With ageing, changes occur that affect the quality and quantity of sleep. These changes could cause sleep disorders in older adults, causing severe consequences for health and quality of life. However, in Mexico there are no studies addressing the prevalence of sleep disorders in older adults.
ObjectiveTo determine the prevalence of sleep disorders, daytime sleepiness and clinical symptomatology in older adults seen at the UNAM Sleep Disorder Clinic in the General Hospital of México.
Materials and methodsA retrospective analysis of 191 medical records and 148 polysomnographic records from adults over 65 years old who were seen at the UNAM School of Medicine Sleep Disorder Clinic from 2009 to 2013 was performed.
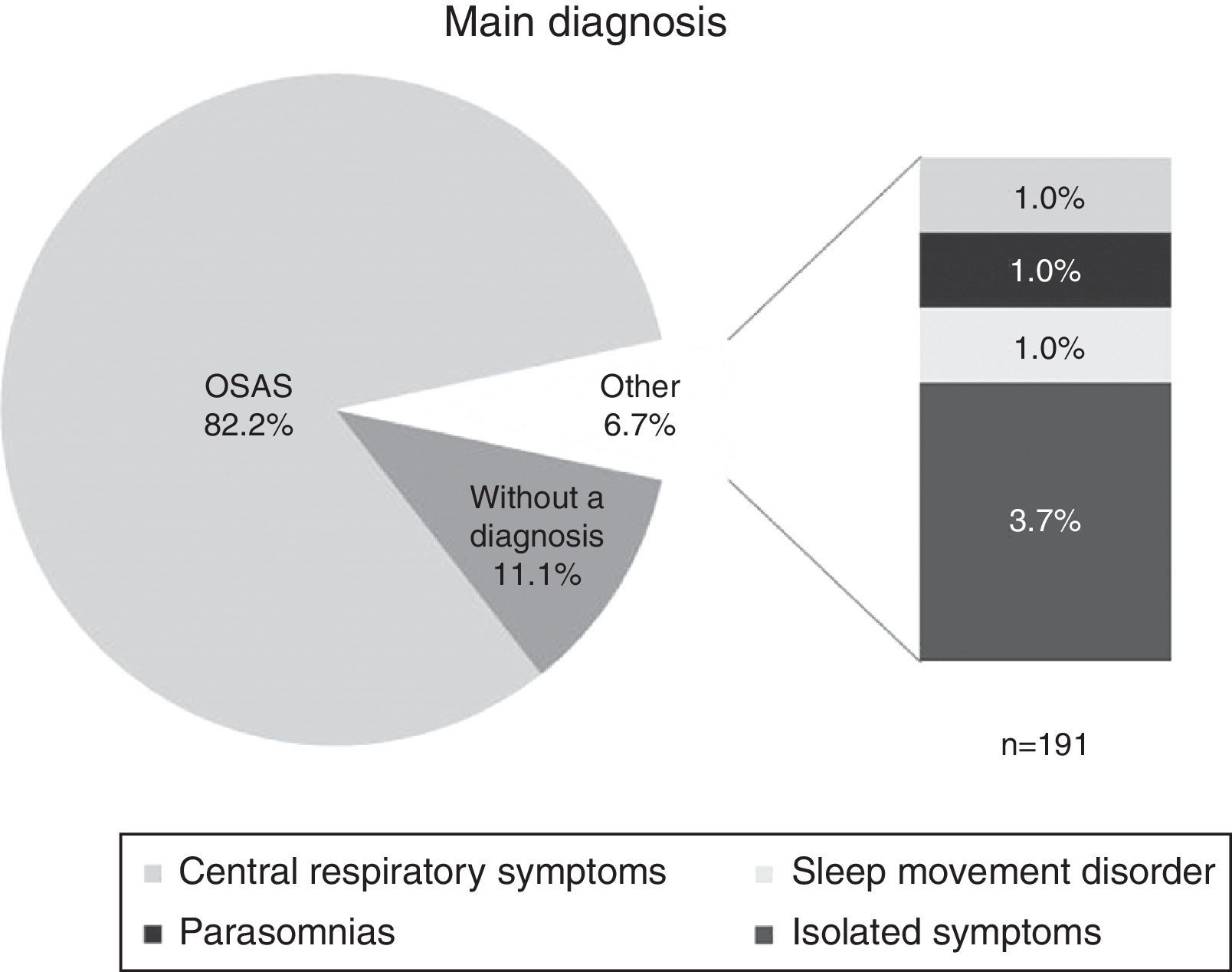
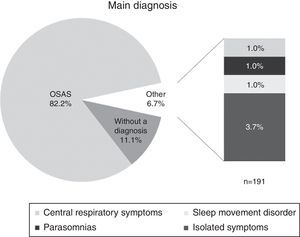
Results82.2% of patients were diagnosed with Obstructive Sleep Apnoea Syndrome (OSAS). The associated factors found were night-time awakenings (89%), medical comorbidities (84.5%), difficulty waking (70.7%), being overweight or obese (69.1%), among others. Of the total sleep time, they spent 14.2% in N1, 53.8% in N2, 16.1% in N3 and 15.4% in REM. Moreover, a REM sleep latency of 160min was found.
