| GAPDH reverse | CATGGTTCACACCCATGACG | ||
| β2-MG | CCTCCATGATGCTGCTTACATGTC | 221pb | 96°C – 45s 55°C – 45s 72°C – 45s 30 cycles |
| β2-MG | ATGTCTCGCTCCGTGGCCTTAGCT | ||
| B7-1 forward | CCTCTCCATTGTGATCCTGG | 566pb | 96°C – 1min 55°C – 45s 72°C – 1min 35 cycles |
| B7-1 reverse | GGCGTACACTTTCCCTTCTC | ||
| B7-2 forward | GACCTGCTCATCTATACAC | 464pb | 96°C – 1min 55°C – 45s 72°C – 1min 30 cycles |
| B7-2 reverse | CTTCATCAGATCTTTCAGG | ||
| B7-H1 forward | ACTGGCATTTGCTGAACGCA | 301pb | 96°C – 1min 57°C – 45s 72°C – 1min 35 cycles |
| B7-H1 reverse | TACACCCCTGCATCCTGCAAT | ||
| B7-H2 forward | GCAAACCAGTGAGTCGAAAACC | 102pb | 96°C – 1min 60°C – 45s 72°C – 1min 35 cycles |
| B7-H2 reverse | GGTGACATCAGGGCTCGGT | ||
| B7-H3 forward | AGCACTGTGGTTCTGCCTCACA | 172pb | 96°C – 1min 53°C – 45s 72°C – 1min 35 cycles |
| B7-H3 reverse | CACCAGCTGTTTGGTATCTGTCAG | ||
| B7-H4 forward | TTAGGCTTGGTCCATGAGTTCA | 143pb | 96°C – 1min 62°C – 45s 72°C – 1min 35 cycles |
| B7-H4 reverse | TCTGTGAGTTGCACGTTTTTCAG | ||
| B7-H6 forward | GACCCTGGGACTGTCTACCA | 143pb | 96°C – 1min 55°C – 45s 72°C – 1min 35 cycles |
| B7-H6 reverse | ATAGGCCACCAATGAATGGA |
The mRNA levels of the genes B7-2, B7-H1, B7-H3 and B7-H4 were measured using an assay with SYBR Green (Applied Biosystems, Foster City, CA, USA). GAPDH was used as a control gene, with all the samples analysed in triplicate in each reaction. The assay was also performed with a negative control in order to assess any contamination of the reagents. Relative levels of gene expression were calculated using the 2−ΔΔCT method, with a sample of normal bone marrow as a calibrator. The median was used as the cut-off between high and low expression.
