Nowadays acute postoperative pain persists as a high prevalence symptom. The incidence, intensity and duration of postoperative pain vary considerably from one patient to another, from one surgery to another, from one hospital to another, and even from one country to another. It is important to learn about recent developments in central sensitisation, as it plays an important role in postoperative pain. Postoperative pain is mainly nociceptive somatic, in response to surgical damage. The surgical trauma and pain cause an endocrine response that increases the secretion of cortisol, catecholamines, and other stress hormones. Tachycardia, hypertension, decreased regional blood flow, impaired immune response, hyperglycaemia, lipolysis, and negative nitrogen balance also occur. All this plays an important role in morbidity and mortality in the postoperative period. Buprenorphine is a semi-synthetic opioid derived from thebaine. It has a binding affinity for the mu, kappa and delta receptors, and has a slow dissociation from these receptors. Because of its action on the mu and kappa receptors it can be used as an analgesic, as well as for maintenance therapy in patients with a history of drug abuse. This article will describe the characteristics of acute postoperative pain, the pharmacology of buprenorphine, and its interference in the management of postoperative pain.
En la actualidad el dolor agudo postoperatorio persiste como un síntoma de elevada prevalencia. La incidencia, intensidad y duración del dolor postoperatorio varían considerablemente de uno a otro paciente, de una a otra intervención quirúrgica, de uno otro hospital e incluso de un país a otro. Es importante conocer acerca de los recientes avances en la sensibilización central, ya que juega un papel importante en el dolor postquirúrgico. El dolor postoperatorio es principalmente nociceptivo somático, respuesta a la agresión quirúrgica. El trauma quirúrgico y el dolor causan una respuesta endocrina que incrementa la secreción de cortisol, catecolaminas y otras hormonas del estrés. También se produce taquicardia, hipertensión, disminución del flujo sanguíneo regional, alteraciones de la respuesta inmune, hiperglicemia, lipólisis y balance nitrogenado negativo. Todo esto juega un importante papel en la morbimortalidad en el periodo postoperatorio. La buprenorfina es un opioide semisintético derivado de la tebaina. Se vincula con afinidad hacia los receptores mu, kappa y delta, y tiene una lenta disociación sobre esos receptores por su acción en los receptores mu y kappa se puede usar como analgésico, asi como el mantenimiento y terapia de pacientes con historia de abuso de drogas. Este articulo describirá las características del dolor agudo postoperatorio, la farmacología de la buprenorfina y la injerencia de ésta en el manejo algológico de dolor postquirúrgico.
The most complete definition of pain is the one backed by the IASP (International Association for the Study of Pain), which says: “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Merksey & Bogduk, 1994).1
Specifically, postoperative pain is that caused after surgery; characterised by diverse unpleasant sensory, emotional, and mental experiences associated with autonomic, endocrine-metabolic, physiological, and behavioural responses.2,3
According to the American Society of Anesthesiologists (ASA), acute surgery-related pain is pain that is present in the surgical patient after a procedure.4
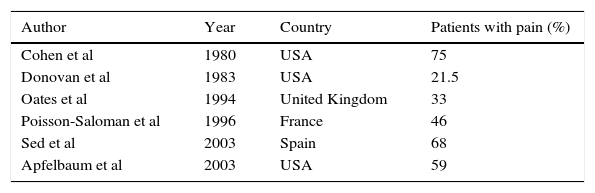
Epidemiology of postoperative painNowadays acute postoperative pain persists with a high prevalence. Despite advances in regional techniques, drugs, and studies conducted by specialised groups to decrease the incidence of pain in the postoperative period, in both developed and developing countries postoperative pain is reported at rates exceeding 70%, although they vary considerably from one patient to another, from one surgery to another, from one hospital to another, and even from one country to another. Cadavid and Chaustre4 indicate that the incidence of moderate to severe pain in the postoperative period is between 8.4% and 47.0%. Some studies that demonstrate the prevalence of pain are mentioned in Table 1.
