Introduction
Osteoblasts are mononucleate cells responsible for bone formation (osteoblastosis). Bone metastasis caused by various types of cancers (breast, prostate, lung, etc.) is one of the diseases of the skeletal system in which there is high osteoblastic activity. The effects of this disease can be palliated with the use of diphosphonates, non-hormonal agents with affinity for osteoblastic activity sites, which act as osteoblastosis inhibitors1-3.
In nuclear medicine, diphosphonates are used both as diagnostic and therapeutic agents. In diagnosis, they are usually administered in the form of methyl diphosphonate labeled with 99mTc (99mTc-MDP) for routine bone scanning and treatment follow-up of chemotherapy and/or radiotherapy of bone metastases4,5. As a therapeutic agent, the ethylenediaminetetramethylenephosphonate labeled with 153Sm (153Sm-EDTMP) and the hydroxyethylidenediphosphonate labeled with 186re (186Re-HEDP) are among the most commonly used. These compounds have been successfully employed alone or combined with other diphosphonates, in the treatment of secondary bone metastases of breast, lung, kidney and prostate cancer6-12.
Another disease of the skeletal system, where osteoblastosis is present, is the arthrosis, a degenerative joint disease associated to aging. It is the most common type of arthropathy and one of the most disabling disorders in developed countries. It is the second leading cause of long term disability, following cardiovascular disease. The estimated annual cost in the United States is more than $33 billion, due to the treatment of the disease and the work absenteeism caused by it. Characteristic symptoms are: deep pain that worsens over time, morning stiffness, snapping and limitation of movement. The most commonly affected joints are the hips, knees, cervical and low lumbar vertebrae, proximal and distal inter-phalanges and the first carpal-metacarpal and tarsus-metatarsus joint1-3. As the histological mechanism that governs the arthrosis is similar to that in bone metastasis (this mechanism being the osteoblastosis), and the therapeutic action of the diphosphonates is to act on this mechanism, it is expected that if diphosphonates have therapeutic action on bones metastasis, they will have it on the arthrosis, as well. The 153Sm-EDTMP has been approved by the Food and Drug Administration (FDA) for pain palliation in bone metastasis. However, it has been employed, together with the 186Re-HEDP for pain palliation in other bone diseases such as inflammatory arthritis, refractory rheumatoid arthritis and multifocal arthritis when conventional treatments have failed13,14. So far, there are to our knowledge no published works on the use of radiolabeled diphosphonates, specifically 153Sm-EDTMP in the treatment of arthrosis.
Conventional treatments for arthrosis are based on the administration of analgesics such as acetaminophen or nonsteroidal anti-inflammatory analgesic drugs (NSAIDs). These drugs are toxic and cause damage to the gastric and duodenal mucosa. They are frequently suspended due to side effects and the patient motility is still compromised by pain. Surgery and / or prosthesis are not always an option, due to medical or economic reasons. It's for those patients that the 153Sm-EDTMP pain palliation therapy is proposed. The use of this compound in the treatment of bone metastases produces pain palliation in 30% to 90% of the patients with an average of 65% and its effect lasts from one to 18 months. If similar effects are found in patients with arthrosis, their quality of life would be significantly improved.
On the other hand, patients who claim pain in the bones and have already been diagnosed with cancer, are strong candidates to be suffering from bone metastases. The conventional procedure to follow is to perform a bone scanning using 99mTc-MDP and once metastases are confirmed, the palliative treatment with 153Sm-EDTMP starts about a week after. The 153Sm radionuclide is a beta-gamma emitter which decays with a half-life of 46.284 hours. This radionuclide enhances the analgesic effect of the diphosphonate because of its beta emission (maximum energy of 810 keV and average of 233 keV). But it also has a gamma emission with energy of 103 keV, which could be useful for bone imaging. In these patients, if a bone scan is performed, using 153Sm-EDTMP (following the same protocol 99mTc-MDP), once the bone metastases diagnosis is confirmed, a 153Sm-EDTMP therapeutic dose can be immediately completed, optimizing treatment time and cost.
The aim of this work is to explore the efficient and safe use of systemically administered 153Sm-EDTMP in two cases. Firstly, as a bone scanning agent for patients suspected to have bone metastases, prior to its use as a palliative for pain. Secondly, as an analgesic treatment for patients with arthrosis, to whom conventional treatments have not been effective and/or have produced undesirable side effects, after verifying that the compound is uptaken in the injured joint. In the first case, the diagnostic efficiency of the compound is evaluated, based on image quality, compared to the conventional 99mTc-MDP agent. In the second case, the palliative effect on pain and joint motility enhancement is assessed, while monitoring patients' hematology to assure no toxicity to the bone marrow. This study was carried out with the knowledge and signed consent of the patients involved.