DiscussionCaring for the elderly is a challenge for healthcare systems. The study of sleep disorders is important because of its effects on health and quality of life, so understanding the clinical characteristics of this population will improve the diagnosis, management and referral of these patients.
Con el envejecimiento, ocurren cambios que afectan la calidad y cantidad de sueño, pudiendo generar trastornos de sueño en adultos mayores, los cuales tienen severas consecuencias para la salud y calidad de vida. Sin embargo, en México no existen estudios que aborden la prevalencia de trastornos de sueño en adultos mayores.
ObjetivoDeterminar la prevalencia de trastornos de sueño, somnolencia diurna y sintomatología clínica en adultos mayores que acuden a la Clínica de Trastornos de Sueño de la UNAM en el Hospital General de México.
Materiales y métodosSe realizó un análisis retrospectivo de 191 expedientes clínicos y 148 registros polisomnográficos de adultos mayores de 65 años que acudieron a la Clínica de Trastornos de Sueño, de la Facultad de Medicina de la UNAM del 2009 a 2013.
ResultadosEl 82.2% de los pacientes fueron diagnosticados con Síndrome de Apnea Obstructiva del Sueño (SAOS). Los factores asociados encontrados fueron despertares nocturnos (89%), comorbilidades médicas (84.5%), dificultad para despertar (70.7%), sobrepeso u obesidad (69.1%), entre otros. Del tiempo total de sueño, pasan 14.2% en N1, 53.8% en N2, 16.1% en N3 y 15.4% en MOR, además, se encontró una latencia a sueño MOR de 160min.
DiscusiónLa atención del adulto mayor es un reto para los sistemas de salud. El estudio de los trastornos de sueño es importante por los efectos que producen en la salud y calidad de vida, por lo que comprender las características clínicas de esta población permitirá mejorar el diagnóstico, manejo y referencia de estos pacientes.
Sleep is an active state during which biochemical, hormonal, and metabolic changes which are necessary for the body to work properly take place.1 As we age, changes occur that affect the quantity and quality of sleep, such as a decrease in the efficiency and total sleep time, increase in superficial sleep (stages 1 and 2 of NREM sleep),2 as well as a decreases in the slow-wave and REM sleep duration. Changes also occur in the density of the sleep spindles and sleep fragmentation.3
Physiologically, the age-related changes cause variations in the sleep–wake cycle,4 which is regulated by two basic mechanisms: the circadian cycles and homeostatic processes. The suprachiasmatic nucleus (SCN) of the anterior hypothalamus regulates important homeostatic rhythms such as the sleep–wake cycle and cortisol and melatonin levels, among others.5
Sleep architecture in the elderly includes a decrease in the amplitude of the sleep–wake circadian rhythm (phase advance) and a tendency for that rhythm to become desynchronised internally, which is associated with a decrease in body temperature and decreased melatonin production6 caused by the decrease in SCN neurons.7,8
During ageing, variations are seen in the sleep-related cholinergic and serotonergic pathways,9 causing the number of neurons in the amygdala, hippocampus, basal ganglia, locus coeruleus, and grey matter to decrease. In turn, the risk of cerebral bleeding, the number of interneuron connections, and reflexes are reduced.10,11 Likewise, the levels of acetylcholine secreted in the basal nucleus region, laterodorsal tegmental nucleus, and pedunculopontine nucleus in the brain stem and histamine in the mammillary tubercle of the hypothalamus degenerate, which in turn has repercussions on the sleep architecture.12
The changes in sleep architecture in the elderly are usually the result of an incapacity to maintain continuity of sleep.13 Medical conditions, stress, consumption of psychoactive substances such as alcohol and benzodiazepines, changes in routine, and a sedentary lifestyle, among many others, can result in sleep disorders.14,15
It has been described that sleep disorders in the elderly are common, along with self-medication with sleeping pills, which makes these cases difficult to detect.16 Sleep disorders in the elderly can have different origins, such as medical disorders (such as sleep apnoea), abnormal sleep–wake patterns (circadian disorders), decreased amount of sleep (insomnia), or poor sleep quality (sleep fragmentation or daytime sleepiness).17 These conditions have severe consequences for health, such as increasing the risk of being depressed, cardiovascular complications such as hypertension or coronary heart disease, and cognitive deterioration.18,19 They increase the risk of falling20 and are even associated with an increase in mortality.21 Lack of sleep is correlated with an increase in the risk of glucose intolerance, which in turn is a predisposing factor for diabetes.22 Excessive sleepiness can induce disorientation and delirium, and increase the risk of accidents and injuries,23,24 in addition to being correlated with less functional recovery during physical rehabilitation.25
