Postural control is considered the basis for the development of motor skills in people with DS. Therefore, the analysis of postural control could guide the rehabilitation of these patients.
ObjectiveTo analyse the postural control in children, adolescents and adults with Down syndrome (DS).
Material and methodsA case–control study. The sample was composed of twenty-two children aged 6–11 years old (10 DS, 12 TD), twenty-three adolescents between 12 and 18 years old (11 DS, 12 TD), and twenty-four young adults 19 and 25 years old (12 DS, 12 TD). Postural control was measured on a force platform in condition of open eyes (OE) and closed eyes (CE) where the centre of pressure (COP) variables were calculated. People with DS and typically developing (TD) were compared.
ResultsNo significant differences were observed in children. In adolescents and adults the COP variables were significantly higher in the groups with DS in OE and CE (P<0.05). In people with DS there were no significant differences between children, adolescents and adults in any of the COP variables. In people with TD significant differences when comparing children, adolescents and adults (P<0.05).
ConclusionsIndividuals with DS have a deficit of postural control and low development of this skill as the individual matures in age.
El control postural se considera la base del desarrollo de habilidades motoras en personas con síndrome de Down (SD). Por ello el análisis del control postural podría orientar su rehabilitación.
ObjetivoAnalizar el control postural en niños, adolescentes y adultos con SD comparativamente con los que presentan un desarrollo típico (DT).
Material y métodoEstudio de casos y controles. La muestra fue compuesta por 22 niños entre 6 a 11 años de edad (10 SD; 12 DT), 23 adolescentes entre 12 y 18 años de edad (11 SD; 12 DT), y 24 adultos jóvenes entre 19 y 25 años de edad (12 SD; 12 DT). El control postural fue medido sobre una plataforma de fuerza en situación de ojos abiertos (OA) y ojos cerrados (OC) calculándose las variables del centro de presión (COP).
ResultadosEn niños, no hubo diferencias estadísticamente significativas. En adolescentes y adultos las variables del COP fueron significativamente mayores en los grupos con SD en OA y OC (p<0,05). En personas con SD no hubo diferencias significativas entre niños, adolescentes y adultos en ninguna de las variables del COP. En personas con DT se observaron diferencias significativas al comparar rangos etarios (p<0,05).
ConclusionesLas personas con SD presentan un déficit del control postural y un bajo desarrollo de esta habilidad a medida que van madurando en edad.
Down syndrome (DS) is a chromosome disorder caused by the presence of an extra copy of chromosome 21.1 That children with DS are characterised by delayed motor development and difficulty in performing functional motor tasks is well known.2 Children with DS present hypotonia, ligamentous hyperlaxity, delayed latency in muscle activation and deficits in posture control.3 These motor area problems continue until adult life.4
Various authors believe that postural control is the basis for the development of motor skills in individuals with SD.5–7 Postural control is considered a complex motor skill derived from the interaction of multiple sensorimotor processes to control the body in space.8 This includes an interaction between the sensory system, the central nervous system (CNS) and the motor system. Maintaining postural control depends on the sensory systems and their ability to integrate the information in the CNS to generate a motor response appropriate for the environmental requirements.9 There are disagreements as to sensory input to postural control due to the physiological maturation of these systems. Some studies indicate that the sensory systems mature between 7 and 10 years of age, when the response patterns are similar to those of adults.3,10,11 Other authors suggest that sensory system maturation is reached around the age of 14–15.9 In individuals with typical development (TD), it has been established that as individuals grow, postural control gradually improves and reaches its most advanced development point in the early stages of adult life.10,11 This has not been researched in individuals with DS.
The universally accepted method for quantifying postural control is through centre of pressure (COP) (the same acronym is used in Spanish and English) displacement using a force platform that senses postural oscillations.12 From COP measurements, variables such as displacement area, velocity and medial-lateral (ML) (side-to-side) and anterior-posterior (AP) (forward-backward) components.12 The greater the value of these variables, the worse the postural control. Children and adolescents with DS have been shown to have poor postural control.5–7,13–15 In adults with DS, few studies have focused on alterations in this motor skill.5 In Latin America, there are no studies analysing postural control in individuals with DS.
