The superior vena cava syndrome (SVCS) is a rare pathological process caused by the superior vena cava obstruction (SVCO).
AimTo know the main causes of SVCS in a third level hospital.
Material and methodsObservational, prospective and descriptive study in 31 patients with SVCS treated between June 2013 and December 2014. Yale, Stanford scores and tumour biopsy were obtained at diagnosis.
ResultsThe main causes of SVCS were malignant tumours: lung cancer (22.5%) and lymphoma (16.1%). For lung cancer, the most common was non small cells (57.1%) and lymphoma was non-Hodgkin (80%).
ConclusionsThe main causes of SVCS are advanced malignant tumours like lung cancer and lymphomas, benign obstruction causes are relatively rare.
El síndrome de vena cava superior (SVCS) es un proceso patológico poco frecuente causado por la obstrucción de la vena cava superior (VCS).
ObjetivoConocer las principales causas de SVCS en un hospital de tercer nivel.
Material y métodoEstudio observacional, prospectivo y descriptivo en 31 pacientes con SVCS atendidos durante junio de 2013 a diciembre de 2014. Se utilizó la escala de Yale, Stanford y biopsia de tumour al momento del diagnóstico.
ResultadosLa principal causa de SVCS fue por tumores malignos: cáncer broncogénico (22.5%) y linfoma (16.1%). Del cáncer pulmonar, el más común fue el de células no pequeñas (57.1%) y para el linfoma fue el no Hodgkin (80%).
ConclusionesEl SVCS es principalmente ocasionado por tumores malignos avanzados como el cáncer de pulmón y los linfomas, los casos debidos a una obstrucción benigna son relativamente raros.
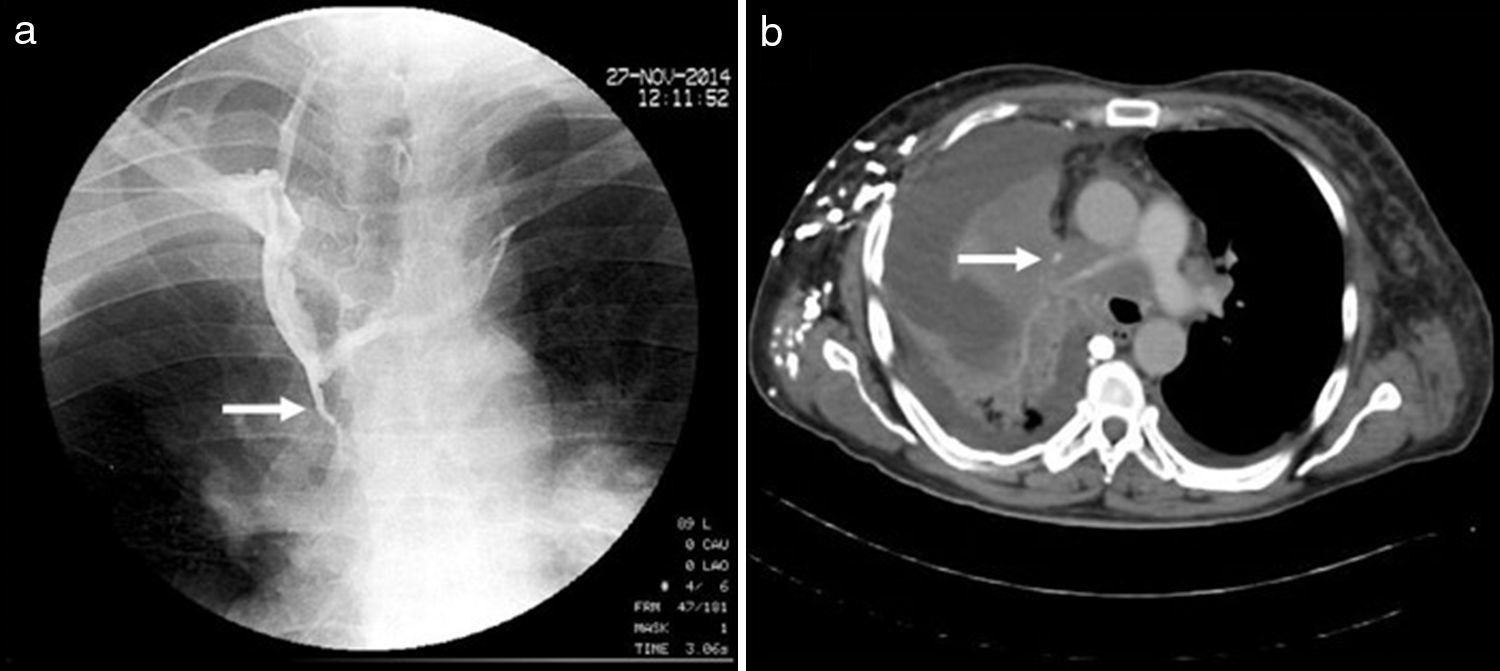
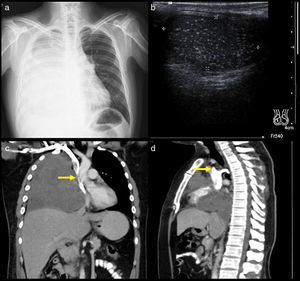
Superior vena cava syndrome (SVCS) is mechanical obstruction of the superior vena cava (SVC) due to venous thrombi or extrinsic compression by intrathoracic tumours in most cases.1 The main associated symptoms are: dyspnoea, collar of Stokes, neck vein distension and superficial venous network on the chest. The severity of the signs and symptoms presented depends on the anatomical level and the time to obstruction of the SVC.2,3 Before the use of antibiotics, tuberculosis and aortic aneurysms due to syphilis were the two main causes of SVCS. Currently, the causes of SVCS may be malignant or benign.4 The diagnosis of SVCS is clearly made by consistent clinical findings such as a superficial venous network and sometimes Horner's syndrome (Fig. 1). Imaging studies such as cavography (Fig. 2) and a chest computed tomography (CT) scan help to confirm the level and severity of the SVCS in order to decide upon a surgical plan, direct radiotherapy or place a stent. However, tumour biopsy is the gold standard as it confirms the histological type.5 The following study was conducted to determine the clinical signs and main causes of SVCS.