Methods and materials
Patient selection, inclusion, non-inclusion and exclusion criteria to the study
Patients, who participated in this study, expressed their agreement through a signed written consent. The research protocol was approved by the research and Bioethics Committee of the School of Medicine of the Autonomous University of the State of Mexico (Universidad Autónoma del Estado de México, UAEMex), and by the Scientific and Bioethics research Committee of the Oncology Center of the institute of Social Security of the State of Mexico and Municipalities (Centro Oncológico Estatal del Instituto de Seguro Social del Estado de México y Municipios, COE-iSSEMyM).
The patient inclusion criteria were the following: male or female non pregnant, of at least 55 years of age, with diagnosed arthrosis (joint pain and reduced motility) or, with suspected bone metastases. In the case of patients with arthrosis, their response to previous conventional treatments must have been unsatisfactory.
The criteria of non-inclusion and exclusion of the study were: history of hereditary cancer in first degree relatives (in order to decrease the probability of stochastic effects); additional pathologies such as diabetes, liver and kidney diseases as well as disorders of the urinary and reproductive systems; discontinuity in treatment follow-up appointments; dropping out of the study due to personal reasons or complications of the illness.
Sampling
A non-probabilistic, of opportunity and sequential sample of 10 patients (5 female and 5 male), was selected, with a diagnosis of arthrosis. Patients` average age was 68 years, median 70 years, range from 58 to 77 years and standard deviation of 7.3 years. In addition, a male patient of 56 years with bone metastasis secondary to prostate cancer was included.
Study protocol
Bone scanning image acquisition using 99mTc-MDP and 153Sm-EDTMP in the patient with bone metastasis and in patients with arthrosis
In order to compare the uptake and the diagnostic efficiency through image quality, of the proposed radiopharmaceutical, an activity of 740MBq of 99mTc-MDP was administered to each patient through one minute of intravenous infusion, followed by oral administration of 2,000 ml of water to facilitate elimination through urine. After 3 hours, a bone scan was performed, using a double detector, variable angle, Siemens e.cam gamma camera, with the detectors in a 180º angle, during 12-15 minutes.
Two weeks later, a second set of images were acquired in the same patients, administering 740MBq of 53Sm-EDTMP, following the same protocol as for 99mTc-MDP. The bone scans obtained with both radiopharmaceuticals were qualitatively compared in order to evaluate the uptake of the 153Sm-EDTMP.
Static images acquisition using 99mTc-MDP and 153Sm-EDTMP in the patient with bone metastasis and in patients with arthrosis
In order to quantitatively determine the activity uptake, three hours after administrating to each patient 740MBq of 99mTc-MDP and 53Sm-EDTMP, respectively, a set of static images was acquired using the same equipment as previously described. in the case of the metastatic patient, the images were of the patient's left shoulder (where the metastasis was located) and of the upper third of the humerus, in order to consider the background activity. In the case of the patients with arthrosis, the images were of the patients' knees and lower third of the femur. In both cases, the images were taken placing the affected area at 7 cm from the anterior detector and 15cm from the posterior one during 5 minutes.
Administration of the 153Sm-EDTMP therapeutic dose in patients with arthrosis
A therapeutic dose of 37MBq per kg of body weight was systemically administered to the patient with arthrosis, in order to reach the range of total injected activity from 2.1 to 2.8GBq. This injection was followed by oral administration of 2,000 ml of water to facilitate elimination through urine. Presence of radioactivity in the patient's urine was monitored during 6 hours, after which, the patient is discharged.
Bone marrow toxicity evaluation in patients with arthrosis
Evaluation of the bone marrow toxicity of the treatment was performed by hematology studies, during an 8-week follow-up. Five ml of peripheral blood of each patient were analyzed weekly, determining red blood cells, neutrophils, lymphocyte and platelet counts and were compared to the levels prior of 153Sm-EDTMP administration. Myelosuppression was considered when a significant decrease was registered, in comparison with the reference levels.