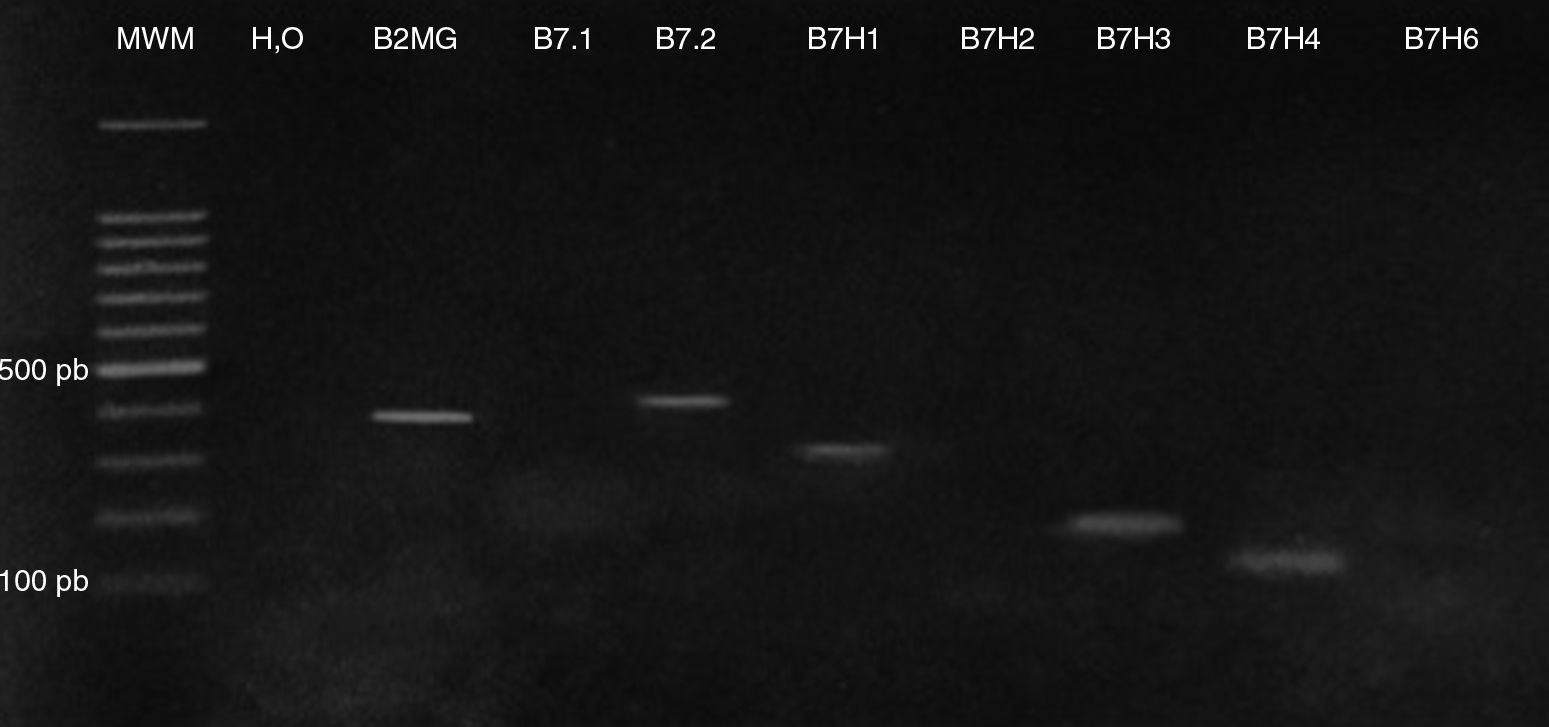
ResultsExpression of costimulatory molecules in cell line MM1.R through RT-PCRThe expression of genes that code for costimulatory molecules B7-1, B7-2, B7-H1, B7-H2, B7-H3, B7-H4 and B7-H6 in the multiple myeloma cell line MM1.R was analysed through endpoint RT-PCR. The results showed that there was only expression of one of the positive costimulatory genes (B7-2). Otherwise, 3 genes with inhibitory effects (B7-H1, B7-H3 and B7-H4) were expressed (Fig. 1).
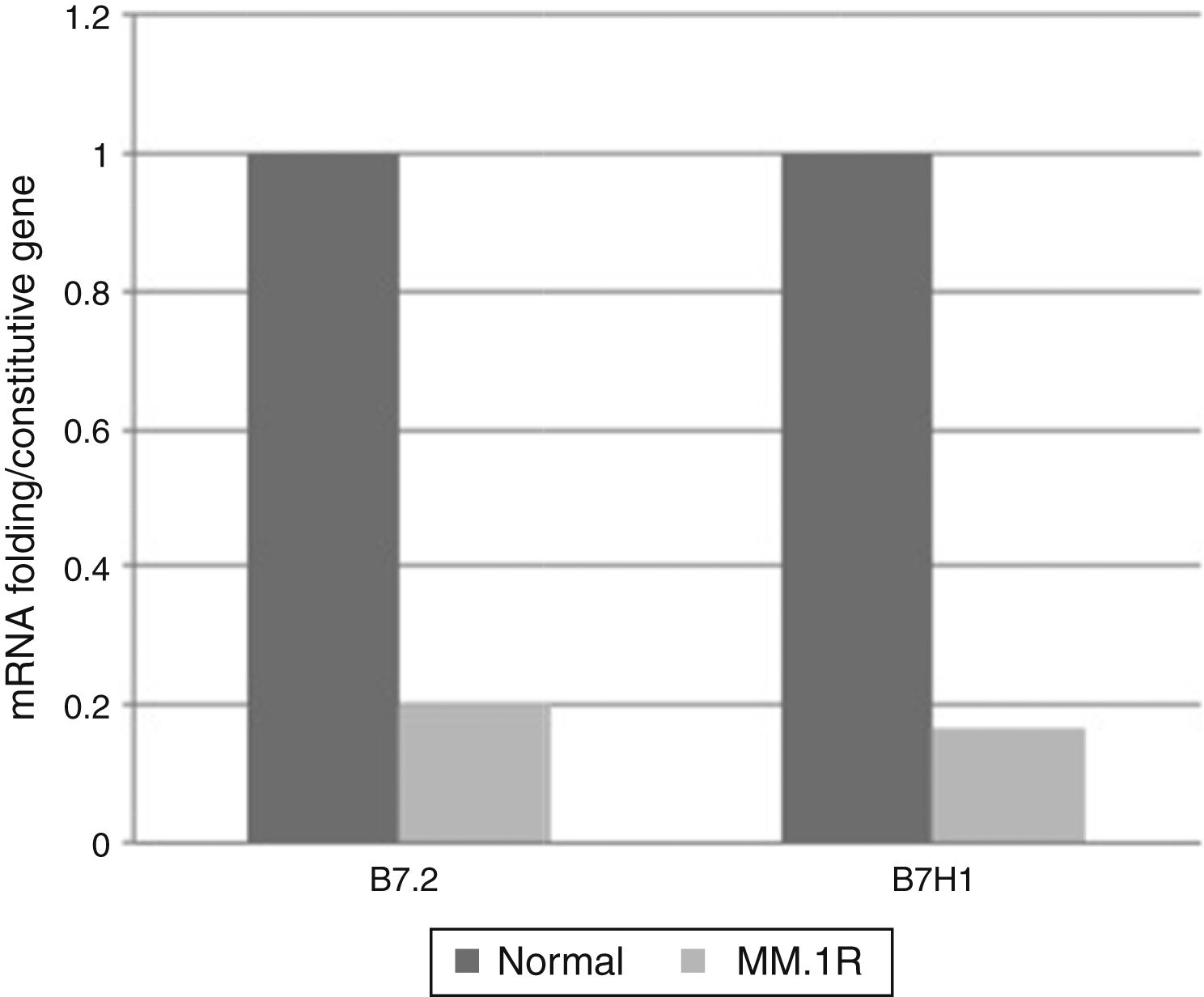
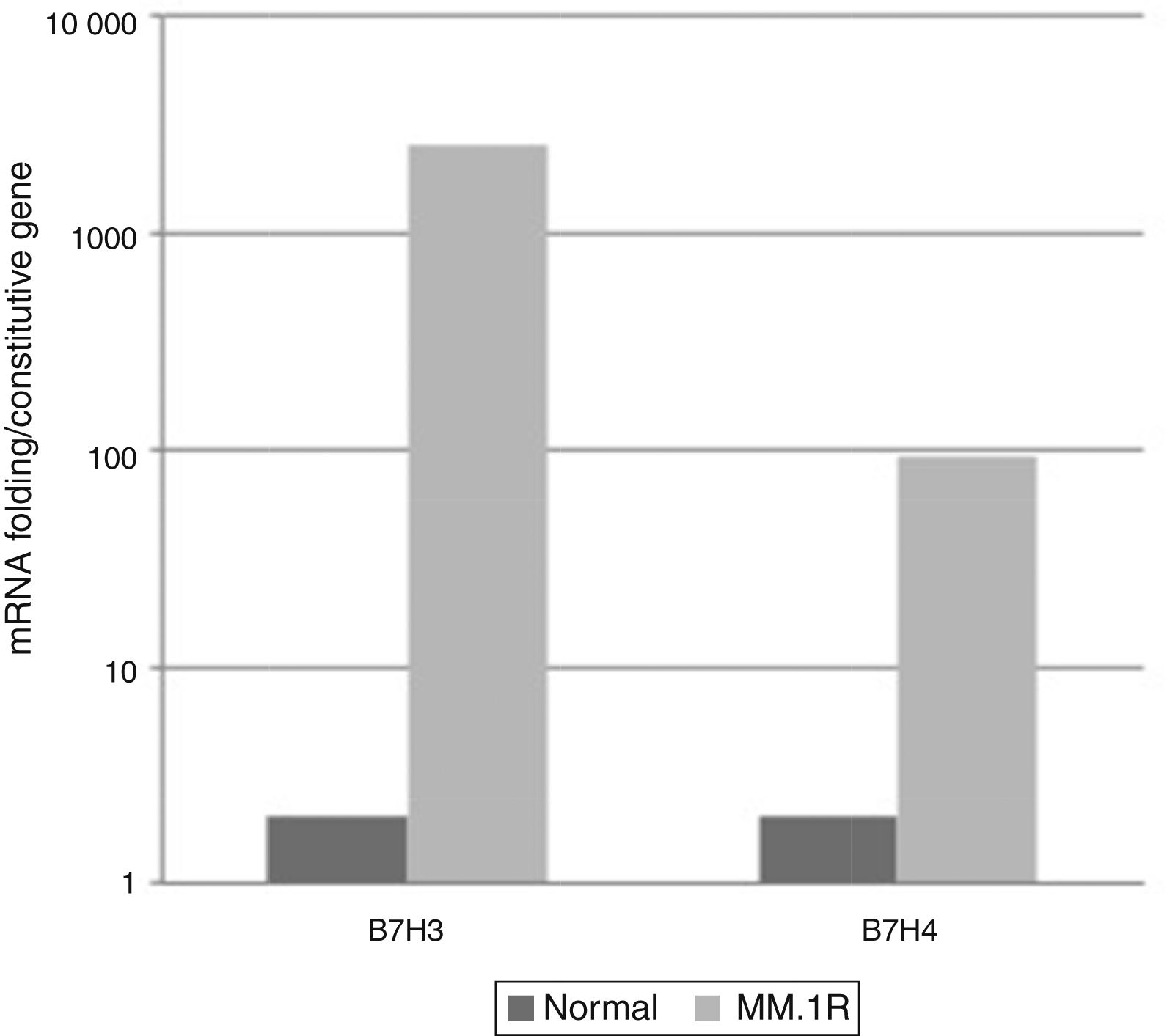
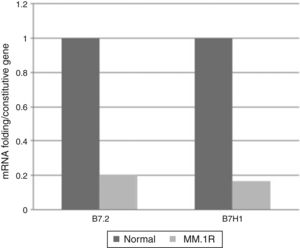
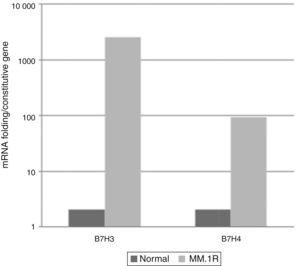
Levels of expression of costimulatory molecules in cell line MM1.R through quantitative RT-PCRFrom the pattern of expression previously analysed, the relative expression levels of genes B7-2, B7-H1, B7-H3, B7-H4 were analysed in cell line MM1.R by quantitative RT-PCR. The results showed reduced expression of costimulatory molecules B7-2 and B7-H1 and increased expression of B7-H3 and B7-H4 compared with the control (Graphs 1 and 2).
Costimulatory molecules are part of a system of secondary signalling mechanisms that are vital for maintaining the delicate balance between immunological potency and autoimmunity suppression.19 By interacting with ligands in the effector cells in immune responses, these transmembrane proteins trigger the stimulation or inhibition effects of this response. Tumour cells use deregulation of the expression of these molecules on their surfaces to carry out immune evasion, which involves the cell death of cytotoxic T cells and the recruitment of regulatory T cells.20–21
