A new species of the paracalanid copepod genus BestiolinaAndronov, 1991 is described from Laguna Mandinga, a coastal lagoon system of the eastern coast of the southern Gulf of Mexico. Bestiolina mexicana n. sp. most closely resembles B. sinica (Shen & Lee, 1966) and B. arabicaAli, Al-Yamani, and Prusova, 2007 but can be distinguished from these and other known congeners by the number and presence of posterior surface spinules on the second and third exopodal segments of female legs 2–4, and the number of spinules on the anterior surface of the second endopodal segment of legs 2–4. This is the only species of the genus with 3, 0, 0 spines on the exopodal segments of leg 2 and 2, 3 spines on the second endopodal segments of legs 3 and 4, respectively. The mandible blade of B. mexicana n. sp. has 2 large supplementary teeth which have not been hitherto observed in the other species of the genus. This is the smallest species in the genus and has some reductions in the armature of the mouthparts. The new species has been known to occur only from this lagoonal system in the Gulf of Mexico and it is the first species of Bestiolina recorded in the northern Atlantic Ocean. The presence of a member of this primarily Indo-Malayan genus in the northwestern Atlantic probably represents the result of its final divergence and is explained by dispersal processes related to the Pliocene conditions.
Se describe una especie nueva de copépodo paracalánido del género BestiolinaAndronov, 1991 de la laguna Mandinga, un sistema de lagunas costeras en la costa oriental del sur del golfo de México. Bestiolina mexicana n. sp. se asemeja más estrechamente a B. sinica (Shen & Lee, 1966) y B. arabicaAli, Al-Yamani and Průšová, 2007 pero puede ser distinguida de estos y otros congéneres conocidos por el número y la presencia de espínulas en la superficie posterior de los segmentos exopodales segundo y tercero de las patas 2-4 de la hembra, y el número de espínulas en la superficie anterior del segundo segmento endopodal de las patas 2-4. Esta es la única especie del género con 3, 0, 0 espinas en los segundos segmentos exopodales de las patas 2 y con 2, 3 espinas en los segundos segmentos endopodales de las patas 3 y 4, respectivamente. La hoja mandibular de Bestiolina mexicana n.sp. posee 2 grandes dientes complementarios que no se han observado hasta ahora en otras especies del género. Esta es la especie más pequeña del género y muestra algunas reducciones en la armadura de las piezas bucales. La especie nueva se presenta sólo en este sistema lagunar en el golfo de México y es la primera especie de Bestiolina registrada en el Atlántico norte. La presencia de un miembro de este género, primariamente indo-malayo en el Atlántico noroeste, probablemente refleja el resultado de su divergencia final y se explica por los procesos de dispersión relacionados con las condiciones del Plioceno.
The calanoid copepod family Paracalanidae is known to contain 8 genera, some of which are among the most common planktonic copepods in estuarine and coastal waters of tropical and subtropical latitudes (Kimmerer, 1984; Rakhesh, Raman, & Sudarsan, 2006). The paracalanid genus BestiolinaAndronov, 1991 currently includes 7 species: B. inermis (Sewell, 1912), B. similis (Sewell, 1914), B. sinica (Shen & Lee, 1966), B. zeylonica (Andronov, 1972), B. amoyensis (Li & Huang, 1984), B. arabicaAli et al., 2007, and the recently described B. coreanaMoon, Lee, and Soh, 2010. Most of them are deemed as estuarine, coastal forms and are widely distributed in tropical and subtropical Asian and Indo-Pacific waters; their origin and current distributional pattern clearly corresponds to the Indo-Malayan region (Ali et al., 2007; Moon et al., 2010). Except for a record of B. similis in the South African coast, species of this genus have not been hitherto recorded in the Atlantic Ocean (Moon et al., 2010; Razouls, de Bovée, Kouwenberg, & Desreumaux, 2005–2015) and its presence in the Northwestern tropical Atlantic was unlikely.
