Chromobacterium violaceum is a Gram-negative, facultative, anaerobic, non-sporing, fermentative and oxidase-positive coccobacillus. Human infections caused by Chromobacterium violaceum are infrequent. There are a few reported cases in several countries. Those infections appear after a skin contact with soil or contaminated water. Infections can progress to necrotizing metastatatic lesions and multiple abscesses of the lung, liver, skin, spleen, lymph nodes and brain with fatal septicemia. Infections caused by this bacterium have a high lethality rate, with a low recovery rate among survivors. It has been described that this bacterium is resistant to several antibiotics.
Materials and methodsChromobacterium violaceum was isolated, cultivated, and identified from oysters from thirteen different restaurants in Mexico City and antimicrobial susceptibility testing was performed.
ResultsFour isolations of Chromobacterium violaceum were obtained from oysters. Three of them corresponded to biotype 36 and all of them had a confidence factor of 0.987. All isolations were resistant to Carbencillin, Cephalothin and Cephotaxime. Two isolations were also resistant to Ampicillin and one was resistant to Amikacin. All isolations were sensitive to Ceftriaxone, Chloramphenicol, Gentamicin, Netilmicin, Nitrofurantoin, Pefloxacine and Trimethoprim-Sulfamethoxazole.
ConclusionsThe presence of Chromabacterium violaceum in food obtained from salt marshes that usually are eaten fresh (like oysters) means a high potential risk for human health because this bacterium has been associated with high morbidity and mortality. In our report, this bacterium showed resistance to several antibacterial agents. Patients should be cautions when eating fresh seafood, and healthcare personnel should suspect an infection caused by Chromobacterium violaceum if the patient presents with sepsis, enteritis or abscesses and they have risk factors for acquiring this bacterium in order to provide prompt treatment and reduce the morbidity and mortality associated with the infection. This work represents the first isolation, cultivation and identification of Chromobacterium violaceum in Mexico.
Chromobacterium violaceum es un cocobacilo Gram negativo, facultativo, anaeróbico, no formador de esporas, fermentativo y oxidasa positivo. Las infecciones en humanos causadas por Chromobacterium violaceum son infrecuentes. Existen pocos reportes de casos en varios países. Estas infecciones aparecen después del contacto de la piel con el suelo o con agua contaminada. Las infecciones pueden progresar a lesiones necrotizantes metastásicas y múltiples abscesos en pulmón, hígado, piel, bazo, ganglios linfáticos y cerebro y septicemia letal. Las infecciones causadas por esta bacteria tienen una alta tasa de letalidad, con una baja tasa de recuperación entre los sobrevivientes. Se ha descrito que esta bacteria es resistente a varios antibióticos.
Material y métodosSe aisló, cultivó e identificó Chromobacterium violaceum en ostras de trece distintos restaurantes de la Ciudad de México y se realizaron estudios de susceptibilidad antimicrobiana.
ResultadosCuatro aislamientos de Chromobacterium violaceum fueron obtenidos de ostras. Tres de los aislamientos correspondieron al biotipo 36 y todos tuvieron un nivel de confianza de 0.987. Todos los aislamientos fueron resistentes a Carbenicilina, Cefalotina y Cefotaxima. Dos aislamientos fueron también resistentes a Ampicilina y uno fue resistente a Amikacina. Todos los aislamientos fueron sensibles a Ceftriaxona, Cloranfenicol, Gentamicina, Netilmicina, Nitrofurantoína, Pefloxacina y Trimetoprim-Sulfametoxazol.
