The aim of this study was to investigate monogenean fauna in gills of Geophagus camopiensis, Pterophyllum scalare, Satanoperca jurupari, and Satanoperca acuticeps in a tributary from the Amazon River system in Brazil. A total of 2,148 monogenean specimens were collected from 140 fish examined from March 2012 to March 2013, and 84.3% of these fish were parasitized by 1 or more species. Such monogeneans were: Sciadicleithrum geophagi, Sciadicleithrum juruparii, Gussevia spiralocirra and Gyrodactylus sp. However, only G. camopiensis was parasitized by more than 1 species of monogenean, while S. jurupari and S. acuticeps were parasitized by the same species. Prevalence, mean intensity and mean abundance varied among host species and the highest levels of infection were by G. spiralocirra followed by S. geophagi, both parasites with aggregated dispersion. Abundance of monogeneans was not influenced by the size of the host. In G. camopiensis, the infection levels by S. geophagi did not vary during the rainy or drainage seasons. This is the first study on monogenean infections for G. camopiensis and S. acuticeps.
El objetivo de este estudio fue investigar la fauna de monogéneos en las branquias de Geophagus camopiensis, Pterophyllum scalare, Satanoperca jurupari y Satanoperca acuticeps en un afluente del sistema del río Amazonas en Brasil. De marzo de 2012 a marzo del 2013 se examinaron 140 peces, en los cuales se encontraron 2,148 monogéneos, el 84.3% de los peces resultaron parasitados por una o más especies: Sciadicleithrum geophagi, Gyrodactylus sp., Gussevia spiralocirra y Sciadicleithrum juruparii. Sin embargo, solo G. camopiensis albergaba más de una especie de monogéneos, mientras S. jurupari y S. acuticeps resultaron infectadas por la misma especie. La prevalencia, la intensidad media y la abundancia variaron entre especies de hospedero y los niveles más altos de infección fueron causados por G. spiralocirra, seguido por S. geophagi, ambos parásitos con dispersión agregada. La abundancia de monogéneos no resultó influenciada por el tamaño del hospedero. En G. camopiensis, los niveles de infección por S. geophagi no variaron durante la estación seca o la temporada de lluvias. Este es el primer estudio de infecciones de monogéneos en G. camopiensis y S. acuticeps.
Monogeneans are helminth ectoparasites parasitizing mainly fish. They can fix to body surfaces, fins, swabs or nasal cavities of the hosts. However, a few species are endoparasites, inhabiting the stomach, intestine or urinary bladder of fishes. They have a direct life cycle and consequently a high reproduction rate. Thus, many ectoparasite species are pathogenic to the host, causing serious problems for fish farms (Boeger & Viana, 2006; Cohen, 2013). They are parasites with high host specificity if compared to other helminths (Bellay, Ueda, Takemoto, Lizama, & Pavanelli, 2012; Boeger & Viana, 2006; Braga, Araújo, & Boeger, 2014; Poulin, 1992), and some monogenean species may have a seasonal infection pattern (Boeger & Viana, 2006; Neves, Pereira, Tavares-Dias, & Luque, 2013; Tavares-Dias, Oliveira, Gonçalves, & Silva, 2014).