Prevalence of acute postoperative pain. Modified from: Zaragoza F et al. Dolor posoperatorio. Madrid 2005.
| Author | Year | Country | Patients with pain (%) |
|---|---|---|---|
| Cohen et al | 1980 | USA | 75 |
| Donovan et al | 1983 | USA | 21.5 |
| Oates et al | 1994 | United Kingdom | 33 |
| Poisson-Saloman et al | 1996 | France | 46 |
| Sed et al | 2003 | Spain | 68 |
| Apfelbaum et al | 2003 | USA | 59 |
It has been described that during the postoperative period after intrathoracic, upper abdominal, and to a lesser extent, kidney surgery, movements that put pressure on the incision (deep breathing, cough, and body movement) aggravate the intensity of the pain.5
The type of incision also has a large influence, and it has been demonstrated that a transverse abdominal incision damages the nerves less, which causes less pain.
Pathophysiology of postoperative painIt is important to learn about central sensitisation as it plays an important role in postoperative pain, which is primarily nociceptive somatic as the result of surgical damage.6
After the damage caused to the nociceptive receptors during the surgery, a hyperalgesic state occurs. This is divided into primary hyperalgesia, resulting from the sensitisation of the peripheral nociceptors, and secondary hyperalgesia, which is associated with sensitisation of the spinal cord and the central nervous system.7,8
After nociceptive stimuli, primary mediators such as prostaglandins, leukotrienes, serotonin, and bradykinins are released. These primary mediators stimulate the release of peptides such as calcitonin gene-related peptide (CGRP) and substance P at the site of injury. Vasodilation is induced by histamine, the release of nerve growth factor, and the sympathetic effect reflex release of norepinephrine, known as “inflammatory soup”.8
The peripheral nociceptor impulses travel through delta and C synapse fibres in laminae II and V in the spinal cord. The C fibres also make synapses in spinal cord lamina I, known as second order neurons.8
There are two types of second order neurons in the spinal cord: the first, in lamina I, respond to impulses in the C fibres; the second, located in lamina V, respond to both harmful stimuli, mainly from Ad fibres, and non-harmful stimuli. Neurotransmitters such as glutamate and aspartate present in lamina V cause fast synaptic transmission. This happens by binding to and activating kainate receptors (KAR), receptors regulating Na+ and K+, the influx of ions, and amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Both AMPA and KAR are nearly impermeable to Ca+ ions. Once the AMPA and KAR receptors are activated, NMDA priming begins.9
The N-methyl-d-aspartate (NMDA) receptors are located postsynaptically in the posterior horn of the spinal cord. They are responsible for mediating the reaction caused by polysynaptic discharge of the primary nociceptive afferent fibres. Activating these NMDA receptors is related with transmission in nociceptive afferent fibres, possibly A-delta and C fibres.9
The NMDA receptors are associated with learning and memory, neural development and plasticity, as well as states of acute and chronic pain. They intervene in initiating and maintaining central sensitisation, associated with damage or inflammation to peripheral tissues.9
As for modulation, endogenous and exogenous opioids can act on the presynaptic terminals of primary afferent nociceptors via the mu opioid receptor which indirectly blocks the calcium channels and opens the potassium channels. Inhibiting calcium from entering the presynaptic terminals and releasing potassium results in hyperpolarisation and inhibits the release of pain neurotransmitters, and therefore in analgesia.10
Activation of the cortical descending neural pathways involves releasing neurotransmitters: beta-endorphins, enkephalins, dynorphins. These peptides modulate pain, even in stressful situations.10
The activation of the descending pathways by endorphins takes place through specific receptors: opioids. This system is activated around the periaqueductal grey matter of the midbrain. These neurons are projected to the medullary reticular formation and the locus coeruleus where serotonin and norepinephrine are produced, respectively. The descending fibres are then projected to the dorsolateral funiculus of the spinal cord dorsal horn for synapsis with the primary afferent neuron.11
The descending pain modulating neurons play the role of releasing neurotransmitters in the spinal cord, such as serotonin and norepinephrine. They activate interneurons that release opioids in the spinal dorsal horn. Releasing serotonin and norepinephrine inhibits the release of pain transmitters in the nociceptive afferent signals and inhibits cellular second order pain transmission. Administering opioids activates the opioid receptors in the midbrain. Moreover, activating the opioid receptors in the second order pain transmission cells prevents ascending transmission of the pain signal, activating the opioid receptors in the C fibre central terminals in the spinal cord prevents the release of pain neurotransmitters, and activating the peripheral opioid receptors inhibits the activation of nociceptors and inhibits the cells that release inflammatory mediators.11
Predictors of postoperative painThe predictive factors of postoperative pain resulting from various surgical procedures are mainly preoperative pain, anxiety, age, and the type of surgery (abdominal, orthopaedic, thoracic, long duration).12 Obesity and gender are inconsistent factors, that is, no consensus has been reach in studies as to whether or not they are predisposing factors.