The purpose of this study was to analyse postural control in children, adolescents and adults with DS.
MethodThis was a case–control observational study. The sample was non-probabilistically selected and based on convenience. All the participants with DS and the minors with TD were authorised by their legal guardians by informed consent. The adults with TD read and signed an informed consent voluntarily.
ParticipantsThe sample consisted of 22 children between 6 and 11 years old (10 DS; 12 TD), 23 adolescents between 12 and 18 years old (11 DS; 12 TD), and 24 young adults between 19 and 25 (12 DS; 12 TD). Both the participants with DS and those with TD had to be able to understand simple instructions and walk independently. The following were exclusion criteria for both groups: musculoskeletal lesions, lower limb surgery, pain in any part of the body at the time of evaluation, vestibular disorders, uncorrected visual disorders and use of technical aids for walking.
ProcedurePostural control was measured with a 40cm×40cm ArtOficio force platform (Artoficio Ltda., Santiago, Chile). The data were acquired with a sample rate of 40Hz. Igor Pro software version 5.01 (WaveMetrics Inc., Oregon, USA) was used to calculate COP variables. Postural control was measured with participants’ eyes open (EO) and eyes closed (EC). Each measurement lasted 30s. The participants were told to stand as quietly as possible, with their arms relaxed along their trunks and their feet shoulder width apart for the 30s. Each condition (EO and EC) was tested 3 times and the results were averaged for the COP variables. Based on COP displacement in ML and AP directions, the following variables were obtained: COP area (m2), COP velocity (m/s), COP velocity in ML direction (ML velocity) (m/s) and COP velocity in AP direction (AP velocity) (m/s).
Statistical analysisThe statistical software SPSS 14.0 (SPSS 14.0 para Windows, SPSS Inc., IL, USA) was used for the analyses and the mean and standard deviation were calculated for all the variables. Data distribution and homogeneity of variance were established with the Shapiro–Wilk and Levene tests, respectively. Student's t-test for independent samples was used to compare postural control between the groups with DS and TD according to age range, and the ANOVA test to compare among children, adolescents and adults. Effect size was calculated using Cohen's d, considering a small (0.2), moderate (0.5) or strong (0.8) effect. An alpha level of 0.05 was used for all the analyses.
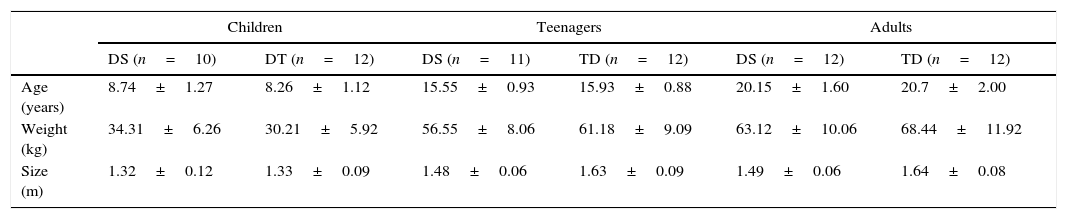
ResultsTable 1 presents the basal characteristics of the sample (age, weight and size).
Baseline characteristics of participants (mean and standard deviation).
| Children | Teenagers | Adults | ||||
|---|---|---|---|---|---|---|
| DS (n=10) | DT (n=12) | DS (n=11) | TD (n=12) | DS (n=12) | TD (n=12) | |
| Age (years) | 8.74±1.27 | 8.26±1.12 | 15.55±0.93 | 15.93±0.88 | 20.15±1.60 | 20.7±2.00 |
| Weight (kg) | 34.31±6.26 | 30.21±5.92 | 56.55±8.06 | 61.18±9.09 | 63.12±10.06 | 68.44±11.92 |
| Size (m) | 1.32±0.12 | 1.33±0.09 | 1.48±0.06 | 1.63±0.09 | 1.49±0.06 | 1.64±0.08 |
kg: kilograms; m: meters; DS: Down syndrome; TD: typical development.