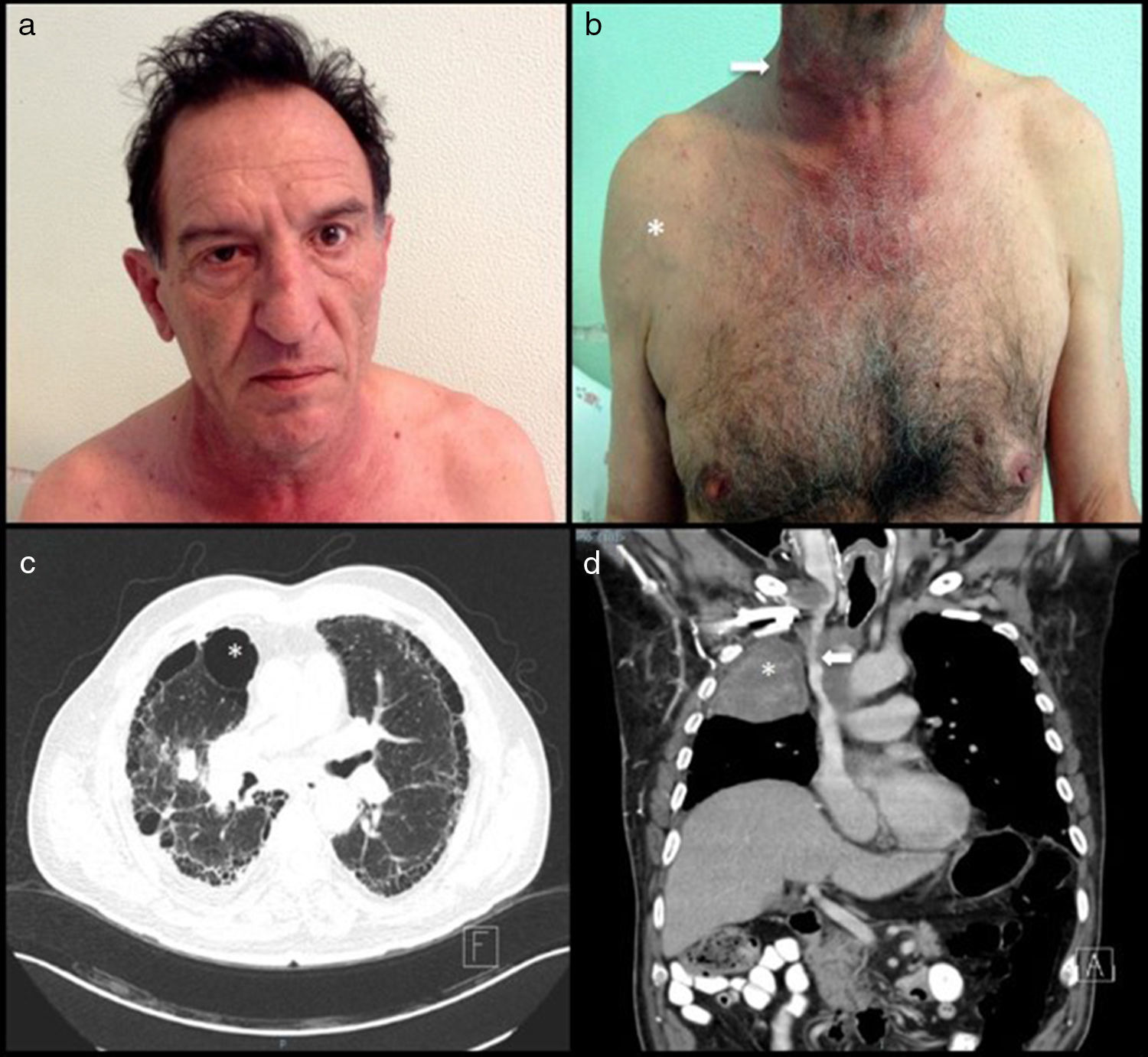
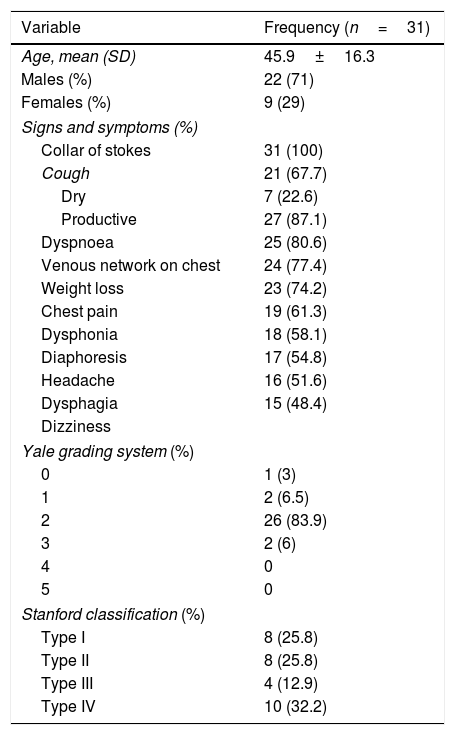
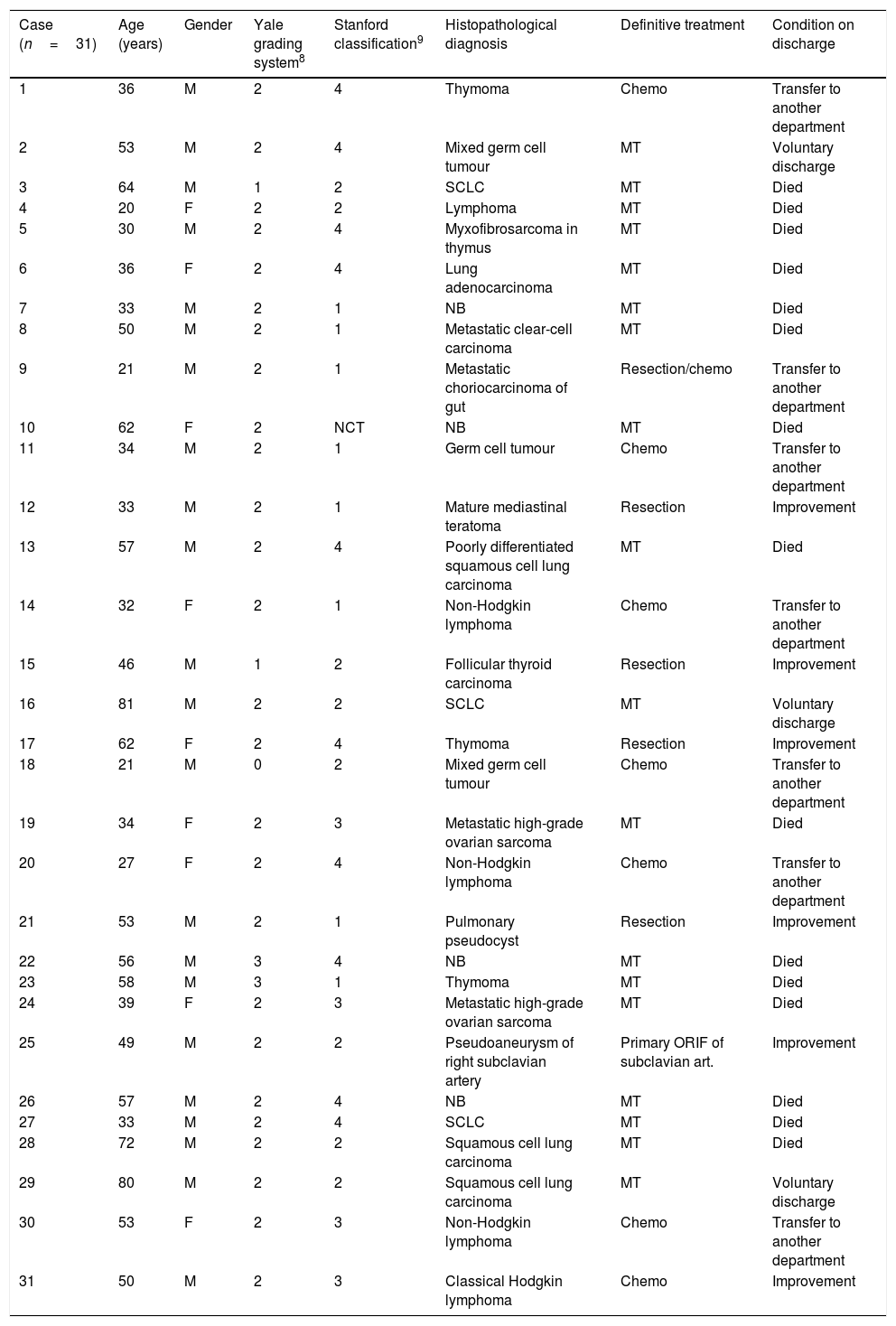
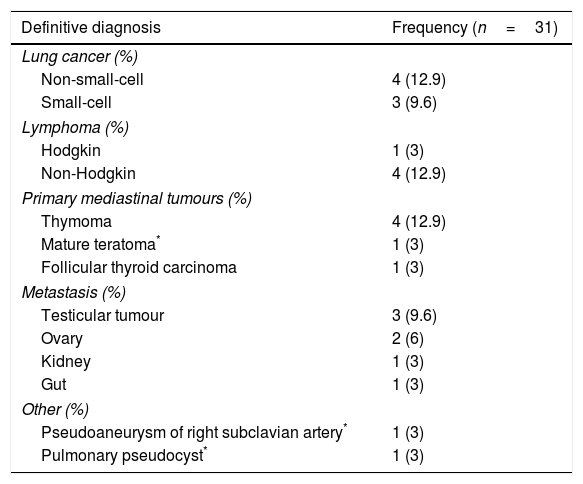
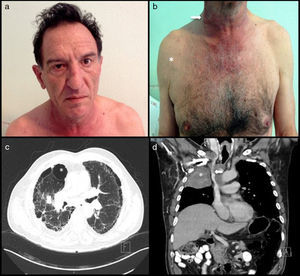
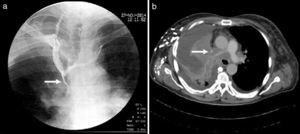
A 57-year-old man and chronic smoker with bullous emphysema and Horner's syndrome. (a) Eyelid pseudoptosis and mild oedema on the right half of the face. (b) Neck vein distension (arrow) and collateral venous network with oedema in the right hemithorax (asterisk). (c) Lung parenchyma with subpleural bullae and septal thickening (asterisk). (d) SVC compression (arrow) due to a right-side Pancoast tumour (asterisk).
A descriptive cross-sectional study was conducted in which consecutive cases of patients clinically diagnosed with SVCS admitted to the “Dr. Alejandro Celis” pulmonology department at Hospital General de México “Dr. Eduardo Liceaga” were enrolled from June 2013 to December 2014. The Yale grading system6 was used to classify patients based on the severity of their signs and symptoms on admission. A chest CT scan was performed with administration of iodinated contrast medium (Xenetix® 300) in order to confirm the degree and level of obstruction according to the Stanford classification.7 The definitive treatment—surgery, radiotherapy or chemotherapy—was determined according to the final diagnosis and based on the