The analysis of zooplankton samples collected in the coastal lagoon system of Mandinga, state of Veracruz, Mexico, in the southern Gulf of Mexico, revealed the presence of male and female copepods that were tentatively identified as paracalanids. The taxonomic examination of these specimens showed that they belong to an undescribed species of the genus BestiolinaAndronov, 1991. The new species is here described based on both adult male and female specimens. The unexpected occurrence of this predominantly Indo-Malayan calanoid genus in a coastal estuarine system of the Northwestern Atlantic is discussed.
Materials and methodsZooplankton samples were obtained at dawn of May 1st, 2015 with hand nets (mesh sizes 100 and 200μm) in shallow areas (depth: 0.6–1.2m) of the lagoonal system of Mandinga, Veracruz, Mexico (19°02′55.2″ N, 96°04′36.0″ W). Environmental parameters were measured with a Hanna Instruments EC/TDS and temperature tester model HI 98130. Temperature of the water during the sampling was 25°C, salinity 23.5psu and slightly alkaline (pH=7.6). Samples were placed in a bucket with 5l of water; copepods were isolated alive 5h after the collection, then fixed in 4% formaldehyde buffered with borax (30g/l of formaldehyde at 40%) and preserved in a 5% glycerine/70% ethanol solution. Several adult male and female Bestiolina were taxonomically examined in the laboratory; specimens were prepared, dissected and examined following Reid (2003). Dissected specimens/appendages were mounted in semi-permanent slides with glycerine sealed with Entellan®, a commercial, fast drying mounting medium and sealant. Drawings were prepared at 1,000× magnifications with the aid of a camera lucida mounted on a standard Olympus BX51 compound microscope equipped with Nomarski DIC. One male specimen was prepared for SEM examination with a TOPCON SM-510 microscope at facilities of ECOSUR in Tapachula, Mexico. The process included dehydration of specimens in progressively higher ethanol solutions (60, 70, 80, 96, 100%), critical point drying, and gold-palladium coating (20nm) following usual methods. This hitherto unknown species was described and illustrated following the current standards for the taxonomic study of the genus (Moon et al., 2010). The morphological terminology follows Huys and Boxshall (1991). The type specimens were deposited in the collection of zooplankton held at El Colegio de la Frontera Sur (ECO-CH-Z), in Chetumal, Mexico and in the Museum Support Center (MSC) of the National Museum of Natural History, Smithsonian Institution, Suitland, Maryland (USNM).
DescriptionOrder CalanoidaSars, 1901–1903
Family ParacalanidaeGiesbrecht, 1893
Bestiolina Andronov, 1991
Bestiolina mexicana sp. nov.
Bestiolina mexicana sp. nov., holotype adult female from the Gulf of Mexico. A, habitus in dorsal view; B, same, lateral view; C, rostrum, semi-lateral view; D, fifth pedigerous somite with row of spinules (arrowed) and urosome, lateral view; E, fifth leg, ventral view; F, fifth leg of one paratype specimen showing slight asymmetry. Scale bars: A, B=100μm, C–F=10μm, D=50μm.
Bestiolina mexicana sp. nov., holotype adult female from the Gulf of Mexico. A, antennule segments 1–14; B, antennule segments 15–23; C, labrum, ventral view; D, mandible with palp; E, gnathal blade with dentition (supplementary monocuspidate teeth indicated with asterisks); F, antenna. Scale bars: A, B=100μm, C, E=10μm, F, D=50μm.
Bestiolina mexicana sp. nov., holotype adult female from the Gulf of Mexico. A, maxillule; B, maxilla; C, maxilliped. Allotype adult male. D, habitus, lateral view; E, rostrum, semi-lateral view; F, mandible palp; G, antenna, a: exopod, b: detail of second endopodal segment; H, maxilliped; I, fifth legs; J, detail of distal and subdistal segments of leg 5. Scale bars: A–C=50μm, D=100μm, E, G a, b, J=10μm, F, H, I=50μm.