ConclusionesLa presencia de Chromobacterium violaceum en alimentos obtenidos de marismas los cuales son ingeridos frescos (como las ostras), representa un alto potencial de riesgo para la salud humana, ya que esta bacteria se ha asociado con enteritis aguda, septicemia y con la formación de abscesos diseminados. En nuestro reporte, esta bacteria demostró una resistencia a varios agentes antimicrobianos. Los pacientes deben de ser cuidadosos al ingerir pescados y mariscos frescos, y el personal de salud debe de sospechar una infección causada por Chromobacterium violaceum si el paciente se presenta con sepsis, enteritis o abscesos, y si tiene los factores de riesgo para adquirir esta bacteria, con el objetivo de dar un tratamiento oportuno y reducir la morbi-mortalidad asociada con esta infección. Este trabajo representa el primer aislamiento, cultivo e identificación de Chromobacterium violaceum en México.
Chromobacterium violaceum is a Gram-negative, facultative, anaerobic, fermentative and oxidase-positive coccobacillus.1–4 This bacterium is frequently found in the soil and water as part of the normal flora of tropical and subtropical climates. It is not part of the normal flora of the human.4–6
The first reported case of Chromobacterium violaceum infection in humans is from Malaysia and Lesslar described it in 1927 in a patient with fatal septicemia and a liver abscess.7 Eleven years later, Black and Shahan described the first case in the United States.8,9 Since then, only 150 cases have been reported. Human infections caused by Chromobacterium violaceum are infrequent and there are few cases reported in Brazil, Argentina, Cuba, India, Japan, Nigeria, Singapore, Taiwan, Sri Lanka, United States of America, Vietnam, Australia and other countries.1–16
The most common mode of entry of the bacteria appears to be the contact of injured skin and subcutaneous tissue with contaminated soil or water.3,17,18 The infection of Chromobacterium violaceum usually starts as a limited infection of the skin at the point of contact with the bacterium. Then it progresses to necrotizing metastatatic lesions and multiple abscesses of the lung, liver, skin, spleen, lymph nodes and brain.19–27 Fatal septicemia has been associated clinically in adults along with high fiver, diffuse abdominal pain, microabscesses with diffuse location in different organs, neutrophilia, alterations in hepatic and kidney function tests, altered mental status, thermodynamic changes and death.10,16,17,25,28–30 Other pathologies that are reported include chronic granulomatosis, cellulitis, osteomyelitis, septic spondylitis, diarrhea, peritonitis, conjunctivitis and periorbial and ocular infection.2,30–32,21 Of the reported cases most of them have a high lethality, with a low recovery rate among survivors.13,17,33
Chromobacterium violaceum can be misidentified with Burkholderia pseudomallei by some identification methods, so care must be taken during the diagnostic evaluation.34 There are some reports in which antimicrobial therapy was successful, particularly with ciprofloxacin together with Amikacin, Piperacillin-Tazobactam and Trimethoprim-Sulfametoxazole as reported in some cases.13,17,33–35 Nevertheless in many of the cases there have been reports of defects in empirical treatment.10,16,25
It has been reported that Chromobacterium violaceum is a bacterium that has multiple antimicrobial resistances, and since infection by this bacterium is rare, there are no clinical trials evaluating different treatments. Among the antibiotics to which the resistance has been observed in vitro are Ampicillin, Cephalothin, Cefoxitin, Cefriaxone, Cephotaxime and Ceftazidime. On the other hand, it appears to be sensitive in vitro to Gentamicin, Amikacin, Tobramycine, Chloramphenicol, Tetracycline, Trimethoprim-Sulfametoxazole, Ciprofloxacin, Imipenem and Cefepime.13,17,33–35 Antibiotics that have been used successfully to treat infection caused by Chromobacterium violaceum include Amikacine, Ciprofloxacine, Co-trimoxazole and Pefloxacin.13,17,33–38
The objectives of this work were to isolate and identify the bacteria Chromobacterium violaceum from oysters for the first time in Mexico and to perform a bacterial sensitivity and resistance investigation.
Materials and methodsWe performed a transverse, observational, descriptive study in order to isolate, cultivate and identify Chromobacterium violaceum from oysters in Mexico.