Some authors have proposed that many of the age-related changes observed in sleep may be masked by conditions such as depression or undiagnosed sleep apnoea26 and that objective sleep measurements, such as polysomnography, enable more precise measurements of the changes in sleep architecture to be obtained, compared to clinimetric scales and self-reporting.27
Obstructive Sleep Apnoea Syndrome (OSAS) is a very common pathology among individuals over 30 with a prevalence of 2%–5% in the general population, although it is not clear if this prevalence increases or decreases with age.28,29 In Mexico City, it has been reported that the prevalence of OSAS is 7.7% in the general 18–65 year-old population.30 However, the prevalence of OSAS and other sleep disorders in the elderly is unknown. Therefore there is a special interest in knowing the prevalence and health profile of the elderly who have sleep disorders, since identifying the major associated problems and factors will enable primary care physicians to be able to make more precise diagnoses and provide a comprehensive treatment that improves the health and quality of life of elderly patients.
The objective of this study was to determine the prevalence of sleep disorders, daytime sleepiness and clinical symptomatology in elderly adults seen at the UNAM Sleep Disorder Clinic in the General Hospital of México.
Materials and methodExperimental designThis study is an observational, retrospective analysis of the medical records of the UNAM School of Medicine Sleep Disorder Clinic. The files corresponding to the years 2009–2013 were selected, according to the following inclusion criteria:
- a)
Patients over 65 years old.
- b)
Patients who were diagnosed by polysomnography.
Duplicate records, those corresponding to CPAP (Continuous Positive Airway Pressure) device titration, or those who were diagnosed by simplified respiratory testing were excluded. After applying these criteria, 191 medical records were selected.
Based on the registered records, a second analysis was performed on those who had access to the polysomnography report (n=148), to assess the sleep architecture, sleep efficiency, latency, heart rate, oxygen saturation, arousals, snoring, and apnoea indices.
ProcedureThe records were coded and registered in Microsoft Excel 2010 (version 14.0.7) and then a subsequent frequency analysis in SPSS (version 20.0) was performed. To analyse sleep architecture, the means and standard deviations of the sleep parameters generated by the polysomnography testing were obtained. While entering the medical records, no personal data was collected that would enable the patients to be identified, in accordance with the provisions of Mexican legislation regarding medical research.
ResultsBased on the 191 medical records, 23 were from 2009 (12%), 28 from 2010 (14.7%), 87 from 2011 (45.5%), 16 from 2012 (8.4%), and 37 from 2013 (19.4%). By calculating the frequencies of the variables in the records, it was found that 10.6% of the values were lost when completing the record by variable, ranging from 3.7% to 24.6%.
Regarding the sociodemographic variables of the study sample, 45.5% (n=87) of the patients were female and 54.5% (n=104) were male; 44.5% (n=85) were 60–69 years old, 51.8% (n=99) 70–79 years old, and only 3.7% (n=7) over 80 years old. As for their civil status, 61.8% (n=118) were married, 34.0% (n=65) were single, and 4.2% (n=8) did not respond. 33.0% (n=63) worked at home, 26.7% (n=51) were retired or receiving a pension, 17.8% (n=34) stated they were professionals, 15.2% (n=29) worked as an employee, and 1.6% (n=3) were unemployed at the time of the test, while 5.8% (n=11) did not respond.
The main diagnosis stated in the record is shown in Fig. 1. 82.2% of the patients were diagnosed with Obstructive Sleep Apnoea Syndrome (OSAS), 6.7% had other diagnoses (central respiratory symptoms, sleep movement disorders, or isolated symptoms), and 11.1% were not diagnosed. As for the severity of the main diagnosis, 6.8% were classified as mild, 15.8% as moderate, and 58.1% as severe, while 19.3% were not classified by severity.
The medical history and substances consumed by the patients are shown in Table 1. In the medical histories it was found that 49.8% presented three to four comorbidities and 35.6% presented one to two comorbidities. It is also striking that 40.8% had one or two treatments while 30.9% were not being treated at the time of the test. 38.2% of the elderly patients were overweight and 30.9% were obese, with a Body Mass Index (BMI) over 25.0kg/m2.