severity of each patient. Patients were followed up during their hospital stay until they were discharged. The data obtained were analysed using the SPSS® programme, version 22. Demographic data were analysed using frequency tables and proportions; quantitative variables were analysed using means with standard deviations.
ResultsOf a total of 950 patients admitted to the “Dr. Alejandro Celis” pulmonology department during the study period, 31 (3.2%) had a clinical diagnosis of SVCS: 22 (71%) males and 9 (29%) females, with an age range of 20–81 years and a mean age of 45.9±(SD: 16.3). Of all patients with SVCS, 18 (58.1%) were admitted from the emergency department, 8 (25.8%) were admitted from the pulmonology outpatient department and 5 (16.1%) were admitted from other departments. The main clinical signs on admission were: collar of Stokes (100%), cough (90.3%), dyspnoea (87.1%), superficial venous network on the chest (80.6%), weight loss (77.4%) and chest pain (74.2%). The Eastern Cooperative Oncology Group (ECOG) scale8 to assess the clinical status of the cancer patient was used, and 20 (64.5%) patients had a score greater than or equal to 2. Regarding the Yale SVCS severity grading system,6 26 (83.9%) patients had a score of 2 (moderate) and two (6.5%) had a score of 3 (severe). According to the Stanford classification,7 patients were divided into four