Acquisition of samplesSamples of oysters were obtained directly from 13 restaurants in different locations of Mexico City. The samples were refrigerated, sealed and then transported in thermal boxes made of Styrofoam with gel refrigerants to the microbiology laboratory at the Anahuac University North Campus. The shells of the oysters were washed and disinfected. Then, in aseptic conditions the oysters were opened and the liquid was recollected completely with a sterile disposable syringe.
Microbiological culture0.1ml of liquid content obtained from the oyster was inoculated in Alkaline Peptone water (Merck, Cat. 101800) with a pH of 9. 6h later, the second inoculums were made in Alkaline Peptone water for another 6h.
The third inoculums were made in Thiosulfate Citrate Bile Sucrose Agar (TCBS, Merck, Cat. 110263) and incubated for 24h at 37°C in aerobic conditions. The colonies obtained in TCBS were cultivated in 5% blood agar for 24h at 37°C in aerobic condition.
Bacterial identificationThe cultures obtained in blood agar colonies were stained in Gram (Golden Bell Reactives TM DMACA TM, Cat. 261181) and indole (BBL TM DMACA TM, Cat. 261187) and biochemical tests for bacterial identification (BD BBL TM Crystal Identification System Enteric/Non-fermenter ID Kit, Cat. 245000).
The oxidase test was performed as the following: three drops of the reagent were added to the Whatman filter paper number 1 in sterile conditions and 3–4 pure colonies were pleated over it. After 10–30s the reading was performed and the results were interpreted: the violet color or purple color as a positive and without a change as a negative.
The indole test was achieved as the following: 3 drops of a reagent were deposited in Whatman filter paper number 1 in sterile conditions and 3–4 pure colonies were pleated over it. The reading took place after 2min and the results interpreted: blue-green color as a positive and pink or no change of color as a negative.
For the biochemical identification test, the pure colonies obtained in blood agar were taken and then deposited in one tube. The inoculums were adjusted to one scale of Mac Farland 1.0 in Nephelometer (BBL TM Crystal Spec tm Nephetometer Cat. 245009). The bacterial suspension was transferred then in sterile conditions to the Crystal system and incubated without CO2 with 40–60% of humidity at 37°C for 18h. (Table 1)
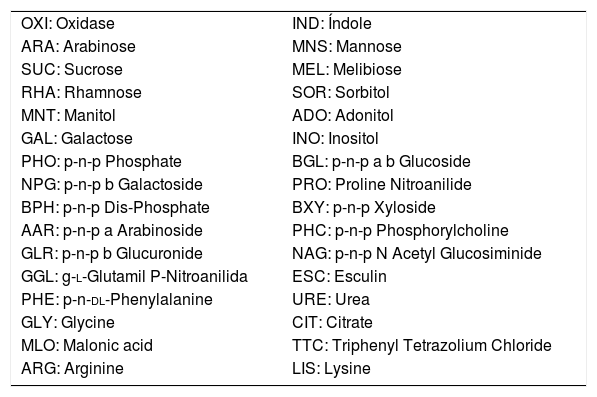
Biochemical test of BD BBL™ Crystal Identification System Enteric/Nonfermenter ID Kit and its abbreviations.
| OXI: Oxidase | IND: Índole |
| ARA: Arabinose | MNS: Mannose |
| SUC: Sucrose | MEL: Melibiose |
| RHA: Rhamnose | SOR: Sorbitol |
| MNT: Manitol | ADO: Adonitol |
| GAL: Galactose | INO: Inositol |
| PHO: p-n-p Phosphate | BGL: p-n-p a b Glucoside |
| NPG: p-n-p b Galactoside | PRO: Proline Nitroanilide |
| BPH: p-n-p Dis-Phosphate | BXY: p-n-p Xyloside |
| AAR: p-n-p a Arabinoside | PHC: p-n-p Phosphorylcholine |
| GLR: p-n-p b Glucuronide | NAG: p-n-p N Acetyl Glucosiminide |
| GGL: g-l-Glutamil P-Nitroanilida | ESC: Esculin |
| PHE: p-n-dl-Phenylalanine | URE: Urea |
| GLY: Glycine | CIT: Citrate |
| MLO: Malonic acid | TTC: Triphenyl Tetrazolium Chloride |
| ARG: Arginine | LIS: Lysine |
The results were read in BD BBLTM Crystal Auto Reader (Cat. 245300) applying BD BBL TM Crystal TM MIND Software (Cat. 441010).