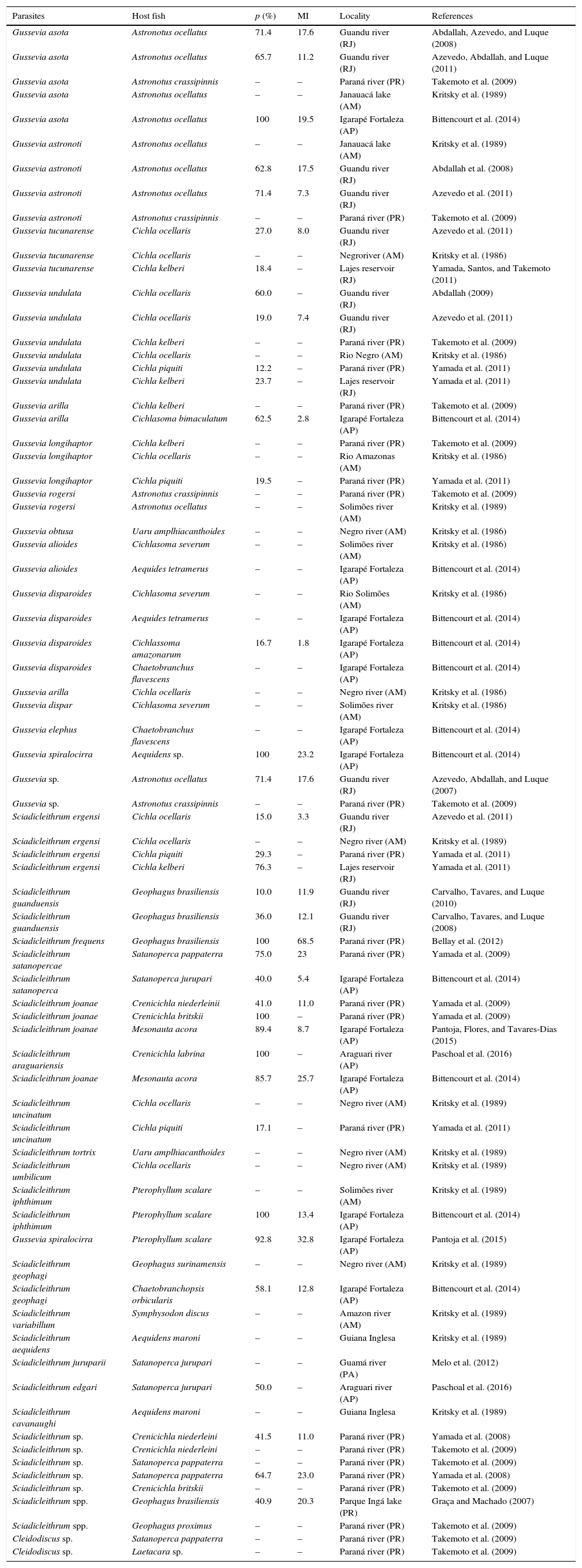
Neotropical cichlids are often parasitized by species of Gussevia Kohn and Paperna, 1964, SciadicleithrumKritsky, Thatcher, and Boeger, 1989, Trinidactylus Hanek, Molnar and Fernando, 1974, TucunarellaMendoza-Franco, Scholz, and Rozkosna, 2010 (Braga et al., 2014; Melo, Santos, & Santos, 2012; Mendoza-Franco & Vidal-Martínez, 2005; Pariselle et al., 2011; Paschoal, Scholz, Tavares-Dias, & Luque, 2016). However, Brazilian cichlids have been mostly parasitized by Gussevia and Sciadicleithrum species and infection levels are highly variable (Table 1). Therefore, Dactylogyridae species are the most frequent monogeneans in these freshwater cichlids.
Monogenean species on native cichlids in Brazil.
| Parasites | Host fish | p (%) | MI | Locality | References |
|---|---|---|---|---|---|
| Gussevia asota | Astronotus ocellatus | 71.4 | 17.6 | Guandu river (RJ) | Abdallah, Azevedo, and Luque (2008) |
| Gussevia asota | Astronotus ocellatus | 65.7 | 11.2 | Guandu river (RJ) | Azevedo, Abdallah, and Luque (2011) |
| Gussevia asota | Astronotus crassipinnis | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia asota | Astronotus ocellatus | – | – | Janauacá lake (AM) | Kritsky et al. (1989) |
| Gussevia asota | Astronotus ocellatus | 100 | 19.5 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia astronoti | Astronotus ocellatus | – | – | Janauacá lake (AM) | Kritsky et al. (1989) |
| Gussevia astronoti | Astronotus ocellatus | 62.8 | 17.5 | Guandu river (RJ) | Abdallah et al. (2008) |
| Gussevia astronoti | Astronotus ocellatus | 71.4 | 7.3 | Guandu river (RJ) | Azevedo et al. (2011) |
| Gussevia astronoti | Astronotus crassipinnis | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia tucunarense | Cichla ocellaris | 27.0 | 8.0 | Guandu river (RJ) | Azevedo et al. (2011) |
| Gussevia tucunarense | Cichla ocellaris | – | – | Negroriver (AM) | Kritsky et al. (1986) |
| Gussevia tucunarense | Cichla kelberi | 18.4 | – | Lajes reservoir (RJ) | Yamada, Santos, and Takemoto (2011) |
| Gussevia undulata | Cichla ocellaris | 60.0 | – | Guandu river (RJ) | Abdallah (2009) |
| Gussevia undulata | Cichla ocellaris | 19.0 | 7.4 | Guandu river (RJ) | Azevedo et al. (2011) |
| Gussevia undulata | Cichla kelberi | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia undulata | Cichla ocellaris | – | – | Rio Negro (AM) | Kritsky et al. (1986) |
| Gussevia undulata | Cichla piquiti | 12.2 | – | Paraná river (PR) | Yamada et al. (2011) |
| Gussevia undulata | Cichla kelberi | 23.7 | – | Lajes reservoir (RJ) | Yamada et al. (2011) |
| Gussevia arilla | Cichla kelberi | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia arilla | Cichlasoma bimaculatum | 62.5 | 2.8 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia longihaptor | Cichla kelberi | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia longihaptor | Cichla ocellaris | – | – | Rio Amazonas (AM) | Kritsky et al. (1986) |