categories: type I (25.8%), type II (25.8%), type III (12.9%) and type IV (32.2%) (Table 1). After they had been clinically diagnosed with SVCS, 18 (58.1%) patients died during hospitalisation due to the advanced state of their disease on admission. Of those who died, one did not undergo a CT scan and four died without a biopsy (Table 2). However, in all other patients with a tumour biopsy, the most common diagnoses were stage IV lung cancer (22.5%) and lymphoma (16.1%) (Fig. 3). In patients diagnosed with lung cancer, non-small-cell lung cancer (NSCLC) was confirmed in 57.1%, and, in these, squamous cell carcinoma was the most common. In patients with lymphoma, non-Hodgkin lymphoma (80%) was the most prevalent. The following primary mediastinal tumours were found: thymoma (16.1%), mature teratoma (3.2%) and thyroid cancer (3.2%). In 7 (22.4%) patients, SVCS was secondary to metastasis of extrathoracic tumours (Fig. 4), such as testicular (9.6%), ovarian (6.4%), kidney (3.2%) and intestinal (3.2%). Of the three cases of SVCS of benign aetiology, one was due to a vascular lesion secondary to placement of a catheter (Mahurkar®) for haemodialysis, one was due to a pulmonary pseudocyst and one was due to a mature mediastinal teratoma. This series found no benign cases of SVCS secondary to mediastinal fibrosis due to tuberculosis or histoplasmosis (Tables 2 and 3).