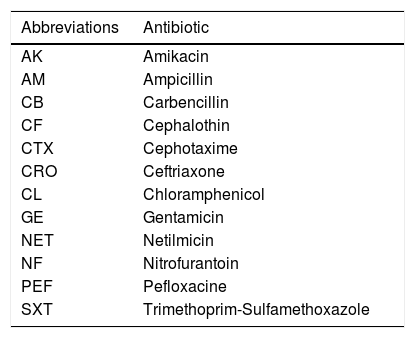
Antimicrobial susceptibility test4–5 colonies from the pure colonies obtained in blood agar were resuspended in a physiological solution and adjusted to one standard of Mac Farland of 0.5. The bacterial suspension was then transferred to the Muller Hinton medium (BD, Cat. 221177) using a sterile hyssop followed by Multidisc Gram Negative analysis (Bio Rad, Cat, 71080280). The analysis was performed in less then 15min taken from the last inoculation and to the plate. The plates were incubated at 37°C for 18–20h without CO2 (Table 2).
ResultsBacterial identificationSmall, light violet 2mm colonies isolated from TCBS medium were composed of Gram-negative bacillus. The pure colonies of 3–4mm approximately were obtained from blood agar. The biochemical identification test and the antimicrobial susceptibility tests were realized.
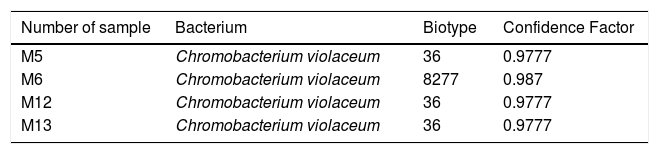
Among the samples taken from 13 different restaurants, 4 samples resulted positive to Chromobacterium violaceum, all isolations having a high confidence factor (Table 3).
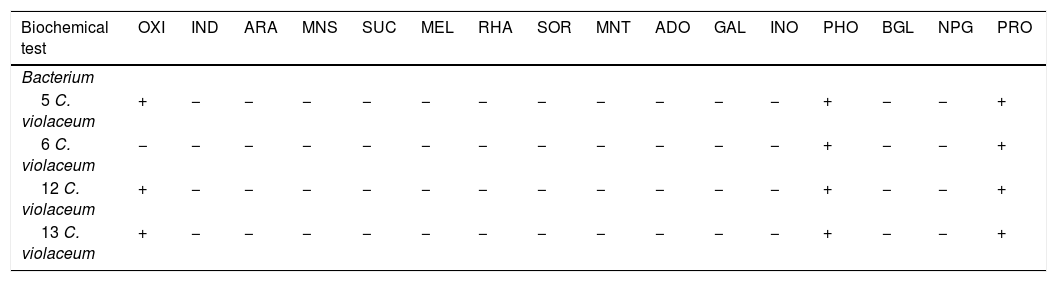
The biochemical profile between the four isolations of Chromobacterium violaceum was similar (Table 4). However, the differences in two substrates between 3 isolations of biotype 8277 were detected. The biotype 8277 showed that the positive oxidase reaction is weaker than the positive Malonic acid reaction, and could be different in this biotype without mentioning that it is a different species, as a confidence factor is 0.987.