Consequences of postoperative painSurgical trauma and pain cause an endocrine response that increases the secretion of cortisol, catecholamines, and other stress hormones, causing tachycardia, hypertension, decreased regional blood flow, impaired immune response, hyperglycaemia, lipolysis, and negative nitrogen balance. All this plays an important role in morbidity and mortality in the postoperative period.13
We divide the responses to postoperative pain by system:
Respiratory systemThoracic and upper abdominal injuries or surgical incisions cause changes in the respiratory system. Inadequate postoperative pain control decreases the flow volume, increases the respiratory rate, changes lung function, decreases vital capacity, or reduces the functional residual reserve volume, which translates clinically to an increase in the frequency of atelectasis and accumulation of bronchial secretions, promoting the onset of hypoxaemia, pneumonia, and respiratory failure.
There are multiple publications that demonstrate that combined anaesthesia, along with minimally invasive surgical techniques, adequate pain treatment, and early respiratory physiotherapy, is essential for significantly decreasing pulmonary complications in the immediate postoperative period.14
Cardiovascular systemPain increases the heart rate, blood pressure, and myocardial contractility, therefore the myocardial demand for oxygen increases. Adequate postoperative pain treatment decreases the activity of the sympathetic system, myocardial ischaemia, and haemodynamic instability.14
Furthermore, adequate postoperative analgesia allows the patient to become ambulatory more quickly which decreases the risk of vein thrombosis.
Digestive systemSympathetic hyperactivity causes ileus and increases the incidence of nausea, vomiting, and intestinal secretions.15
Endocrine-metabolic systemThe hormone release induced by surgical damage happens as a consequence of stimulating the central and peripheral autonomic nervous system, which induces the release of catabolic hormones (cortisol, glucagon) and catecholamines, and inhibits anabolic hormones (insulin, testosterone). The injured tissues contribute to the humoral response by releasing multiple mediators, such as cytokines, leukotrienes, prostaglandins, nitric oxide, endotoxins, etc. The clinical effect that occurs is hypermetabolism with proteolysis and hyperglycaemia, hypernatraemia, hypokalaemia, and hypomagnesaemia, which can increase the release of antidiuretic hormone (ADH) and aldosterone.16
Immune and infectious functionThe immune function is altered after major surgery, which involves several factors such as neuroendocrine response, hypothermia, and blood transfusion. The clinical consequences of these immunological changes are an increase in susceptibility to complications from infection.16
Although postoperative morbidity has fallen in recent decades, major surgery continues to be associated with cardiac, respiratory, thromboembolic, intestinal, infectious, and neuroendocrine alterations. Efforts have been aimed at improving each of these specific prognosis variables (preoperative review, antithrombotic and antimicrobial prophylaxis, etc.).