Bestiolina mexicana sp. nov. from the Gulf of Mexico, SEM-prepared male specimen. A, cephalic area showing proximal segments of antennule, ventral view, rostral filaments arrowed; B, first antennule segment and antenna, ventral; C, first leg, ventral view, row of setules on second segment arrowed; D, surface of second and third endopodal segments of the second leg, ventral view.
Body robust, widest at pedigerous somites 2–3, anterior end of cephalosome rounded, tapering distally. Body length 0.65–0.69mm, average=0.67mm (n=7). First pedigerous somite separated from cephalosome by complete suture. Rostral projections (Fig. 1C) thick, strongly developed. Posterior outer margins of fifth pedigerous somite rounded, bearing rows of minute spinules (Fig. 1A, B, arrowed in D). Urosome with 4 free somites. Genital double-somite symmetrical in dorsal view, with moderately expanded outer margins; somite strongly protuberant in lateral view (Fig. 1D). Anal somite slightly shorter than preceding 2 urosomites together. Caudal rami symmetrical, almost as long as anal somite, each ramus bearing 5 caudal setae, innermost being shortest.
Antennule (Fig. 2A, B) 23-segmented, reaching midlength of anal somite (Fig. 1A). Ancestral segments II–IV and XXVII–XXVIII fused. Armature (seta=s, aesthetasc=ae, spine=sp.) considering ancestral segmentation (in Roman numerals) as follows: I-IV-7s+ae, V-1s+ae, VI-1s+ae, VII-1s+ae, VIII-1s+ae, IX-1s+ae, X-XI-1s+sp, XII-1, XIII-1s+ae, XIV-1s, XV-1s, XVI-1s, XVII-1s+ae, XVIII-1s, XIX-1s, XX-1s+ae, XXI-1s+ae, XXII-1s, XXIII-2s, XXIV-2s, XXV-2s, XXVI-2s, XXVII–XXVIII-4s+ae.
Antenna (Fig. 2F) with short, subquadrate coxa, separated from basis by complete suture, bearing short, lightly setulated seta. Basis with 2 unequally long distal setae. Endopod 2-segmented, first segment with 2 unequally long setae and row of spinules along distal margin; second antennulary segment bilobed, subterminal lobe with 9 setae, apical lobe with 6 setae. Exopod 8-segmented, segments 2–4 partially fused, with incomplete suture; segments 1–7 each armed with 1 seta, terminal segment with 4 apical setae.
Labrum (Fig. 2C) well developed, bordered by row of strong spinules arranged in tight pattern.
Mandible (Fig. 2D, E) with gnathal blade heavily sclerotized, with 4 strong medial teeth, dorsal teeth bicuspidate, large anterior tooth separated from main blade by wide diastema, tooth furnished with pair of strong secondary monocuspidate teeth (asterisks in Fig. 2E). Palp biramous, basis with 4 subequal setae. Exopod relatively short, 5-segmented, setal formula as: 1, 1, 1, 1, 2; endopodal ramus 2-segmented, first segment with 4 subequal setae, second segment with 11 setae.
Maxillule (Fig. 3A) with praecoxal arthrite bearing 14 setal elements on and around distal margin, 4 of them setiform, 10 stout, spiniform. Coxal endites armed with 3, 3 setae, respectively; coxal epipodite with 9 setae. Basis with 4 setae on endite; endopod and exopod with 14 and 11 setae, respectively.
Maxilla (Fig. 3B) with well-developed, subquadrate praecoxal endite armed with 5 setae, 2 succeeding coxal endites each armed with 3 setae. Basis with 4 setae; endopod 3-segmented, first segment with 1, second with 3 and small terminal segment with 5 setae, 3 of them apical.
Maxilliped (Fig. 3C) slender, relatively long, with elongate syncoxa, armed with 4 groups of setal elements, proximalmost with 1, second with 2, third with 3, and distalmost group with 4 setae; rows of minute spinules at insertion of second and fourth setal groups. Basis with 5 setae. Endopod 6-segmented, setal formula as: 2, 3, 3, 2, 4, 4.