Biochemical profiles of Chromobacterium violaceum strains isolated from oysters.
| Biochemical test | OXI | IND | ARA | MNS | SUC | MEL | RHA | SOR | MNT | ADO | GAL | INO | PHO | BGL | NPG | PRO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterium | ||||||||||||||||
| 5 C. violaceum | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + |
| 6 C. violaceum | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + |
| 12 C. violaceum | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + |
| 13 C. violaceum | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + |
| Biochemical test | BPH | BXY | AAR | PHC | GLR | NAG | GGL | ESC | PHE | URE | GLY | CIT | MLO | TTC | ARG | LYS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterium | ||||||||||||||||
| 5 C. violaceum | + | − | − | + | − | + | + | − | − | + | + | + | − | + | + | + |
| 6 C. violaceum | + | − | − | + | − | + | + | − | − | + | + | + | + | + | + | + |
| 12 C. violaceum | + | − | − | + | − | + | + | − | − | + | + | + | − | + | + | + |
| 13 C. violaceum | + | − | − | + | − | + | + | − | − | + | + | + | − | + | + | + |
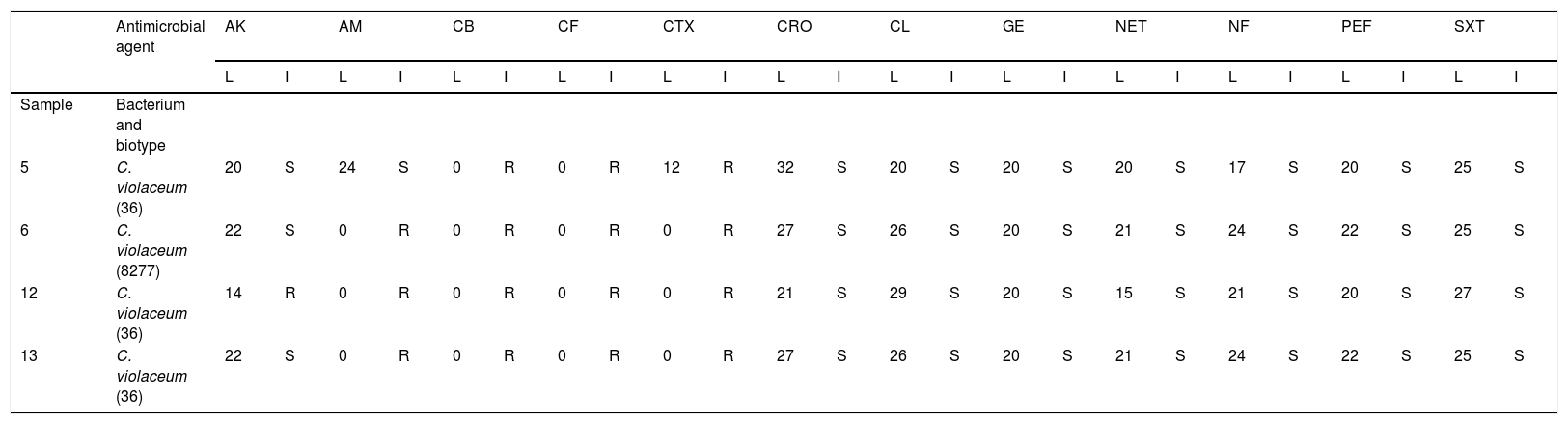
As it is shown in Table 5, all isolations were resistant to Carbenicillin, Cephalothin y Cephatoxime. Two isolations from biotype 36 (samples 12 and 13) and from biotype 8277 (sample 6) were resistant to Ampicillin; another isolation from biotype 36 (sample 5) was sensitive. With respect to Amikacin, only sample 12 (the biotype 36) resulted resistant. Finally, all isolations were sensitive to Ceftriaxone, Chloramphenicol, Gentamicin, Netilmicin, Nitrofurantoin, Pefloxacine and Trimethoprim-Sulfamethoxazole. In this case, no correlation was observed between antimicrobial resistance and the biotype studied.