Pain assessmentTo be able to treat acute postoperative pain, it must be objectively assessed. For this, scales and methods are available, which include, among others17:
- •
Visual Analogue Scale (VAS)
- •
Numeric rating scale
- •
McGill Questionnaire
The “Visual Analogue Scale” (VAS), designed by Scott Huskinson in 1976, is the most frequently used measurement method to evaluate pain in many centres. It consists of a 10cm line that represents the continuous spectrum of the pain experience. The line can be vertical or horizontal and it ends in a right angle at its ends. Descriptions only appear at the ends: “no pain” at one end and “the worst pain imaginable” at the other, with no descriptions along the line. Its main advantage comes from the fact that it does not contain numbers or descriptive words. Patients are not asked to describe their pain with specific words. Instead they are free to indicate the intensity of their pain along a continuous line in relation to its two ends. The VAS is a simple, solid, sensitive, validated, and reproducible instrument that is useful for reassessing pain in the same patient on different occasions.18,19
VAS 1–3: Can be satisfactorily treated without opioid analgesics using non-steroidal anti-inflammatory drugs (NSAIDs).20–22
VAS 4–6: Can be treated with weak opioid analgesics with a ceiling effect (tramadol, buprenorphine, nalbuphine), either in bolus or in continuous infusion; these analgesics can also be used in combination with NSAIDs, or, if necessary, adjuvant drugs such as ketamine can be used concomitantly.20–22
VAS 7–10: the intense pain can be managed with powerful opioids (morphine and fentanyl citrate), either as a continuous infusion, with patient-controlled analgesia techniques, or regional anaesthesia techniques. Similarly, if necessary they can be used in combination with NSAIDs or adjuvant drugs.20–22
History of opioidsThe first-line treatment for postoperative pain is opioids. These compounds are known from the curative preparations obtained from the Papaver somniferum poppy plant, from which opium is extracted, and they have been used for centuries to alleviate pain. Poppy cultivation has been documented since Mesopotamia (3400 BC). The Sumerians referred to it as “the plant of happiness” (Hul Gil). Poppy cultivation passed from the Sumerians to the Assyrians, it continued with the Babylonians, and finally knowledge of it reached the Egyptians. In Greece, Hippocrates (460 BC), the “father of medicine”, recognised its use as a narcotic and recommended it be used to treat diseases in women and during epidemics. By the year 1020 Avicenna considered it as the most powerful of the narcotics and, around 1500, the Portuguese introduced the habit of smoking opium to Europe. By the early 17th century, the ships of Elizabeth I were transporting opium to England and by the middle of that century the East India Company of England took over control of opium production in India, assuming a monopoly over the opium market in 1793. Linnaeus (1707–1778) classified the poppy as Papaver somniferum – sleep inducer – in his book Genera Plantarum (1737).
In 1803, the German pharmacologist W. Sertürner (1783–1841) purified morphine, which is the main alkaloid constituent of the opium extracts and is responsible for causing its analgesic effect. He called it morphine in honour of Morpheus, the god of sleep. In 1827, E. Merck & Company, from Darmstadt in Germany, started its production and marketing. In 1874, the London chemist Alder Wright discovered heroin, which was synthesised and marketed as a treatment for several pulmonary illnesses by the company Bayer in 1897. Although already known and used, it was not until 1925 that Gulland and Robinson demonstrated the chemical structure of morphine. Since then, several different chemical substances derived from morphine have been produced and endogenous opioids began to be discovered, as well as compounds able to antagonise its activity, opening one of the major fields of interest of contemporary neuroscience.
Classification of opioidsOpioids can be classified as natural, semi-synthetic, and synthetic.23 Natural opioids can be divided into two chemical classes: phenanthrenes (morphine and codeine) and benzylisoquinolines (papaverine). Semi-synthetic opioids are derived from morphine, to which numerous changes have been made. Synthetic opioids are classified into four groups: morphine derivatives (levorphanol), biphenyl or methadone derivatives (methadone, pentazocine), benzomorphans (phenazocine and pentazocine), and phenylpiperidine derivatives (meperidine, fentanyl, alfentanil, sufentanil, and remifentanil).24
Characteristics of buprenorphineSynthesised in 1969 by K.W. Bentley, for the treatment of pain, it is classified in the oripavines group. It is a semi-synthetic opioid analgesic derived from thebaine. It has a binding affinity for the mu, kappa, and delta receptors, and has a slow dissociation from these receptors. Although it binds to the mu receptor with a high affinity, the intrinsic binding capacity is lower compared with a complete mu agonist.24 In comparison with morphine, its potency is around 20- to 30-fold with a higher liposolubility25; which, in addition to giving it a higher analgesic potency, makes it an attractive molecule to apply to different routes: intravenous, sublingual, transdermal, etc.22 It can nearly maximally occupy the mu receptor; therefore it decreases the bioavailability of the mu receptor, which is useful for decreasing withdrawal symptoms.23,26 The onset of action depends on the route of administration; from 5 to 10min intravenously, 10 to 20min intramuscularly, and 15 to 45min sublingually. Buprenorphine's duration of action is from 6 to 8h,27 and it has a mean half-life of 4 to 5h.