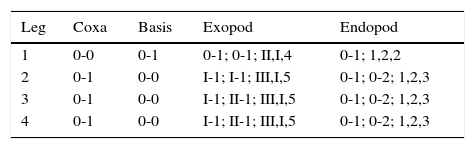
Spine and setal formula of P1 to P4 as follows:
Leg 1 (Fig. 4A) with stout inner basipodal seta. Exopod 3-segmented, endopod 2-segmented. Second exopodal segment with tight row of setules on outer margin. Legs 2–4 with 3-segmented exopods and 3-segmented endopods; coxal plates smooth, narrow, subrectangular. Coxae of legs 2–4 with strong, plumose inner coxal seta. Basipodal seta absent in legs 2–4.
Leg 2 (Fig. 4B) first exopodal segment ornamented with row of 3 spinules at insertion of outer spine. Second endopodal segment with 2 spinules on anterior surface, 4 on posterior surface (not illustrated).
Legs 3 (Fig. 4C) and 4 (Fig. 4D) with 2 and 3 spinules on anterior surface of second endopodal segment, respectively.
Leg 5 (Fig. 1E) typically reduced, represented by pair of symmetrical rounded lobes, some specimens with slight asymmetry, one lobe being smaller (Fig. 1F).
Description of adult maleBody more slender than female (Fig. 3D) and slightly smaller. Total body length 0.63–0.67mm, average=0.64mm (n=4). Cephalosome fused with first pedigerous somite. Posterolateral margins of fifth pedigerous somite rounded, with row of minute spinules (Fig. 3D). Rostral projections thick, tapering distally, as in female (Figs. 3E, arrowed in Fig. 5A). Urosome with 5 somites. Caudal rami symmetrical, about 2 times longer than wide, armed with 5 caudal setae; inner margins of rami lightly setulated.
Antennule (Figs. 3D, 5A, B) 18-segmented, extending to distal part of second urosomite (Fig. 4C): ancestral segments I–IV, V–VIII, and IX–X fused. Segmentation and setation pattern as follows: I–IV-5+5ae, V–VIII-5+4ae, IX–X-2+I+ae, XI–XII-2+ae, XIII-0, XIV-1+ae, XV-0, XVI-2, XVII-0, XVIII-1+ae, XIX-0, XX-1, XXI-1, XXII-0, XXIII-1, XXIV-1+1, XXV-1, XXVI-1+1, XXVII–XXVIII-4+ae.
Antenna (Figs. 3Ga-b, 5B) biramous, coxa and basis completely fused, with single seta; endopod 2-segmented, first segment unarmed, second segment bilobed, ornamented with row of spinules on distal margin at insertion of apical setae. Subdistal lobe with 4 setae, 3 of them apical, 1 subapical (Fig. 3Gb); distal lobe armed with 5 setae; exopod incompletely fused, armed with 5 setae.
Mandible (Fig. 3F) coxal gnathobase absent, basis armed with short papilliform seta; exopod 2-segmented, armed with 6 setae, with partial sutures (arrowed in Fig. 4F); endopod 2-segmented, first segment unarmed, second endopodal segment with 8 setae.
Maxilliped (Fig. 3G) reduced, with 4 segments including long, robust syncoxa, shorter subrectangular basis plus 2-segmented endopod. Syncoxa naked, basis with short seta. First endopodal segment with 3 setae, outermost being thick, bipinnate; second segment with 4 setae, 2 of them thick, bipinnate.
Leg 1. Basipodal seta flexible, curved, uniserially setulated, third segment with proximal row of long, flexible setules (arrowed in Fig. 5C); all other characters as described for female.
Leg 2. Outer spines of exopodal segments relatively longer, narrower than in female. Anterior surface of second endopodal segment smooth (Fig. 5D). All other characters as in female.