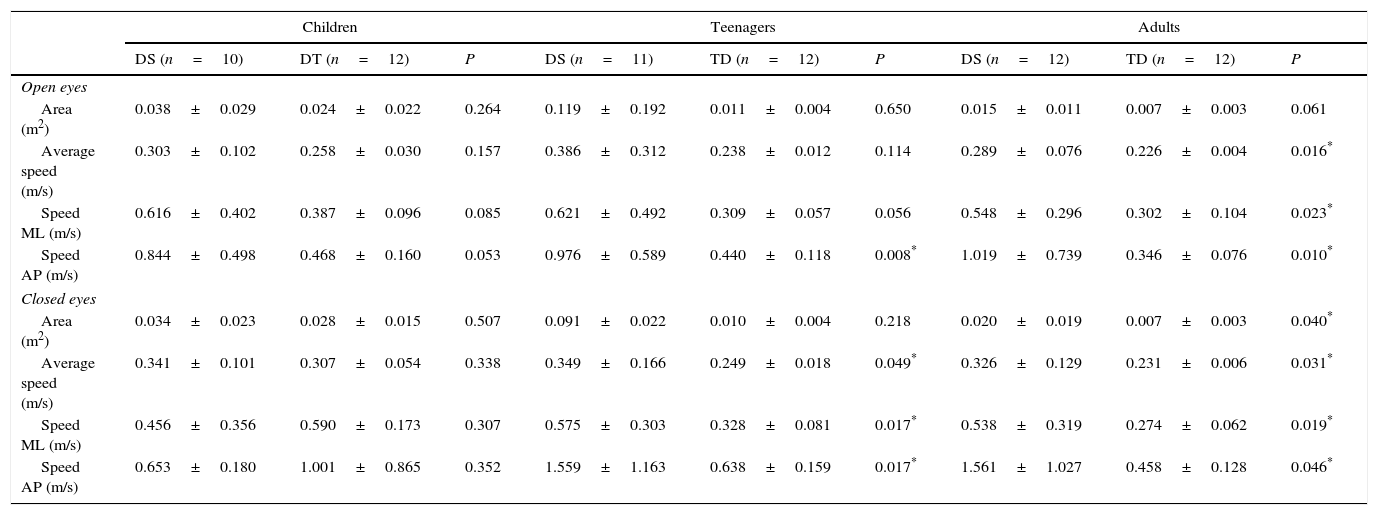
The COP variables increased in value in the individuals with DS compared with those with TD (Table 2). In children, no statistically significant differences were found. In adolescents, the differences were statistically significant in EO AP velocity (P=.008; d=1.26), mean EC velocity (P=.049; d=0.85), EC ML velocity (P=.017; d=1.11) and EC AP velocity (P=.017; d=1.11). In adults, statistically significant differences were observed in mean EO (P=.016; d=1.17), EO ML velocity (P=.023; d=1.28), EO AP velocity (P=.010; d=1.10), EC area (P=.040; d=0.96), mean EC velocity (P=.031; d=1.04), EC ML velocity (P=.019; d=1.15) and EC AP velocity (P=.046; d=1.50).
Comparison of the variables of the COP between DS and TD (average and deviation standard).