Medical history and substances consumed by elderly patients (n=191).
| n | % | ||
|---|---|---|---|
| Medical comorbiditiesa | |||
| 0 | 28 | (14.7) | |
| 1–2 | 68 | (35.6) | |
| 3–4 | 95 | (49.8) | |
| Medical treatmentsb | |||
| 0 | 59 | (30.9) | |
| 1–2 | 78 | (40.8) | |
| 3–5 | 54 | (28.3) | |
| Daily coffee consumption | |||
| Yes | 86 | (45.0) | |
| No | 90 | (47.1) | |
| Don’t know/No answer | 15 | (7.9) | |
| Daily cola consumption | |||
| Yes | 74 | (38.7) | |
| No | 92 | (48.2) | |
| Don’t know/No answer | 25 | (13.1) | |
| Alcohol consumption during the week | |||
| Yes | 20 | (10.5) | |
| No | 126 | (66.0) | |
| Don’t know/No answer | 45 | (23.5) | |
| Alcohol consumption on the weekends | |||
| Yes | 38 | (19.9) | |
| No | 128 | (67.0) | |
| Don’t know/No answer | 25 | (13.1) | |
| Daily smoking | |||
| Yes | 19 | (9.9) | |
| No | 144 | (75.4) | |
| Don’t know/No answer | 28 | (14.7) | |
| Drinks alcohol as hypnotic | |||
| Yes | 5 | (2.6) | |
| No | 164 | (75.4) | |
| Don’t know/No answer | 28 | (14.7) | |
| Uses hypnotic drugs | |||
| Yes | 62 | (32.5) | |
| No | 110 | (57.6) | |
| Don’t know/No answer | 19 | (9.9) | |
| BMI | |||
| Normal weight (BMI 18.5–24.9kg/m2) | 39 | (20.4) | |
| Overweight (BMI 25.0–29.9kg/m2) | 73 | (38.2) | |
| Obese (BMI over 30.0kg/m2) | 59 | (30.9) | |
| Don’t know/No answer | 20 | (10.5) | |
BMI – Body Mass Index.
As for the substances consumed, we see that 45.0% drank coffee daily and 38.7% cola, 32.5% used medications to be able to sleep, 19.9% drank alcohol on the weekends, 10.5% drank alcohol throughout the week, and 9.9% smoked tobacco daily.
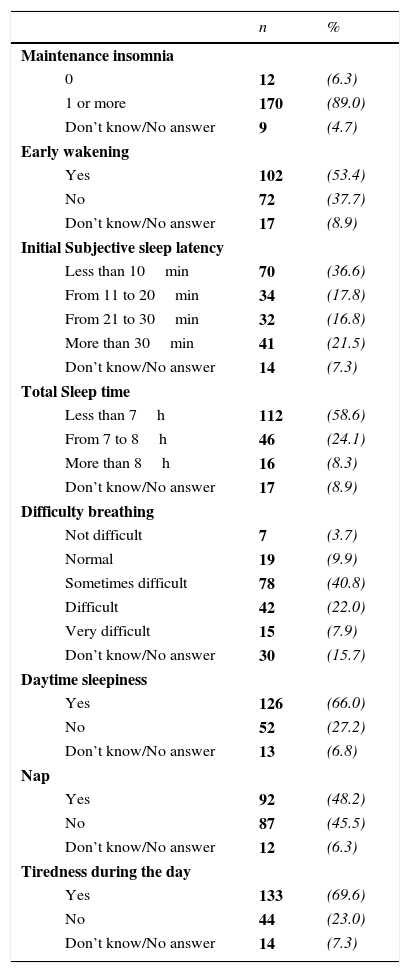
The subjective sleep quality and daytime functioning indicators are described in Table 2. It was found that 89.0% experienced maintenance insomnia, 53.4% woke very early and found it impossible to return to sleep, and 54.4% of the patients took more than 20min to fall asleep. By analysing the subjective sleep time, around 58.6% of the patients mentioned sleeping less than 7h; additionally 70.7% had difficulty waking up, 66.0% mentioned having daytime sleepiness, 48.2% took naps, and 69.6% reported feeling tired during the day. These last two symptoms are associated with daytime sleepiness.