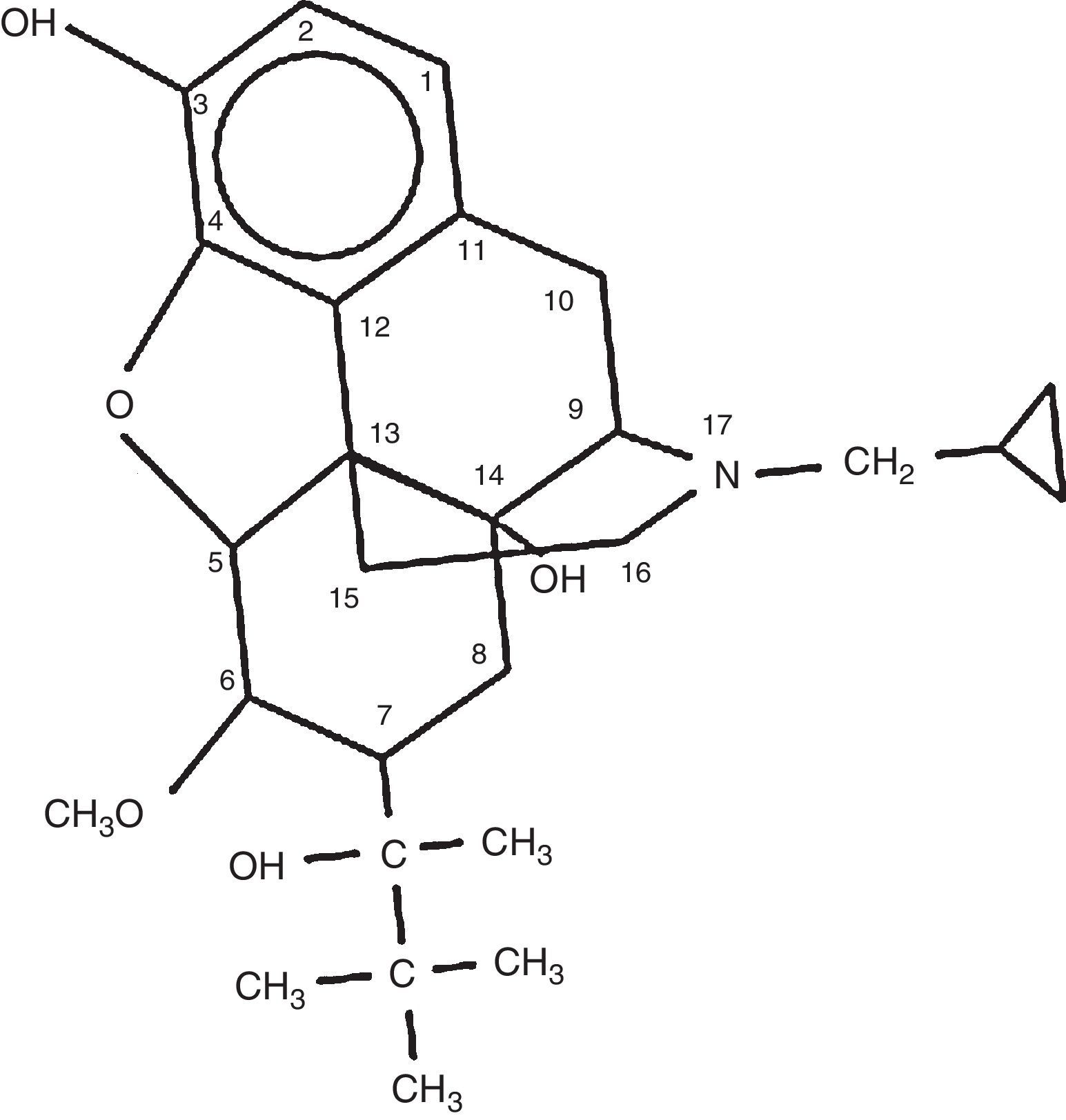
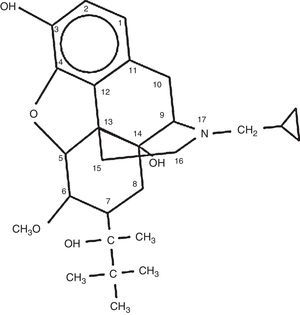
It has a hexacyclic structure that differentiates it from natural opioids such as morphine or codeine, or semi-synthetic derivatives such as heroin, which has a pentacyclic structure. It was first marketed in the United States in the 1980s by the Reckitt & Colman pharmaceutical company, as an analgesic, with the brand name of Buprenex 0.3mg/ml injection.23,28 The chemical structure is shown in Fig. 1.
Sittl29 suggests that buprenorphine has an anti-nociceptive potency approximately 75–100 times higher than that of morphine. Buprenorphine has a dose-dependent effect on analgesia without respiratory depression. At certain doses, 1–3μg/kg body weight, a marked analgesic effect is achieved, but by increasing the dose range adverse effects are encountered, such as the loss of autonomic respiratory control.26
Patients in the recovery room are monitored to prevent haemodynamic, respiratory, and metabolic complications. Buprenorphine has analgesic effects, but there is no respiratory depression, at doses of up to 10μg/kg body weight, therefore it can have a differential effect on respiration and analgesia. It has also been demonstrated to have strong antihyperalgesic effects that can exceed its analgesic effects.30