An analysis of the expression of these molecules in the cell line showed evidence that these cells have developed mechanisms of immune evasion, as with B7-H1 (PD-L1/CD273).22 The molecule B7-H1 is part of the most widely studied co-inhibitory pathway. In this way, the B7-H1/PD-1 pathway indirectly causes the death of effector cells and, therefore, a reduction in immune response.23,24
Another gene involved in generating immune inhibition mechanisms whose expression was confirmed in MM1.R cells was B7-H4 (B7x/B7S1/VTCN1). This is a co-inhibitory molecule whose interaction with its receptor decreases the proliferation and production of cytokines in CD4+ and CD8+ cells, as well as the expansion of neutrophil progenitors. Likewise, overexpression has been associated with different types of tumour, such as breast cancer, renal cancer, ovarian cancer and oesophageal carcinoma, amongst others.25–27 In multiple myeloma, this molecule has not been studied. However, as it is an important mechanism of immune evasion, it is highly likely to be expressed in this pathology. The discovery of this gene in cell line MM1.R concurs with what has been reported in other cancers, because, as mentioned above, the presence and overexpression of this gene has been associated with disease progression. Cell line MM1.R comes from an individual with an advanced stage of the disease (extramedullary); therefore, the finding of B7-H4 is a logical fact.
In addition to inhibitory B7-H1 and B7-H4 genes, two genes were found whose action differs depending on the receptor to which it binds. One of these two genes is B7-2 (CD86), which is part of the costimulatory/co-inhibitory pathway that is the most important in secondary signalling in mounting an immune response. The other gene identified in this assay was B7-H3, which codes for a type 1 transmembrane protein and which on a transcript level is widely expressed in lymphoid and non-lymphoid organs. However, its expression at the protein level is limited to recently activated monocytes, T cells, B cells and NK cells. High levels of expression are aberrant in this gene, and have been associated with conditions of cellular stress, such as cancer or sepsis.28–30 With regard to this molecule, both stimulatory and inhibitory functions have been reported, and to date, specific receptors for this ligand have not been conclusively identified.
The identification of these molecules helps to provide an overview of immune status in this disease. Similarly, these molecules represent alternative therapeutic molecular targets to traditional treatments, such as glucocorticoids, to which myeloma cells generate resistance during disease progression.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingResources facilitated by Hospital General de México's Research Department, registration number DIC/09/204/03/131, DIC/08/204/04/017.
Conflicts of interestThe authors declare that they have no conflicts of interest.