Subjective indicators of sleep quality and daytime functioning reported by elderly patients (n=191).
| n | % | ||
|---|---|---|---|
| Maintenance insomnia | |||
| 0 | 12 | (6.3) | |
| 1 or more | 170 | (89.0) | |
| Don’t know/No answer | 9 | (4.7) | |
| Early wakening | |||
| Yes | 102 | (53.4) | |
| No | 72 | (37.7) | |
| Don’t know/No answer | 17 | (8.9) | |
| Initial Subjective sleep latency | |||
| Less than 10min | 70 | (36.6) | |
| From 11 to 20min | 34 | (17.8) | |
| From 21 to 30min | 32 | (16.8) | |
| More than 30min | 41 | (21.5) | |
| Don’t know/No answer | 14 | (7.3) | |
| Total Sleep time | |||
| Less than 7h | 112 | (58.6) | |
| From 7 to 8h | 46 | (24.1) | |
| More than 8h | 16 | (8.3) | |
| Don’t know/No answer | 17 | (8.9) | |
| Difficulty breathing | |||
| Not difficult | 7 | (3.7) | |
| Normal | 19 | (9.9) | |
| Sometimes difficult | 78 | (40.8) | |
| Difficult | 42 | (22.0) | |
| Very difficult | 15 | (7.9) | |
| Don’t know/No answer | 30 | (15.7) | |
| Daytime sleepiness | |||
| Yes | 126 | (66.0) | |
| No | 52 | (27.2) | |
| Don’t know/No answer | 13 | (6.8) | |
| Nap | |||
| Yes | 92 | (48.2) | |
| No | 87 | (45.5) | |
| Don’t know/No answer | 12 | (6.3) | |
| Tiredness during the day | |||
| Yes | 133 | (69.6) | |
| No | 44 | (23.0) | |
| Don’t know/No answer | 14 | (7.3) | |
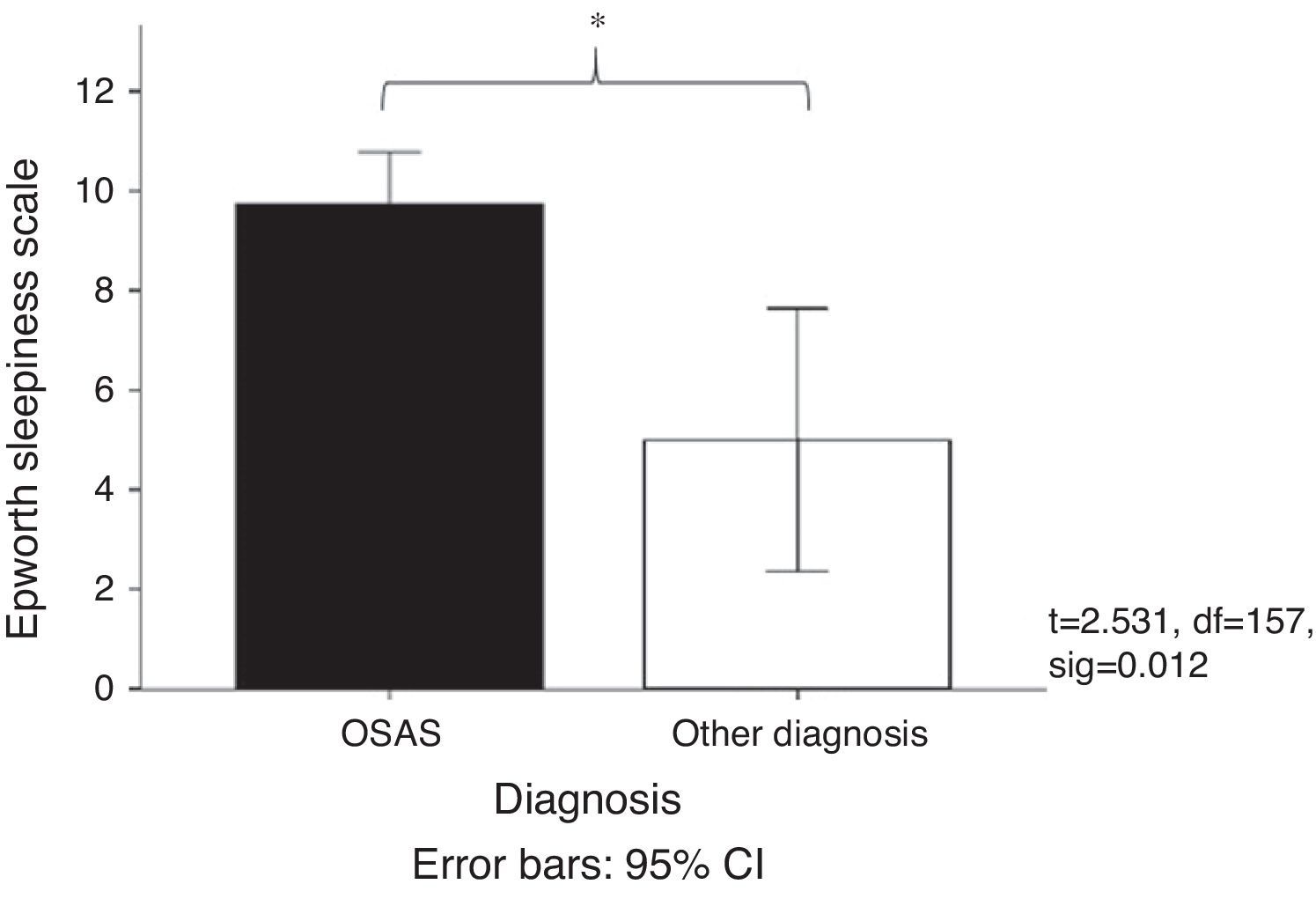
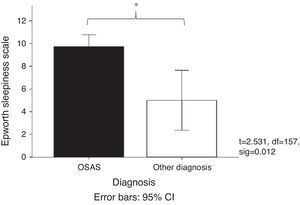
Fig. 2 shows the mean score obtained on the Epworth Sleepiness Scale31 in patients with OSAS (mean=9.47) compared with patients grouped into other diagnoses (mean=5). It was observed that the patients diagnosed with OSAS had a higher sleepiness score. This result coincides with the daytime functioning measurements, since it was observed that 56.5% of the elderly adults took a nap during the day while 29.3% took two or more naps during the day. Regarding the duration of the nap, 34.8% took naps lasting less than 20min, 33.4% took naps longer than 30min, and 16.3% took naps lasting 20–30min.