Pain evaluation in patients with arthrosis
The evaluation of pain due to arthrosis was performed using a qualitative 10 point scale, where 0 represents no pain and 10 the most intense pain, according to the recommendations of the American College of rheumatology15. In each patient, the intensity of pain before administration of the 153Sm-EDTMP (reference level) is compared with the one after, once a week, for 7 weeks. it was considered that there was change in the intensity of pain when a variation of at least 2 points was recorded. A positive variation meant deterioration and a negative one, improvement.
Motility evaluation in patients with arthrosis
in a similar manner, the evaluation of motility was performed also using a qualitative 10 points scale, where 10 represents unrestricted motility. It was considered as an improvement, an increase of at least one point in the scale in comparison with the motility registered before the administration of 153Sm-EDTMP (reference level) and as deterioration, a decrease of at least one point.
Statistical analysis in patients with arthrosis
The statistical analysis was performed with the Student's t Test (for paired samples), where measurements of pain and hematology were taken from the same patient before and after the 153Sm-EDTMP application, once a week, for a 7 week follow-up.
Results
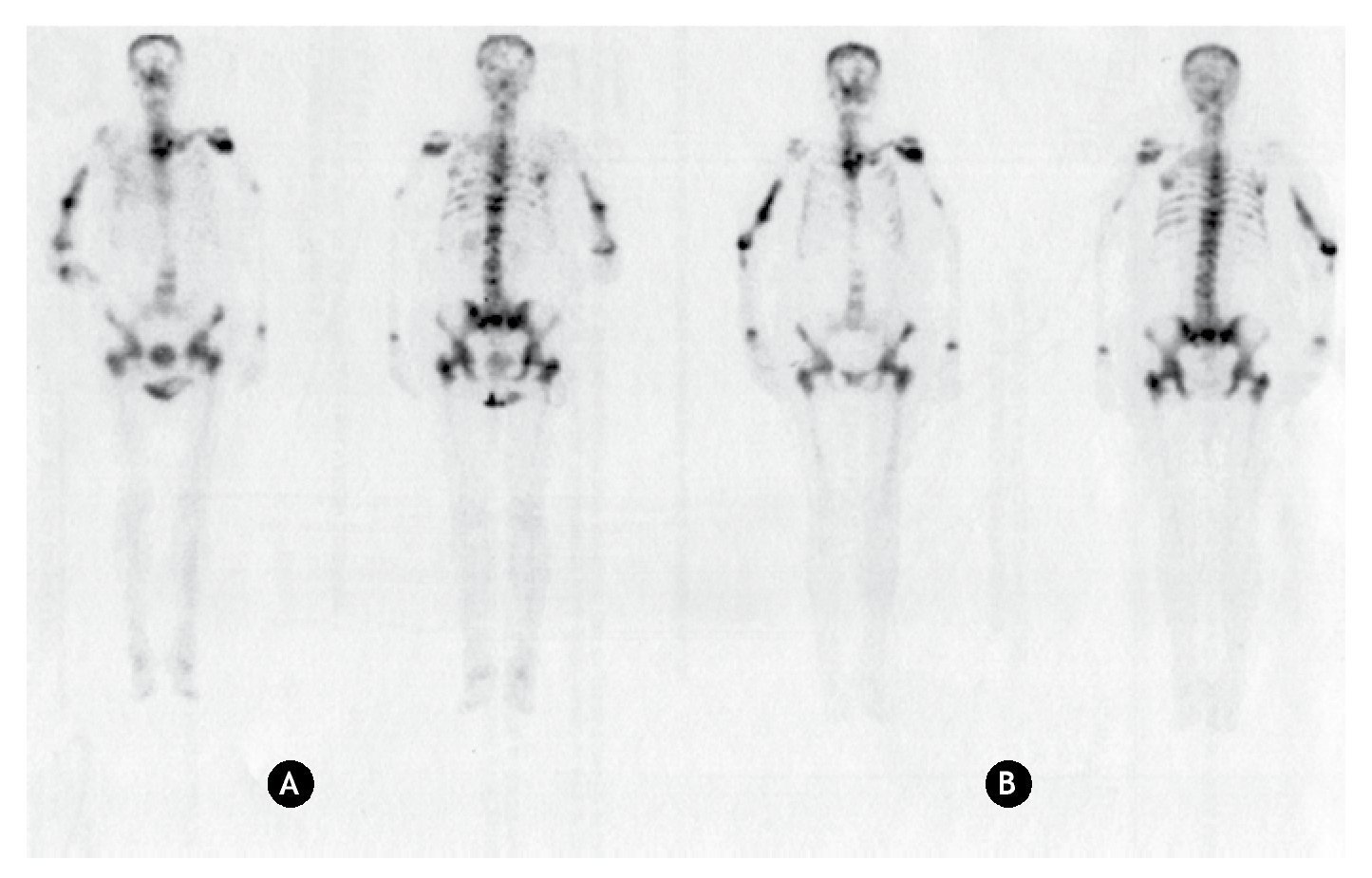
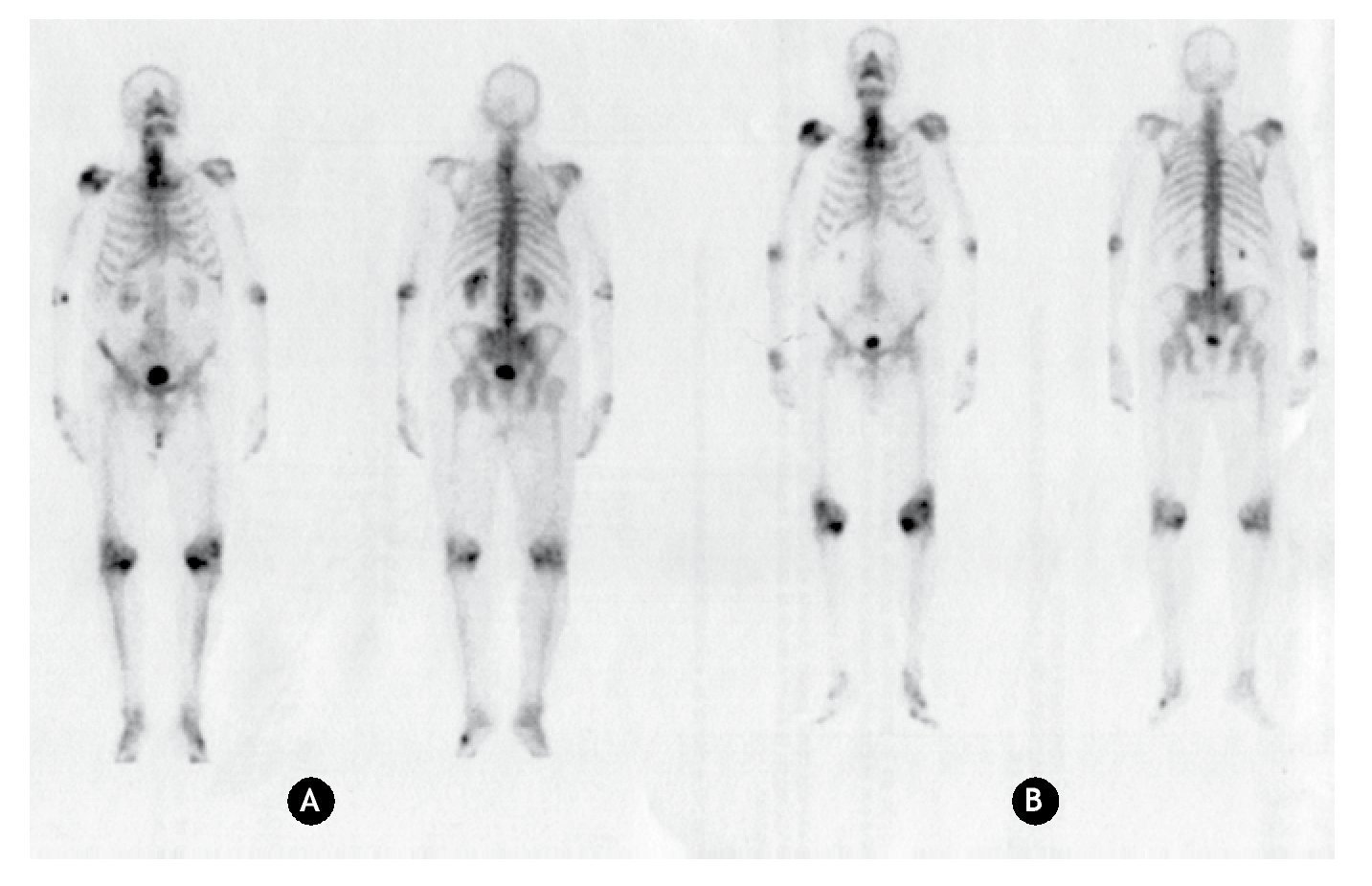
Since 99mTc-MDP is the standard radiopharmaceutical to diagnose bone lesions, bone scans using both 99mTc-MDP and 153Sm-EDTMP were carried in patients to visualize the uptake of these radiopharmaceuticals. Anterior and posterior bone scans acquired using 99mTc-MDP and 153Sm-EDTMP in the patient with bone metastasis and one patient with join arthrosis are shown in figures 1 and 2.