Main signs and symptoms in patients clinically diagnosed with SVCS.
| Variable | Frequency (n=31) |
|---|---|
| Age, mean (SD) | 45.9±16.3 |
| Males (%) | 22 (71) |
| Females (%) | 9 (29) |
| Signs and symptoms (%) | |
| Collar of stokes | 31 (100) |
| Cough | 21 (67.7) |
| Dry | 7 (22.6) |
| Productive | 27 (87.1) |
| Dyspnoea | 25 (80.6) |
| Venous network on chest | 24 (77.4) |
| Weight loss | 23 (74.2) |
| Chest pain | 19 (61.3) |
| Dysphonia | 18 (58.1) |
| Diaphoresis | 17 (54.8) |
| Headache | 16 (51.6) |
| Dysphagia | 15 (48.4) |
| Dizziness | |
| Yale grading system (%) | |
| 0 | 1 (3) |
| 1 | 2 (6.5) |
| 2 | 26 (83.9) |
| 3 | 2 (6) |
| 4 | 0 |
| 5 | 0 |
| Stanford classification (%) | |
| Type I | 8 (25.8) |
| Type II | 8 (25.8) |
| Type III | 4 (12.9) |
| Type IV | 10 (32.2) |
Follow-up of patients diagnosed with superior vena cava syndrome during hospitalisation.
| Case (n=31) | Age (years) | Gender | Yale grading system8 | Stanford classification9 | Histopathological diagnosis | Definitive treatment | Condition on discharge |
|---|---|---|---|---|---|---|---|
| 1 | 36 | M | 2 | 4 | Thymoma | Chemo | Transfer to another department |
| 2 | 53 | M | 2 | 4 | Mixed germ cell tumour | MT | Voluntary discharge |
| 3 | 64 | M | 1 | 2 | SCLC | MT | Died |
| 4 | 20 | F | 2 | 2 | Lymphoma | MT | Died |
| 5 | 30 | M | 2 | 4 | Myxofibrosarcoma in thymus | MT | Died |
| 6 | 36 | F | 2 | 4 | Lung adenocarcinoma | MT | Died |
| 7 | 33 | M | 2 | 1 | NB | MT | Died |
| 8 | 50 | M | 2 | 1 | Metastatic clear-cell carcinoma | MT | Died |
| 9 | 21 | M | 2 | 1 | Metastatic choriocarcinoma of gut | Resection/chemo | Transfer to another department |
| 10 | 62 | F | 2 | NCT | NB | MT | Died |
| 11 | 34 | M | 2 | 1 | Germ cell tumour | Chemo | Transfer to another department |
| 12 | 33 | M | 2 | 1 | Mature mediastinal teratoma | Resection | Improvement |
| 13 | 57 | M | 2 | 4 | Poorly differentiated squamous cell lung carcinoma | MT | Died |
| 14 | 32 | F | 2 | 1 | Non-Hodgkin lymphoma | Chemo | Transfer to another department |
| 15 | 46 | M | 1 | 2 | Follicular thyroid carcinoma | Resection | Improvement |
| 16 | 81 | M | 2 | 2 | SCLC | MT | Voluntary discharge |
| 17 | 62 | F | 2 | 4 | Thymoma | Resection | Improvement |
| 18 | 21 | M | 0 | 2 | Mixed germ cell tumour | Chemo | Transfer to another department |
| 19 | 34 | F | 2 | 3 | Metastatic high-grade ovarian sarcoma | MT | Died |
| 20 | 27 | F | 2 | 4 | Non-Hodgkin lymphoma | Chemo | Transfer to another department |
| 21 | 53 | M | 2 | 1 | Pulmonary pseudocyst | Resection | Improvement |
| 22 | 56 | M | 3 | 4 | NB | MT | Died |
| 23 | 58 | M | 3 | 1 | Thymoma | MT | Died |
| 24 | 39 | F | 2 | 3 | Metastatic high-grade ovarian sarcoma | MT | Died |
| 25 | 49 | M | 2 | 2 | Pseudoaneurysm of right subclavian artery | Primary ORIF of subclavian art. | Improvement |
| 26 | 57 | M | 2 | 4 | NB | MT | Died |
| 27 | 33 | M | 2 | 4 | SCLC | MT | Died |
| 28 | 72 | M | 2 | 2 | Squamous cell lung carcinoma | MT | Died |
| 29 | 80 | M | 2 | 2 | Squamous cell lung carcinoma | MT | Voluntary discharge |
| 30 | 53 | F | 2 | 3 | Non-Hodgkin lymphoma | Chemo | Transfer to another department |
| 31 | 50 | M | 2 | 3 | Classical Hodgkin lymphoma | Chemo | Improvement |
SVCS: superior vena cava syndrome; M: male; F: female; MT: medical treatment; chemo: chemotherapy; radio: radiotherapy; SCLC: small-cell lung cancer; NSCLC: non-small-cell lung cancer; NB: no biopsy; NCT: no CT scan; ORIF: open reduction and internal fixation.