Table 3 presents the sleep-related symptomatology reported by the elderly adults seen between 2009 and 2013. Sleep disordered breathing (58.6%), waking with a feeling of drowning (46.1%), as well as snoring (63.9%), and waking with dry mouth (62.8%) are the most common symptoms in this group; 83.8% also reported nocturia, 31.9% reported waking with headache, and 25.7% reported involuntary movement of the extremities (legs or arms), and 39.3% reported having one or more family members with some sleep disorder.
Sleep-related symptomatology reported by elderly patients (n=191).
| n | % | ||
|---|---|---|---|
| Sleep disordered breathing | |||
| Yes | 112 | (58.6) | |
| No | 60 | (31.4) | |
| Don’t know/No answer | 19 | (9.9) | |
| Waking with feeling of drowning | |||
| Yes | 88 | (46.1) | |
| No | 85 | (44.5) | |
| Don’t know/No answer | 18 | (9.4) | |
| Snoring interrupted by silence | |||
| Yes | 122 | (63.9) | |
| No | 49 | (25.7) | |
| Don’t know/No answer | 20 | (10.5) | |
| Dry mouth upon awakening | |||
| Yes | 120 | (62.8) | |
| No | 53 | (27.7) | |
| Don’t know/No answer | 18 | (9.4) | |
| Headache upon awakening | |||
| Yes | 61 | (31.9) | |
| No | 112 | (58.6) | |
| Don’t know/No answer | 18 | (9.4) | |
| Nocturia | |||
| Yes | 160 | (83.8) | |
| No | 18 | (9.4) | |
| Don’t know/No answer | 18 | (6.8) | |
| Restless Legs Syndrome | |||
| Yes | 49 | (25.7) | |
| No | 114 | (59.7) | |
| Don’t know/No answer | 28 | (14.7) | |
| Periodic extremity movements symptoms | |||
| Yes | 46 | (24.1) | |
| No | 115 | (60.2) | |
| Don’t know/No answer | 30 | (15.7) | |
| Family members with sleep disorders | |||
| Yes | 75 | (39.3) | |