Leg 5 strongly asymmetrical (Fig. 3F, G), right leg as in female, represented by rounded lobe; left leg elongate, 5-segmented, almost as long as urosome. Penultimate segment with pointed process on distal margin; distal segment with short spiniform process and long, slender apical spine.
Taxonomic summaryMaterial examined. Holotype. Adult female, dissected, mounted in glycerin, sealed with Entellan (ECO-CHZ-09308), Laguna de Mandinga, Veracruz, Mexico (19°02′55.2″ N, 96°04′36.0″ W), collected May 1, 2015 by R.J. Almeyda-Artigas. Allotype male, dissected, same site, date, and collector (ECO-CHZ-09309). Paratypes. Two adult females, dissected, same date and collector, 3 adult females, undissected, same locality, date, and collector, ethanol-preserved, vial (ECO-CHZ-09310); 3 adult males, same collection data, undissected, vial (ECO-CHZ-09311). One adult male, 1 adult female, undissected, ethanol-preserved (USNM-1283119). Non-type specimen: 1 adult male, same collection data, processed for SEM analysis.
Etymology. The species name refers to Mexico, the country in which the genus is first reported outside its known geographical distribution, in the Northwestern Atlantic.
RemarksThe specimens examined were identified as members of the paracalanid genus Bestiolina because of the lack of serration on the outer margins of the second and third exopodal segments of legs 2–4, a reduced female leg 5 represented by a pair of simple lobes and an asymmetrical male leg 5 with the left ramus as in the female, with a rounded lobe and a long, 5-segmented left leg furnished with a short apical spiniform process and a relatively long, slender spine (Andronov, 1991; Moon et al., 2010). The species of BestiolinaAndronov, 1991 are in general very much alike in terms of body shape and proportions, body segmentation and leg armature but they can be distinguished by subtle characters. The spinulation pattern on the anterior and posterior surfaces of the exopodal and endopodal segments of legs 2–4 is the most relevant character used to separate species (Ali et al., 2007; Moon et al., 2010), but also there are differences in the presence or absence of minute spinules on the posterior corners of the fifth pedigerous somite, and the structure of the rostrum, among other characters (Moon et al., 2010).
The new species, B. mexicana, was compared with the 7 other known congeners. When running the identification key by Moon et al. (2010), our specimens key down to the couplet B. zeylonica/B. sinica regarding the presence or absence of spinules on the exopodal segments 1–3 of leg 2; the new species has spinules on the first segment only, so far a unique character among the known species of the genus. The new species can be readily distinguished from B. similis (Sewell, 1914) by the structure of the rostrum, which has long, slender rostral filaments (Greenwood, 1976, Figs. a, b; Wellershaus, 1969, Fig. 12), thus contrasting with the relatively strong, stout rostral projections of the new species (Fig. 1C). Based on the comparative work by Moon et al. (2010), the presence of spinules on the posterior margin of the fifth pedigerous somite is a character shared by 5 species: B. coreana, B. amoyensis, B. zeylonica, B. sinica, and also by the new species, B. mexicana. Also, in B. sinica the second endopodal segment of leg 3 has 5 spinules on the posterior surface, thus differing from the new species with none in the same segment (Fig. 4C). The new species has important affinities with B. sinica, including a reduced ornamentation of the exopodal segments of legs 2–4; in the latter species these segments lack spinules (Shen & Lee, 1966, Figs. 13–15) hence differing from B. mexicana with spinules present on the first exopodal segment of leg 2. Also, the second endopodal segments of legs 2–4 have a set of 4 or 5 spinules on each (anterior and posterior) surfaces (Shen & Lee, 1966, Figs. 13–15; Moon et al., 2010). The new species diverges from these patterns in having 3 spinules on the first exopodal segment of leg 2 and 2, 3 spinules of the anterior and posterior surfaces of the second endopodal segments of legs 2–4, respectively. The rostrum is short, weakly developed in B. sinica (Shen & Lee, 1966, Fig. 11) vs. a strong, thick rostrum in the new species.