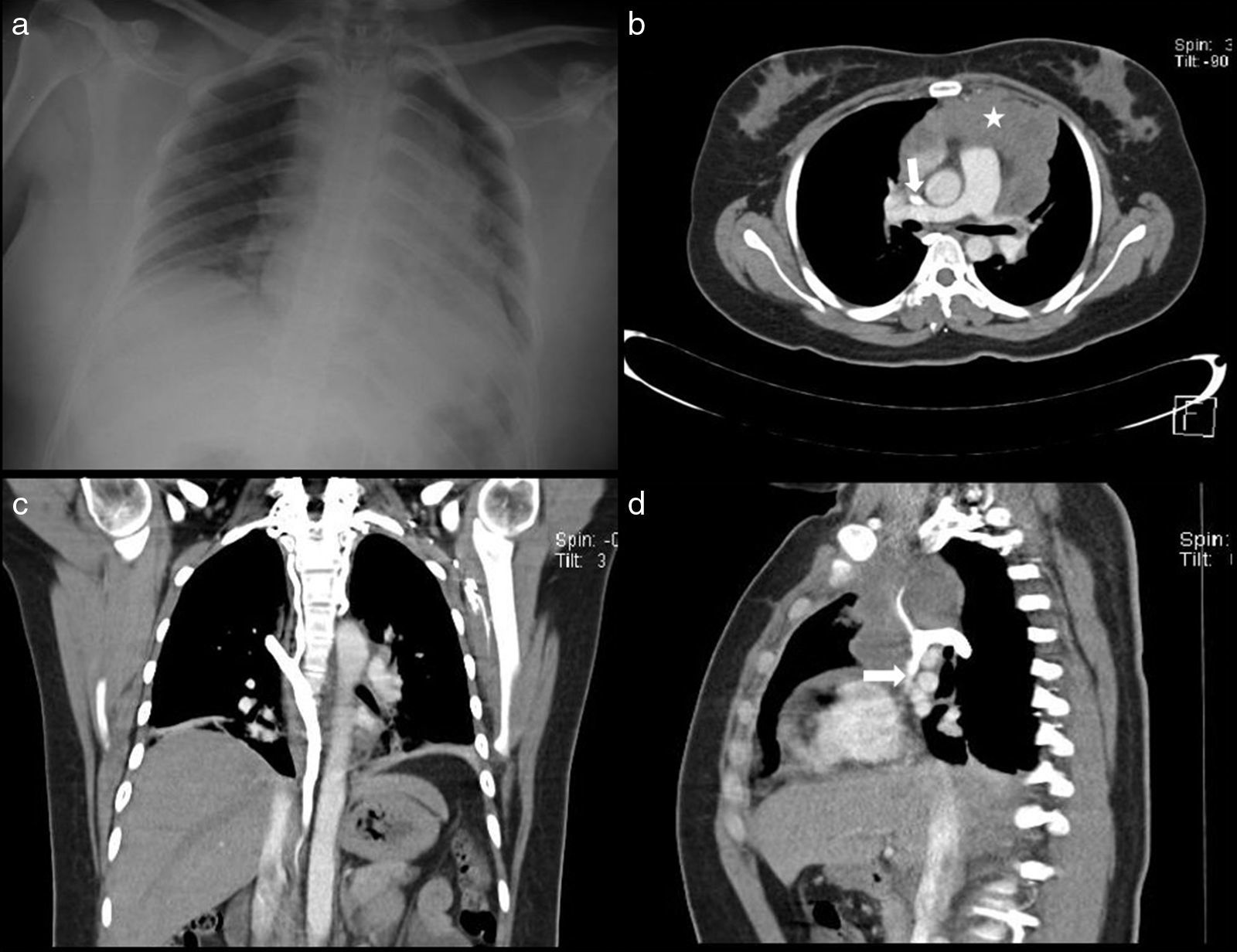
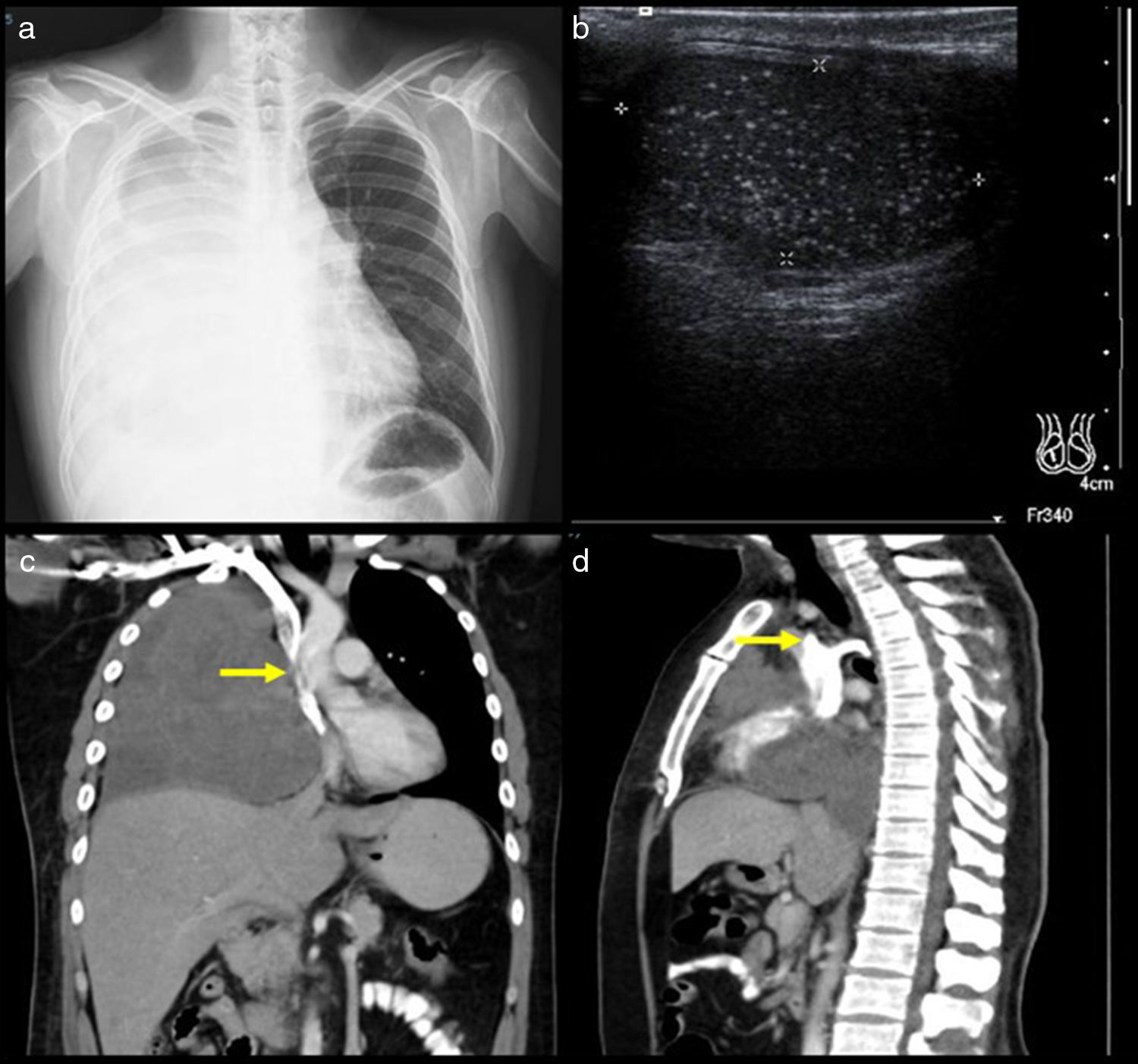
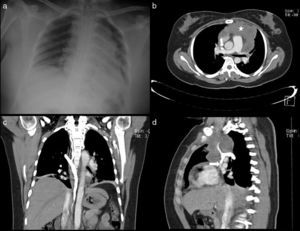
A 34-year-old man diagnosed with metastasis to the mediastinum by a testicular germ cell tumour. (a) Chest X-ray with opacity in the right hemithorax. (b) Testicular ultrasound with multiple nodules, some calcified. (c and d) CT cavography with obstruction of the SVC above the azygos (arrows).