| Children | Teenagers | Adults | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DS (n=10) | DT (n=12) | P | DS (n=11) | TD (n=12) | P | DS (n=12) | TD (n=12) | P | |
| Open eyes | |||||||||
| Area (m2) | 0.038±0.029 | 0.024±0.022 | 0.264 | 0.119±0.192 | 0.011±0.004 | 0.650 | 0.015±0.011 | 0.007±0.003 | 0.061 |
| Average speed (m/s) | 0.303±0.102 | 0.258±0.030 | 0.157 | 0.386±0.312 | 0.238±0.012 | 0.114 | 0.289±0.076 | 0.226±0.004 | 0.016* |
| Speed ML (m/s) | 0.616±0.402 | 0.387±0.096 | 0.085 | 0.621±0.492 | 0.309±0.057 | 0.056 | 0.548±0.296 | 0.302±0.104 | 0.023* |
| Speed AP (m/s) | 0.844±0.498 | 0.468±0.160 | 0.053 | 0.976±0.589 | 0.440±0.118 | 0.008* | 1.019±0.739 | 0.346±0.076 | 0.010* |
| Closed eyes | |||||||||
| Area (m2) | 0.034±0.023 | 0.028±0.015 | 0.507 | 0.091±0.022 | 0.010±0.004 | 0.218 | 0.020±0.019 | 0.007±0.003 | 0.040* |
| Average speed (m/s) | 0.341±0.101 | 0.307±0.054 | 0.338 | 0.349±0.166 | 0.249±0.018 | 0.049* | 0.326±0.129 | 0.231±0.006 | 0.031* |
| Speed ML (m/s) | 0.456±0.356 | 0.590±0.173 | 0.307 | 0.575±0.303 | 0.328±0.081 | 0.017* | 0.538±0.319 | 0.274±0.062 | 0.019* |
| Speed AP (m/s) | 0.653±0.180 | 1.001±0.865 | 0.352 | 1.559±1.163 | 0.638±0.159 | 0.017* | 1.561±1.027 | 0.458±0.128 | 0.046* |
DS: Down syndrome; TD: typical development.
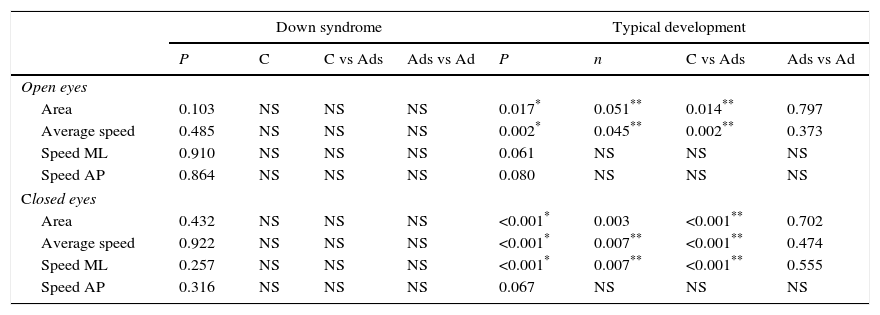
In individuals with DS, there were no statistically significant differences between children, adolescents and adults in any of COP variables, in either the EO or the EC conditions (Table 3).
Results of the comparison of the variables of the COP among children, adolescents and adults with SD and DT.
| Down syndrome | Typical development | |||||||
|---|---|---|---|---|---|---|---|---|
| P | C | C vs Ads | Ads vs Ad | P | n | C vs Ads | Ads vs Ad | |
| Open eyes | ||||||||
| Area | 0.103 | NS | NS | NS | 0.017* | 0.051** | 0.014** | 0.797 |
| Average speed | 0.485 | NS | NS | NS | 0.002* | 0.045** | 0.002** | 0.373 |
| Speed ML | 0.910 | NS | NS | NS | 0.061 | NS | NS | NS |
| Speed AP | 0.864 | NS | NS | NS | 0.080 | NS | NS | NS |
| Closed eyes | ||||||||
| Area | 0.432 | NS | NS | NS | <0.001* | 0.003 | <0.001** | 0.702 |
| Average speed | 0.922 | NS | NS | NS | <0.001* | 0.007** | <0.001** | 0.474 |
| Speed ML | 0.257 | NS | NS | NS | <0.001* | 0.007** | <0.001** | 0.555 |
| Speed AP | 0.316 | NS | NS | NS | 0.067 | NS | NS | NS |
C: children; Ads: adolescents; Ad: adults; NS: not significative.
In individuals with TD, there were statistically significant differences when comparing children, adolescents and adults in the variables EO area (P=.017), mean EO velocity (P=.002), EC area (P<.001), mean EC velocity (P<.001) and EC ML velocity (P<.001). The post hoc test established that these differences existed between children and adolescents, and between children and adults (Table 3). There were no statistically different differences between adolescents and adults in any of the variables.