Antimicrobial susceptibility test.
| Antimicrobial agent | AK | AM | CB | CF | CTX | CRO | CL | GE | NET | NF | PEF | SXT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | I | L | I | L | I | L | I | L | I | L | I | L | I | L | I | L | I | L | I | L | I | L | I | ||
| Sample | Bacterium and biotype | ||||||||||||||||||||||||
| 5 | C. violaceum (36) | 20 | S | 24 | S | 0 | R | 0 | R | 12 | R | 32 | S | 20 | S | 20 | S | 20 | S | 17 | S | 20 | S | 25 | S |
| 6 | C. violaceum (8277) | 22 | S | 0 | R | 0 | R | 0 | R | 0 | R | 27 | S | 26 | S | 20 | S | 21 | S | 24 | S | 22 | S | 25 | S |
| 12 | C. violaceum (36) | 14 | R | 0 | R | 0 | R | 0 | R | 0 | R | 21 | S | 29 | S | 20 | S | 15 | S | 21 | S | 20 | S | 27 | S |
| 13 | C. violaceum (36) | 22 | S | 0 | R | 0 | R | 0 | R | 0 | R | 27 | S | 26 | S | 20 | S | 21 | S | 24 | S | 22 | S | 25 | S |
Note: L, measure in millimeters of diameter of inhibition; I, interpretation; S, sensible; R, resistant.
For the first time in Mexico we isolated, cultivated and identified Chromobacterium violaceum with a high grade of confidence. The bacteria was isolated from oysters, which represent a principle source of disease and could play an important role in the development of infection associated with a high morbidity and mortality, especially because oysters are consumed fresh and raw. In addition to the isolation and identification of the bacteria, the antimicrobial susceptibility tests that were performed showed that this bacterium is resistant to many of the commonly used antibiotics, which should be taken into account when treating a patient infected by Chromobacterium violaceum.
The isolation and identification of a potentially pathogen microorganism with a high mortality rate that was found in seafood which is consumed fresh and raw, is of great importance to the public health.
The principal market in Mexico City supplies most restaurants working with seafood. Therefore we can suppose that bacteria and biotype in these foods have more or less the same pattern of distribution, as it was observed in biotype 36. Therefore, it is obvious that it is necessary to perform more studies about the frequency of those pathogens and other bacteria and make a correlation of those results and diseases associated with the consumption of these foods. In addition, the authorities should have stricter rules and laws regarding the distribution, refrigeration and consumption of seafood, to prevent diseases associated with their consumption.
Since this is the first isolation of Chromobacterium violaceum in Mexico, there is not much information about this bacterium in this country. Also, since the infection cause by this bacterium is uncommon, most of the diagnostic and treatment evidence is taken from case reports. Because of this lack of information, many cases of enteritis and septicemia with multiple abscesses receive inappropriate treatment as this bacterium showed a resistance to many antimicrobials.
Cases of suspected infection of Chromobacterium violaceum should prompt bacterial identification and antimicrobial susceptibility tests so an adequate diagnosis can be made and efficient treatment provided.
There are some limitations of this study. First, the design of the study was transverse, which means that the samples were obtained in a given time. In the future, samples should be obtained at different times, so we can detect if the presence of the bacterium continues along time or there is only a particular time when this bacterium is present. Also, the presence of this bacterium in oysters does not necessarily traduce disease in humans, since we are not certain that the gastrointestinal tract is an entrance via of the bacterium that could cause severe disease. In addition, the sample of this study was small. In a future study a bigger and more representative sample should be used. Furthermore, a genomic assay should be performed to further classify and characterize Chromobacterium violaceum.
Finally, it is indispensable to perform further studies and implement effective hygiene and health practices in the processing of foods of sea origin, particularly those that are consumed fresh and raw, like oysters in order to reduce the risk of infection.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo funding was received for this study.
Conflict of interestThe authors declare that they have no conflict of interests.