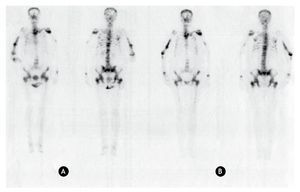
Figure 1 Anterior and posterior bone scanning images of a patient with bone metastasis. A) Using 99mTc-MDP. B) Using 153Sm-EDTMP.
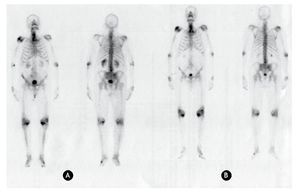
Figure 2 Anterior and posterior bone scanning images of a patient with arthrosis. A) Using 99mTc-MDP. B) Using 153Sm-EDTMP.
In the patient with bone metastasis, similar uptakes in the same sites are observed with both radiopharmaceuticals, leading to the same diagnosis. Consequently, based on a clinical point of view, the diagnostic bone scanning to confirm the presence of bone metastases can be performed either with 99mTc-MDP or 153Sm-EDTMP.
The advantage of using 153Sm-EDTMP as a diagnostic agent can be supported by the fact that, in a patient with suspected bone metastases, the localization of those can be determined with a trace activity of 153Sm-EDTMP, and once confirmed, the activity can be immediately completed to a therapeutic one. In this way the bone scan with 99mTc-MDP would not be necessary, therefore the pain palliation treatment would be optimized in time and cost.
As in the case of bone metastasis, for patients with arthrosis, similar uptakes in the pathologic joints are also observed with both radiopharmaceuticals. Therefore, 153Sm-EDTMP can be used in the treatment of arthrosis because it is uptaken and retained in the injured joins.
In order to evaluate the uptake trend among a healthy knee, a knee with arthrosis, and a site with bone metastases, static images were taken using both 99mTc-MDP and 153Sm-EDTMP. Table 1, shows the ROI/background ratios obtained from the anterior and posterior static images. The bone metastasis site is located in the left shoulder of the patient. It also shows that the ROI/background ratio for both radiopharmaceuticals is very similar, indicating a similar trend uptake in the different regions.
In the case of the metastatic patient, the upper third of the humerus was considered the background value because the metastatic region was the shoulder. For both radiopharmaceuticals, the results are similar. There is significant difference in captured activity, between background and both healthy knee and injured sites. Among injured sites, for each radiopharmaceutical, captured activity is also significantly different, being higher in the metastatic bone, intermediate in the knee with arthrosis and low in the healthy knee. This is due to a higher osteoblastic activity in a joint compared to skeletal bone.
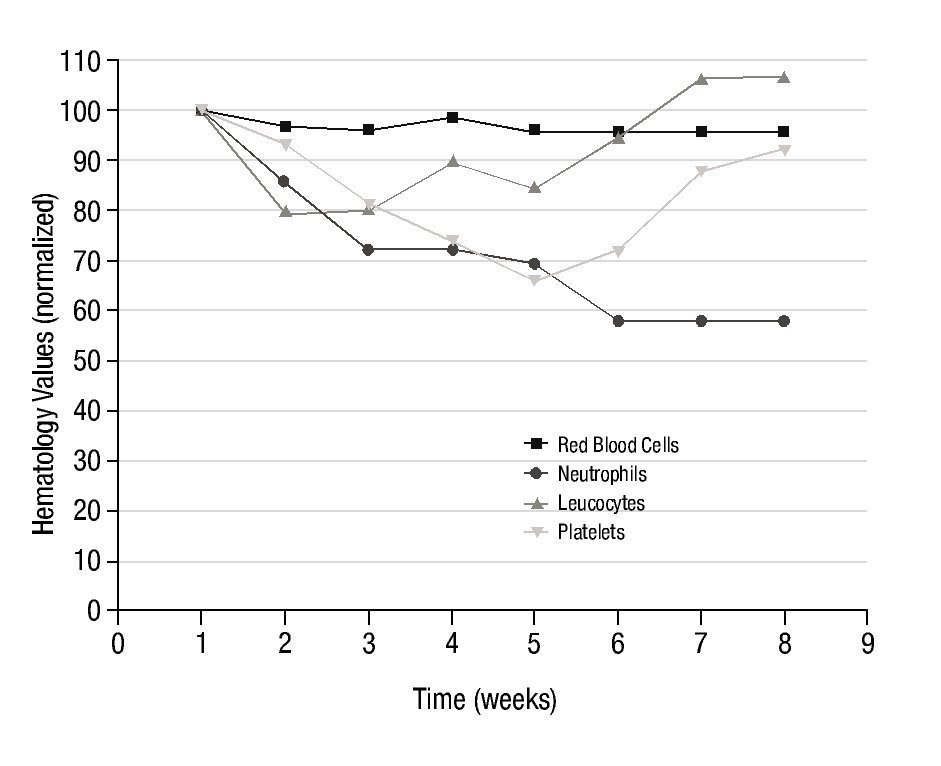
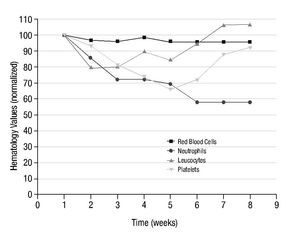
Figure 3 shows the results obtained in the average number of red blood cells, neutrophils, lymphocytes and platelets, in the 10 patients with arthrosis after the systemic administration of a therapeutic dose of 153Sm-EDTMP, during a 7 weeks follow-up.