Another advantage in administering buprenorphine in comparison with drugs such as morphine for postoperative analgesia is the low rate of constipation in patients being treated with it, preventing the higher probability of this event especially when combined with abdominal surgery.
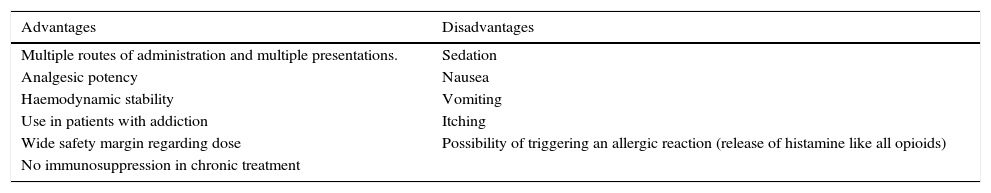
Another problem that is ever more frequent is the treatment of patients with an addiction to opioid or sympathomimetic drugs. Since 1994, buprenorphine has been considered an acceptable alternative in opioid detoxification treatment due to its low potential for overdose, prolonged duration of action, and low frequency of withdrawal symptoms (Table 2).31
Advantages and disadvantages of buprenorphine.
| Advantages | Disadvantages |
|---|---|
| Multiple routes of administration and multiple presentations. | Sedation |
| Analgesic potency | Nausea |
| Haemodynamic stability | Vomiting |
| Use in patients with addiction | Itching |
| Wide safety margin regarding dose | Possibility of triggering an allergic reaction (release of histamine like all opioids) |
| No immunosuppression in chronic treatment |
It is metabolised in the liver and intestine into norbuprenorphine through glucuronidation,32 mostly by liver Cp450.28 Norbuprenorphine is a metabolite of N-dealkylation with one fourth the potency of buprenorphine. Norbuprenorphine can cause 10-times more respiratory depression compared to buprenorphine, but this can be reversed with naloxone.