Bestiolina arabica is another species in which spinules are absent from the exopodal segments, but it has 3, 3 spinules on the second endopodal segment of legs 2 and 3, respectively (Ali et al., 2007, Figs. 3b, c), thus differing from the new species, with a 3, 0 formula (Fig. 4B, C). Also, the shape and width of the rostral projections differ between these 2 species; they are slenderer in B. arabica (Fig. 1c) than in B. mexicana n. sp. (Fig. 1C).
The new species has 3 spinules on the surface of the first exopodal segment of leg 2; this is a character that it shares only with B. zeylonica (Andronov, 1972, Fig. 4; Moon et al., 2010, Table 1). In the other species this segment has 0 or 2 such elements (Moon et al., 2010). Aside of this character, B. zeylonica differs from the new species, B. mexicana, in the ornamentation of legs 2–4; this species has 3, 2 spinules on leg 2 second and third exopodal segments, respectively vs. 0, 0 in the new species. In addition leg 3 exopodal segments 1–3 carry 0, 3, 2 spinules, respectively (Andronov, 1972, Fig. 15; Moon et al., 2010, Table 1) vs. none in B. mexicana (Fig. 4C). Also, the female antennules are distinctively long in B. zeylonica, reaching well beyond the posterior margin of the caudal rami (Andronov, 1972, Fig. 2), whereas antennules barely reach the anterior margin of the anal somite in the new species (Fig. 1A). The new species is the smallest known species of the genus (females 0.65–0.69mm; males 0.63–0.67mm), followed closely by B. zeylonica (0.68–0.70, 0.61, respectively). All other species of Bestiolina range between 0.75–1.02 (females) and 0.7–0.96 (males) (Razouls et al., 2005–2015).
DiscussionThe planktonic copepod fauna of the Laguna de Mandinga was previously surveyed by Álvarez-Silva and Gómez-Aguirre (2000); they recorded 7 species but no paracalanids were present in their samples. When first revealed, the record of a species of Bestiolina in the Northwestern Atlantic was surprising because of the genus strong Indo-Pacific and Asian affinities (Moon et al., 2010). Hence, its presence was first attributed to a relatively recent (at least after 2000) introduction via ballast water, aquaculture, or other human activities, as has been known to occur for different Asian coastal calanoids (Orsi & Ohtsuka, 1999); hence, the first working approach was to identify the introduced species among those previously known. The taxonomical analysis and comparisons of the subtle characters currently used to separate the species of Bestiolina (Ali et al., 2007; Moon et al., 2010) allowed us to confirm that the species from Mandinga is undescribed, different from its Asian congeners. This work allows an increase of the number of known species of Bestiolina to 8 (Ali et al., 2007; Andronov, 1972; Moon et al., 2010).
The new species is the first member of the genus described from the Atlantic Ocean and represents the first record of Bestiolina in the Gulf of Mexico (Suárez-Morales, Fleeger, & Montagna, 2009) but also in the Northwestern Tropical Atlantic. There is one unconfirmed record of B. similis (as Acrocalanus similis) from the California area in the Mexican Pacific coast (Suárez-Morales & Gasca, 1998).