Main causes of superior vena cava syndrome in the “Dr. Alejandro Celis” pulmonology department at Hospital General de México “Dr. Eduardo Liceaga”.
| Definitive diagnosis | Frequency (n=31) |
|---|---|
| Lung cancer (%) | |
| Non-small-cell | 4 (12.9) |
| Small-cell | 3 (9.6) |
| Lymphoma (%) | |
| Hodgkin | 1 (3) |
| Non-Hodgkin | 4 (12.9) |
| Primary mediastinal tumours (%) | |
| Thymoma | 4 (12.9) |
| Mature teratoma* | 1 (3) |
| Follicular thyroid carcinoma | 1 (3) |
| Metastasis (%) | |
| Testicular tumour | 3 (9.6) |
| Ovary | 2 (6) |
| Kidney | 1 (3) |
| Gut | 1 (3) |
| Other (%) | |
| Pseudoaneurysm of right subclavian artery* | 1 (3) |
| Pulmonary pseudocyst* | 1 (3) |
Note: Four patients died before undergoing a tumour biopsy.
Superior vena cava syndrome is an uncommon disease. This study demonstrated that malignant intrathoracic tumours (NSCLC) are its main cause. Moreover, its diagnosis is clinical due to symptoms secondary to obstruction of the SVC, including collar of Stokes, cough and dyspnoea. The first description of SVCS was made by William Hunter in 1757. However, it was William Stokes in 1837 who described in detail the clinical signs and symptoms of a patient with lung cancer who had paraesthesia on the right side of the chest and in the shoulder, in addition to cough, dyspnoea, facial oedema, dysphonia, dysphagia and distension of the external jugular vein. These symptoms were present in the majority of our patients.9 Anatomically, the SVC is formed by the union of two brachiocephalic veins (right and left), and empties into the right atrium after receiving blood flow from the azygos system.10,11 The SVC is a low-pressure vein; even so, its obstruction leads to an increase in this pressure up to 21mmHg, which triggers the classical symptoms described.12 SVCS is more common in men. The main malignant causes are lung cancer and mediastinal lymphoma. The main benign causes are mediastinal fibrosis due to infections (tuberculosis and histoplasmosis) and vascular lesions secondary to placement of central venous catheters and cardiovascular devices such as pacemakers.4 Only 2–4% of patients with lung cancer end up developing the syndrome. When it occurs, it is associated with a poor prognosis and decreased short- or medium-term survival. This explains the high mortality in this series of patients.13 As described by Rice et al.,4 this study found that lung cancer was the main cause of SVCS, but the squamous cell variety of NSCLC was the most prevalent histological type, followed by non-Hodgkin lymphoma and primary mediastinal tumours—thymus, thyroid and germ cell tumours. The symptoms depend on the time and the level of obstruction of the SVC and whether the obstruction is above or below the outlet of the azygos vein. Cases of severe obstruction of the SVC lead to life-threatening complications: airway collapse due to tracheal oedema and neurological decline secondary to cerebral oedema. No patient had any of these complications.6 In Mexico, most information on this disease is limited to case reports.14 A descriptive study of 458 mediastinal tumours by Ibarra et al.15 reported that 84 (18.34%) developed SVCS and that 80 of them were malignant tumours. Clinical data such as collar of Stokes, neck vein distension and dyspnoea help to make the clinical diagnosis of SVCS; however, the degree and level of obstruction are obtained by means of imaging studies such as a plain chest CT scan or a chest CT scan with contrast. When SVCS is caused by a thoracic tumour, a biopsy of the causal process reveals the histological type. Treatment depends on the aetiology and on the severity of the signs and symptoms. Therefore, SVCS is considered an oncological emergency requiring immediate treatment when accompanied by airway invasion or compression, or neurological decline due to cerebral oedema.16 In the case of malignant tumours, treatment is conservative with measures such as elevation of the head of the bed to 45°, supplementary oxygen as needed, diuretics and sometimes corticosteroids intravenously for sensitive tumours (lymphoma and thymoma). Radiotherapy improves the symptoms in the short term due to a decrease in tumour volume, and chemotherapy is the treatment of choice when the histological type of the tumour is known.3 If the syndrome has a benign cause, surgery may be a good option due to its low percentage of complications. However, the endovascular approach for placement of stents is the first-line treatment, since the procedure has minimal complications and improvement is immediate due to a significant decrease in SVC pressure after stents are placed. Unfortunately, few centres in Mexico have the equipment needed to perform this procedure.12
ConclusionsThe diagnosis of SVCS is essentially clinical. Imaging studies reveal the degree and extent of vena cava stenosis and facilitate the choice of the site where a biopsy should be performed in order to determine the histological type of the causative process and consider radiotherapy or chemotherapy. SVCS is mainly caused by advanced malignant tumours such as lung cancer and lymphoma. Cases due to benign obstruction are relatively rare. SVCS carries a poor prognosis in most cases and represents a therapeutic challenge.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.