Figure 3 normalized hematology values in 10 patients with arthrosis, during 7 weeks of follow-up, after 153Sm-EDTMP administration.
Despite count variations shown in Figure 3 with respect to pre-treatment levels, after the 7 weeks period, some values were fully reestablished and others were stabilized with a tendency to reach normal levels. No clinically significant toxic effect on bone marrow was observed, since none of the patients required specific corrective therapeutic intervention.
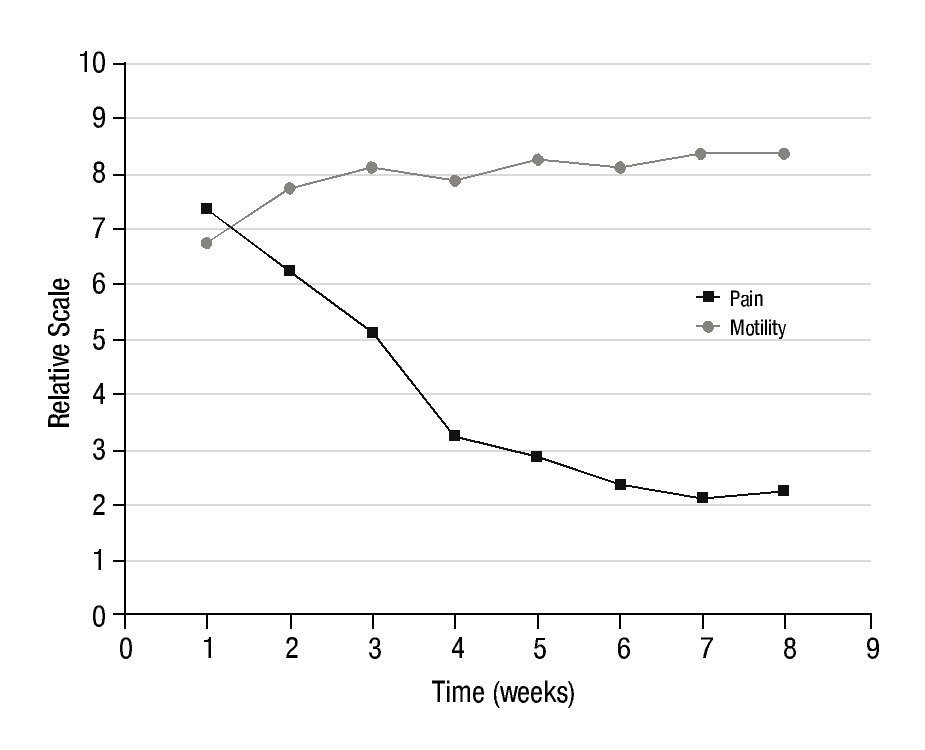
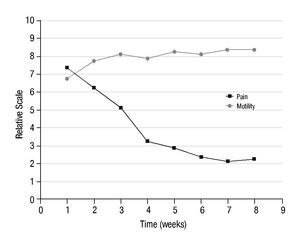
Figure 4 shows the evolution of the average level of pain and motility in the 10 patients with arthrosis, during the 7 weeks of follow-up, after completing the administered activity of 153Sm-EDTMP to the therapeutic level.