Based on the zoogeographic analysis presented by Moon et al. (2010), it is clear that the genus Bestiolina had its radiation core in the Indo-Malayan region and different biogeographic processes allowed it to spread and colonize new geographic areas in the region. This scenario did not include the occurrence of the genus in the Northwestern Tropical Atlantic (NWTA). A similar pattern is known for the distribution of the genus Tortanus Giesbrecht in Giesbrecht and Schmeil, 1898, another widespread brackish water coastal calanoid that is most diverse in the Indo-Pacific region but with representatives in the NWTA (Ohtsuka, 1992; Ohtsuka & Reid, 1998). There are at least 4 species of this genus reported from the NWTA, all of them members of the subgenus Acutanus: T. (Acutanus) setacaudatusWilliams, 1906, T. (A.) compernisGonzález and Bowman, 1965, T. (A.) angularisOhtsuka, 1992, and T. (A.) ecornatusOhtsuka and Reid, 1998 (Ohtsuka, 1992; Ohtsuka & Reid, 1998). The first species has been recorded in the Gulf of Mexico and the area of Florida (Suárez-Morales et al., 2009) and remarkably, also in the Mandinga lagoonal system (Álvarez-Silva & Gómez-Aguirre, 2000). The second species is known only from Bahía Fosforescente, the type locality in Puerto Rico; the third species is distributed along the Caribbean coast of Belize and Mexico (Ohtsuka & Reid, 1998; Suárez-Morales, 1994) and T. ecornatus is known from Jamaica. According to the phylogeographic analysis by Ohtsuka and Reid (1998), these 4 species of the Acutanus clade represent the final divergence of the radiation of the groups of species of Tortanus and is characterized by reductions on the armature of the antennule, antennae and swimming legs. Overall, this diversity represents the result of speciation and successful dispersal of this clade of Tortanus along the coastal areas of the NWTA. When explaining the presence of this group of species of Tortanus in the Caribbean and adjacent areas of the NWTA, Ohtsuka and Reid (1998) discarded the hypothesis of a Tethyan origin and provided an explanation involving more recent paleogeographic processes. Particularly, the Indo-Malayan planktonic species reaching the NWTA could have arrived through the Panama Isthmus during the Middle Miocene (12–15 MYA) or even earlier, from the late Oligocene (24 MYA). At that time the Panama passage was open and many islands (proto-Antilles) were moving to the east (Iturralde-Vinent & MacPhee, 1999), thus explaining the occurrence of Tortanus in Puerto Rico and Jamaica. In addition, the dominant flow for dispersion of planktonic forms at that time was westward to eastward, particularly after the Miocene (Van der Spoel, 1983). So the conditions were adequate for the tropical ancestors of these species to disperse and colonize the Caribbean coastal systems and then to have a regional diversification after the closing of the Panama passage in the Pleistocene. According to Ohtsuka, Yoon, and Endo (1992), and Ohtsuka, Ueda, and Lian (1995), marine organisms belonging to the initial East Asian endemic group evolved as taxa highly adapted to brackish water and thus are dominant in estuarine, coastal systems of East Asia; these initial endemic copepods may be able to adapt to new habitats, thus explaining their ability to disperse and colonize. The same general pattern appears to explain the occurrence of Bestiolina in the Northwestern Atlantic, particularly in the southern Gulf of Mexico. If it is assumed that these Indo-Malayan forms radiated at the same time and went through similar processes as those inferred for Tortanus, it is likely that there are other species of Bestiolina dwelling in the insular and continental Caribbean coasts. Hence, it is not that surprising to have a species of Bestiolina occurring in the Northwestern Atlantic. The new species has some interesting reductions in the armature of the antennules and interesting apomorphies like the supernumerary teeth on the gnathal blade but also a reduced set of ornamentation on legs 2–4, which contrast with the regular dentition and the richer spinulation patterns found in the Asian species (Ali et al., 2007; Moon et al., 2010). The finding of this species of Bestiolina strengthens the notion that Asian coastal/estuarine forms had successful interoceanic dispersal processes and were able to colonize the Northwestern Atlantic as a result of pre-Pliocene conditions.
Guadalupe Nieto kindly prepared the male specimen of the new species for SEM analysis and guided our observations at the Laboratorio de Microscopía Electrónica de Barrido in ECOSUR-Tapachula. Rosa Ma. Hernández deposited the type specimen in the collection of zooplankton at ECOSUR-Chetumal and provided catalog numbers. Kathryn Ahlfeld (Smithsonian Institution, National Museum of Natural History) kindly provided the USNM catalog number.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.