| No | 69 | (36.1) | |
| Don’t know/No answer | 47 | (24.6) | |
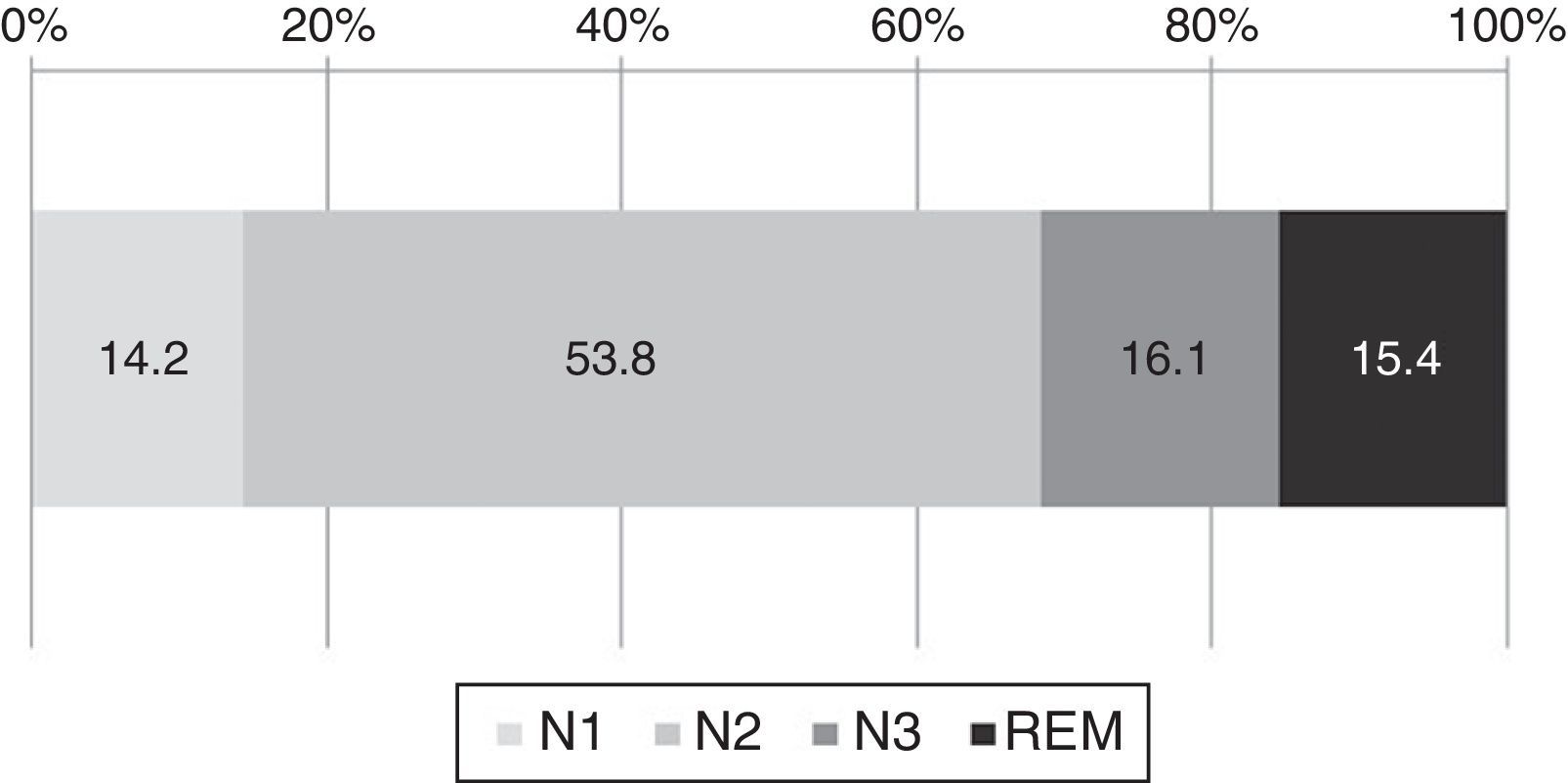
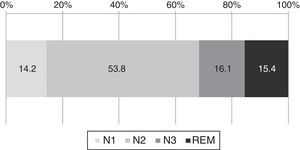
From the 191 medical records, 148 reports had polysomnography. The analysis of those files showed that on average the elderly adults spent 27% of the time in night-time wakefulness. The sleep architecture is shown in Fig. 3. Of the total time asleep, on average 14.2% was spent in N1, 53.8% in N2, 16.1% in N3, and 15.4% in REM.
The sleep latency onset was 32min, and the REM latency was 160min. The awake heart rate was 73.9 beats per minute (bpm), higher than during sleep (64.5 and 65.5bpm in REM and NREM, respectively). The mean oxygen saturation remained relatively stable during sleep, taking values of 88.5% while awake, 87.9% in NREM, and 88.6% in REM sleep, although the lowest saturation recorded was 71.0%. Mean snoring was 240 events (ranging from 0 to 1411) and arousals was 66 (ranging from 0 to 307). The apnoea index (AI) was 15.7 and the apnoea-hypopnoea index (AHI) was 44.8.
DiscussionCaring for the elderly has become a challenge for the Mexican healthcare system. 98% of elderly adults who are seen in the geriatrics department have at least one chronic disease and 99.5% some geriatric syndrome. The most common are a risk of falling, gait problems, and polypharmacy.32 This is similar to what was observed in our study, where 85.4% of the elderly adults presented 1–4 comorbidities. However, the population cared for in the UNAM Sleep Disorder Clinic also had sleep-related symptomatology.
Sleep medicine has gained importance due to the effects they cause on health and quality of life, and vice versa.13 In this study, we found that the most prevalent sleep disorder was OSAS. As this is a referral clinic, more than half of the patients were diagnosed with severe OSAS.