It is excreted by biliary, intestinal, and urinary excretion (although only 15% of the total dose can be found excreted in urine).25 This gives it a great advantage over other opioids that have mostly renal excretion, especially for surgical procedures where often due to the patient or procedure characteristics, patients have acute kidney failure.33
DoseThere are recommended doses to manage postoperative pain by age groups:
From 2 to 12 years, 2–6μg/kg every 6–8h.
≥13 years, 1–3μg/kg in intervals of up to every 6h. Repeat the initial dose (up to 0.3mg) every 30–60min if necessary.34
Adverse effectsThe most common adverse effects are nausea and vomiting and they are dose-related, therefore it has been observed that doses from 1 to 3μg×kg are effective and have low probabilities of nausea and vomiting. Another factor that determines the possibility of this effect is the dose that is administered versus the surgical stimulus since it should not be forgotten that it is a drug that is 30–40 times more potent than morphine.
After nausea and vomiting, the most common effect is constipation, although this has a lower incidence than with other opioids. Although it is an annoying symptom, it tends to improve hours after the last dose, and in the case where the drug is maintained via perfusion, the use of laxatives causes improvement in most cases.
The effects on the nervous system occur in the follow proportions: dizziness, drowsiness, fatigue, confusion, hallucinations,35,36 other diaphoresis effects, dermatitis, itching. Buprenorphine does not have cardiac depressive effects and does not cause immunosuppression in chronic treatment.
It was thought that the release of histamine was responsible for the itching caused by opioids; however those that do not release histamine, such as buprenorphine, also produce it. Facial itching may not necessarily be a direct effect of buprenorphine in the trigeminal nucleus; as it could be a reflex from the neuron transmission at a distant site.37
Respiratory depression: Although it has a lower incidence than other opioids, it is present in 0.1–1% of cases independent of the route of administration.36 It presents as a significant reduction in the stimulating effect exercised by CO2 on ventilation, in addition to as an increase in the apnoeic threshold and the end-expiratory PCO2 at rest. It also reduces the ventilatory impulse in response to hypoxia, increasing the expiration duration during the respiratory cycle. This all translates to a decrease in the respiratory rate with the consequent reduction in flow volume.38
The usual treatment for opioid-induced respiratory depression is naloxone, from 1 to 4μg/kg/h via intravenous perfusion.
Nausea is defined as the subjective sensation of the desire to vomit without expulsive muscle movements, with the following classification:
- 1)
No nausea
- 2)
Mild nausea that does not need treatment
- 3)
Moderate nausea, treatment may be ordered but the patient tolerates it
- 4)
Severe nausea where treatment is necessary
- 5)
Intractable nausea, the patient demands treatment39
The most common adverse effects are nausea and vomiting, and these are dose-related. After nausea and vomiting, the most common effect is constipation. The effects on the nervous system occur in the follow proportions: dizziness, drowsiness, fatigue, confusion, hallucinations, diaphoresis, dermatitis, itching.31,34 Buprenorphine does not have cardiac depressive effects and does not cause immunosuppression in chronic treatment.
Interactions with other drugsThe effects on the central nervous system can be intensified when buprenorphine is administered jointly with other opioids, sedatives, hypnotics, anaesthetics, alcohol, antidepressants, and neuroleptics. CYP3A4 inhibitors (fluoxetine, erythromycin, metronidazole, ketoconazole, HIV drugs, oral contraceptives, amiodarone, omeprazole) increase its effect. Inducers (carbamazepine, dexamethasone, rifampicin) lower its efficacy.35
Buprenorphine and the immune systemUnlike what happens with morphine and fentanyl, which are potent immune system suppressors,27 buprenorphine demonstrates a neutral behaviour on the immune system at the doses used for analgesia. Opioids that have a carbonyl substitution at C6 and a single bond between C7 and C8 (such as buprenorphine) offer advantages due to the absence of immunosuppressive effects. Buprenorphine has no effect on immune cell responses from the spleen (lymphoproliferation, natural killer cell activity, production of interleukin 2 and interferon 4). They confirm that buprenorphine has a neutral immunopharmacological profile among the opioid analgesics,40 which provides a potential advantage for immunocompromised patients, such as those who have undergone surgery or cancer patients.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.