Figure 4 Average level of pain and motility in 10 patients with arthrosis, during the 7 weeks of follow-up, after 153Sm-EDTMP administration.
In all patients, the reduction of pain was notoriously marked and the motility was also improved. The improvement in motility was not as remarkable as the pain reduction but sufficient to show effective analgesic effect of 153Sm-EDTMP in arthrosis.
Discussion
The similar behavior of 153Sm-EDTMP and 99mTc-MDP in bone lesions in patients with metastases or arthrosis is explained by the fact that the carrier molecule in both radiopharmaceuticals is a diphosphonate, which is preferably captured in the sites of higher osteoblastic activity. For this reason the images obtained with both radiopharmaceuticals are very similar and lead to the same diagnosis. However, given the high radiation dose absorbed in the bone marrow due to 740 MBq of 153Sm-EDTMP for bone scanning (1.54 mgy/MBq), this procedure is not recommended for routine exploratory purposes. It is only recommended for patients already diagnosed with cancer and who also complain of bone pain, and therefore suspected to have bone metastases. These patients are candidates to pain palliation treatment with 153Sm-EDTMP therapeutic doses (> 740 MBq).
On the use of 153Sm-EDTMP for pain palliation in patients with arthrosis, an important matter to take into account is the absorbed dose. The range of total injected activity in this work was from 2.1 to 2.8 GBq. in a work reported by Wick et al.16, the incidence of leukemia and other malignant diseases was investigated after 10 weekly injections of about 1 MBq of 224Ra each, in humans. This radionuclide is deposited, as well as 153Sm-EDTMP, in bone. It has a longer half-life (T1/2 = 3.66 d) than 153Sm and decays to radioactive nuclides, unlike 153Sm who decays to stable 153Eu. The cumulative α-dose to the marrow due to 224Ra is 0.56 Gy which represents 11.2 Sv, and the one due to 153Sm is (1.54 mgy/ MBq * 2800, MBq = 4.3 Gy) 4.3 Sv. The value of 2800 MBq is the maximum activity of 153Sm-EDTMP injected in our study for arthrosis pain palliation. Wick et al.16, reported that "the time-dependent cumulative hazard function is nearly proportional to the expected cumulative hazard until about 30 years after 224Ra treatment". The expected cumulative hazard was determined from a population not exposed to the 224Ra treatment. They also found that for exposed patients, the probability of leukemia and myeloid leukemia incidence is approximately 3 - 4 times and about five times higher respectively, 35 - 45 years after the first 224Ra treatment.
Although the treatment with 224Ra is more aggressive to bone marrow that that with 153Sm-EDTMP (224Ra equivalent dose is 11.4 Sv vs. 4.3 Sv for 153Sm), let's assume that the probabilities for cumulative hazard and leukemia are the same. The average age of patients with arthrosis in this study was 68 years. Despite of the low number of patients, this age is very representative of patients with advanced
arthrosis who present disabling pain and had a number of unsuccessful previous treatments. As stated previously, patients with hereditary cancer history, diabetes, liver and kidney diseases as well as disorders of the urinary and reproductive systems were excluded from this study. By 2010, life expectancy in Mexico was 77.8 and 73.1 years for women and men respectively. if these patients are going to suffer from leukemia of any other malignant disease as a consequence of the treatment with 153Sm-EDTMP 30 years later, they would be by then 98 years old. it is most likely that they would have died before that age due to any other condition, while at the present time; their quality of life is greatly improved because of the pain palliation.
In fact, the hematology values reported in this work are in very good agreement with a previous reported study where 153Sm-EDTMP has been systemically administered for pain palliation in refractory rheumatoid arthritis13. in that study, Mikuls et al. injected an activity of 37 MBq/Kg, which is exactly the same activity administered in this work. They found that bone marrow suppression was both mild and transient. in our case, 7 weeks post-injection there was an overall decrease of about 5% in the number of erythrocytes. Erythrocytes show an initial decline of approximately 200,000 cells during the first two weeks post-treatment, with almost full recovery by fourth week. From week 4 to week 5 the same reduction in about 200,000 cells is observed, and the reached number is stabilized. Significant statistical differences (p<0.05) relative to the pre-treatment values, (Student's t Test for paired samples) were found in all weeks excepting week 4. in Mikuls et al. work13, the overall reduction of erythrocytes was 6%.