An important theme regarding elderly adults is polypharmacy, since 69.1% of the sample was taking 1–5 concurrent pharmacological treatments, which could affect the sleep-related symptomatology, depending on the involved drug. Furthermore, the elderly adults reported consuming coffee (45.0%) and cola (38.7%) daily. It has been described that consuming caffeine, present in both drinks, causes a stimulant effect that is directly involved in sleep disorders.33
It is well known that obesity is another risk factor for OSAS34 and we found that 30% of the elderly adults were obese. Therefore comprehensive weight management in primary care becomes necessary. A correlation between obesity, total sleep time, and mood disorders (depression and anxiety) have also been reported in OSAS patients.35
As previously mentioned, 85.4% of the patients had one or more medical comorbidities at the time of testing, and a correlation between OSAS and liver problems,36 changes in blood glucose levels,37 cerebrovascular events,38 and cardiovascular diseases,39 among others, have been documented. In our sample we found a decrease of around 10bpm in the heart rate during sleep, although they were within normal physiological values.40
It has been observed that the elderly adults who went to the clinic reported several sleep-related symptomatologies as the reason for the consultation, such as fragmented sleep, problems falling or remaining asleep, daytime sleepiness, as well as trouble waking up. Napping was present in half of the patients assessed, similar to what is described in the international literature.41 Among the most common symptoms reported are respiratory problems, snoring, feeling of drowning, and dry mouth upon waking, which are characteristics of OSAS.
Regarding the polysomnography assessment, we found that our sample of elderly adults spent an average of 14.2% of the total sleep time in S1, 53.8% in S2, 16.1% in S3, and 15.4% in REM. This data contrasts with what is described for this population3 where it has been reported that healthy adults spend 6.5% in N1, 55% in N2, 9% in N3, and 19% in REM, which indicates that in our sample there is an increase in phases S1 and S3 as well as a decrease in REM sleep, versus the standard mean value (Fig. 3).
REM sleep latency is very important, since changes in REM sleep latency are considered potential biomarkers for a large number of sleep disorders42 and even mood disorders.43 In young adults, it has been described that REM sleep latency typically occurs 90min after the start of sleep, with it decreasing as they age.2 It has also been reported that during acute depressive episodes REM latency shortens.43 Nevertheless, in our study the mean REM sleep latency (160min) is higher than that reported in the literature for this population, a significant piece of data which requires to be examined in depth in future studies. Therefore we are currently conducting studies to analyze the significance of this increase in REM latency in the population of healthy adults and those with OSAS.
By the clinical characteristics of the study population, the elderly have a higher risk of OSAS-related cardiopulmonary complications. 58% reported breathing problems, 63.9% snoring interrupted by silence, and 66% daytime sleepiness. This last point was confirmed by the Epworth Sleepiness Scale, with a score of 9.45 for the patients diagnosed with OSAS while the polysomnography assessment showed a minimum oxygen saturation of 71%, and apnoea/hypopnoea index of 44.8.
A physiological mechanism has been proposed to explain the relationship between OSAS and cardiopulmonary complications,44,45 in which the decreased oxygen concentration increases the activity of the autonomic nervous system, leading to an increase in blood pressure and a decrease in blood supply to the heart, which increases the cardiac workload which could cause ventricular arrhythmia and heart failure. At the same time, insufficient oxygen increases blood pH causing irritation to the pulmonary parenchyma and vasoconstriction, which generates pulmonary oedema and respiratory failure. Based on the above, it is important to identify and assess elderly adults with symptomatology compatible with OSAS, to come to a suitable and specific diagnosis to be able to offer therapeutic alternatives such as CPAP.29
One limitation of this study was the amount of data lost for some variables. Since it was a retrospective study, the loss of some data is expected. Some authors suggest that a greater than 10% loss of data may significantly skewed the analysed data46 and in this study the mean was 10.6%, so we consider that the data presented are representative of the study population.
Another limitation of this study was that by being conducted in a referral clinic, the medical records reviewed were from elderly adults who had sleep disorders, but it would advisable to conduct a study focused on the natural changes in the sleep of the elderly and their physiology.
We can conclude that OSAS is the sleep disorder with the highest prevalence in the elderly adults seen in this sleep clinic (82.2%). Health problems are common in the age group, and this emphasised the need for comprehensive healthcare in the elderly. The prevalence of symptoms and sleep problems was also significant, therefore it is necessary for primary care centers to receive training to be able to diagnose and refer patients to specialised sleep medicine clinicals, which will improve their health and quality of life.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThere was no source of funding.
Conflicts of interestAll the authors declare that they have no conflicts of interest.