DiscussionThe results of this study show that individuals with DS present a deficit in postural control. Statistically significant differences were detected in adolescents and adults, expressed to a greater extent when the eyes were closed. These results coincide with what was reported in previous studies.5–7,13–15
The neuropathological bases of motor disorder in DS are unknown, but cerebellar dysfunction, delayed myelination and proprioceptive and vestibular deficits have been considered to be factors that strengthen motor disorders.16,17 Postural control deficit in individuals with DS has been attributed to musculoskeletal characteristics inherent to ligamentous laxity and hypotonia.5,18 Directly or indirectly, individuals with DS develop abnormal compensatory strategies to rectify these deficiencies.5 These include delay in reaction time, muscle co-contraction and wide joint movements,3 contributing to greater oscillations when standing. In our study, the postural control of the individuals with DS was deficient compared with the group with TD. The greater COP area observed reflects greater postural oscillations; increased mean COP ML and AP velocities show the incapacity of these individuals to control adequately the oscillations in both the ML and AP directions.
Prior studies have reported postural control alterations in children with DS in comparison with a group with DT.5,7,15 In this study, the children with DS presented greater values in all the COP variables, but these differences were not statistically significant between the two groups. Children with TD have been described as having poor postural control compared with adults.10,11 Gatica et al. (2013) found that children had greater postural oscillations than older adults, who are widely known to have deterioration in postural control.19 This could explain why no statistically significant differences were found between children with DS and TD, given that both groups would present poor postural control. The children's postural control deficit might be influenced by the immaturity of the sensory systems. Some authors indicate that the maturation of these systems is reached around 14–15 years of age.9 The immaturity of sensory systems in the children would serve as the basis for the differences found in the group with TD between children and adolescents, and between children and adults.
In adolescents, there were significant differences between DS and TD, shown mainly in EC conditions, coinciding with reports from other studies.5,6 In adults, statistically significant differences between DS and TD were observed in all the COP variables, except in EO area. Previously, only 1 study had described the differences in this age group.5 In our study, the differences between DS and TD became more evident as the age ranges got older. Rigoldi et al. (2011) postulate that in DS motor development alterations gradually progress from infancy through adulthood, which is when the motor anomalies are most evident.5 It is believed that the scarce sensorimotor experiences undergone by children with DS play a role in the poor development of postural control in adolescence and adulthood.7 Such undeveloped sensorimotor skills in individuals with DS would explain the absence of significant postural control differences among children, adolescents and adults. In individuals with TD, the differences between children and adolescents and between children and adults were clear, probably influenced by adequate sensorimotor stimulation.
The greatest differences were found under EC conditions, both in comparing DS with TD and comparing among children, adolescents and adults. When the eyes are closed, there are greater postural oscillations because one of the sensory systems that contributes to postural control is inhibited. Consequently, the possibilities of maintaining stability drop and, in compensation, the solicitation of the vestibular and somatosensory systems increases.20 This makes the test with EC more demanding than the test with EO, and makes the differences between groups more evident.
During the first years of life, early physiotherapy for children with DS has focused on improving control motor and coordination to achieve development milestones. Once the children begin to walk, very few continue receiving physiotherapy.14 Our study results indicate the relevance of receiving early, constant physiotherapeutic treatment to accompany the motor development of individuals with DS until they are adults. Wang et al. (2012) showed that a deficit in balance (reflected in the increase in COP variables) correlated with motor skills essential for voluntary movements.7 These included standing up, dynamic activities such as walking, running and jumping, and muscle strength. Consequently, maintaining proper postural control would favour the development of static and dynamic daily-life activities.
Limitations of this study include the limited sample size and the selection of participants by convenience. These probably restrict the external validity of the study. Despite this, the statistical significance and strong effect size demonstrate the strength of the differences observed in our investigation.
In conclusion, our study results show a deficit in postural control in individuals with DS and poor development of this skill as the individual matures in age.
Conflicts of interestThe authors declare no conflicts of interest.