The overall reduction in the number of neutrophils is about 40%. Since the first week the tendency is to decrease over time until week 6, where the value is stabilized. This 40% of decrease means a reduction of 1,500 cells. Significant statistical differences (p<0.05) relative to the pre-treatment values (Student's t Test for paired samples), were found in all weeks. in Mikuls et al. work13, the overall reduction of neutrophils was also 40%.
The lymphocytic cells show a maximum decrease of 20% in the first week post-injection. This decrease corresponds, in absolute numbers, to approximately 400 cells and is statistically significant (p<0.05). From the third week on, the tendency is to recover the pre-treatment value. By week 6 post-treatment, the pre-treatment number of lymphocytes was fully recovered and exceeded in about 5%.
Finally, platelet count shows a progressive reduction during 4 weeks post-injection reaching a maximum decrease of 35% by week 4. From this week on, the tendency was to reach a full recovery. By week 7 post-injection the platelet value was about 92% on the pre-treatment level. in absolute numbers this means a reduction from 220.000 to 146,000 platelets by week 4 post-injection and a recovery to 202,000 by week 7. Statistically significant differences were observed from week 2 to 6 post-injection. in Mikuls et al. work13, the overall reduction of platelets was 42% reaching this maximum reduction value also at week 4 post-injection.
The variations found in this work on the hematological profile are not only in good agreement with Mikuls et al. work13, but also with other results reported in the literature such as the palliative effect of 153Sm-EDTPM on breast cancer bone metastasis pain, resistant to opioid medication12.
Despite the high dose received by the bone marrow from the 153Sm decay process, the hematological values obtained in this work indicates, as in the case of the referred works, that the systemic administration of 37 MBq/Kg is well tolerated.
In the assessment of the pain, the pre-treatment value of 7 point had a rapid decrease during the 3 weeks post-injection to reach a value of 4 points. During the following weeks the pain reduction was more gradual reaching a final and stable value of 2 points. Statistical differences were significant (p<0.05) starting from the first post-injection week. In the case of joint motility, an average improvement of 3 points in the respective scale was also observed. These results were consistent in all patients, demonstrating the analgesic properties of 153Sm-EDTMP intravenously administered, in accordance with its previously known analgesic properties in radiation synovectomy7-9. These results are also in good agreement with Palmedo et al.14 where 186Re-HEDP was systemically injected to patients with polyarthritis. They found that a single injection led to an improvement of the disease reducing both pain and inflammation in joints.
Arthrosis can be a very disabling disease for adults who are still in working active age. This may become in a serious problem given the level of absenteeism that it causes and the limited quality of life. One alternative for those patients where conventional treatments have failed and refuse synovectomy is the systemic administration of 153Sm-EDTMP. A detailed analysis of the patient´s medical history is required. if the patient is candidate for the treatment, a therapeutic dose of the radiopharmaceutical is administered which is well tolerated by the bone marrow.
Conclusions
Bone scanning using 740 MBq of 153Sm-EDTMP and 99mTc-MDP were carried out in 10 patients with arthrosis and one patient with bone metastases. Both radiopharmaceuticals were administered systemically. images obtained show that both radiopharmaceuticals are uptaken in the same injured sites. images produced by 153Sm-EDTMP are similar to those obtained with the conventional protocol with 99mTc-MDP from a clinical point of view. Therefore, in an effort to optimize in time and cost the pain palliation treatment in patients with suspected bone metastases, secondary to a confirmed primary cancer, a trace activity of 153Sm-EDTMP could be used as a diagnostic agent, prior to the therapeutic dose. Additionally, given that 153Sm-EDTPM is captured by joints injured by arthrosis, a therapeutic activity of 2.1 to 2.8 GBq of 153Sm-EDTPM was administered to patients with arthrosis whom conventional treatments had previously failed. This administration from the dosimetric point of view was well tolerated, and from the clinical one has analgesic effect.
Financial Support
No financial support was received to carry out this research.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Ms Brenda león Mejía, Technician of the nuclear Medicine Department of the Centro Oncológico Estatal, iSSEMyM, for her technical support.
* Corresponding author:
Facultad de Medicina, Universidad Autónoma del Estado de México.
Paseo Tollocan s/n, Colonia Moderna de la Cruz, Z.P.
50180, Toluca, Mex., Mexico.
Phone: (+52) 722 2174564, ext. 109.
Fax: (+52) 722 2174564, ext. 102.
E-mail: ahardy42@yahoo.com.mx (A. E. Hardy-Pérez).
2.
E-mail: ahardy42@yahoo.com.mx (A. E. Hardy-Pérez).