| Gussevia longihaptor | Cichla piquiti | 19.5 | – | Paraná river (PR) | Yamada et al. (2011) |
| Gussevia rogersi | Astronotus crassipinnis | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Gussevia rogersi | Astronotus ocellatus | – | – | Solimões river (AM) | Kritsky et al. (1989) |
| Gussevia obtusa | Uaru amplhiacanthoides | – | – | Negro river (AM) | Kritsky et al. (1986) |
| Gussevia alioides | Cichlasoma severum | – | – | Solimões river (AM) | Kritsky et al. (1986) |
| Gussevia alioides | Aequides tetramerus | – | – | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia disparoides | Cichlasoma severum | – | – | Rio Solimões (AM) | Kritsky et al. (1986) |
| Gussevia disparoides | Aequides tetramerus | – | – | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia disparoides | Cichlassoma amazonarum | 16.7 | 1.8 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia disparoides | Chaetobranchus flavescens | – | – | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia arilla | Cichla ocellaris | – | – | Negro river (AM) | Kritsky et al. (1986) |
| Gussevia dispar | Cichlasoma severum | – | – | Solimões river (AM) | Kritsky et al. (1986) |
| Gussevia elephus | Chaetobranchus flavescens | – | – | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia spiralocirra | Aequidens sp. | 100 | 23.2 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia sp. | Astronotus ocellatus | 71.4 | 17.6 | Guandu river (RJ) | Azevedo, Abdallah, and Luque (2007) |
| Gussevia sp. | Astronotus crassipinnis | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Sciadicleithrum ergensi | Cichla ocellaris | 15.0 | 3.3 | Guandu river (RJ) | Azevedo et al. (2011) |
| Sciadicleithrum ergensi | Cichla ocellaris | – | – | Negro river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum ergensi | Cichla piquiti | 29.3 | – | Paraná river (PR) | Yamada et al. (2011) |
| Sciadicleithrum ergensi | Cichla kelberi | 76.3 | – | Lajes reservoir (RJ) | Yamada et al. (2011) |
| Sciadicleithrum guanduensis | Geophagus brasiliensis | 10.0 | 11.9 | Guandu river (RJ) | Carvalho, Tavares, and Luque (2010) |
| Sciadicleithrum guanduensis | Geophagus brasiliensis | 36.0 | 12.1 | Guandu river (RJ) | Carvalho, Tavares, and Luque (2008) |
| Sciadicleithrum frequens | Geophagus brasiliensis | 100 | 68.5 | Paraná river (PR) | Bellay et al. (2012) |
| Sciadicleithrum satanopercae | Satanoperca pappaterra | 75.0 | 23 | Paraná river (PR) | Yamada et al. (2009) |
| Sciadicleithrum satanoperca | Satanoperca jurupari | 40.0 | 5.4 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Sciadicleithrum joanae | Crenicichla niederleinii | 41.0 | 11.0 | Paraná river (PR) | Yamada et al. (2009) |
| Sciadicleithrum joanae | Crenicichla britskii | 100 | – | Paraná river (PR) | Yamada et al. (2009) |
| Sciadicleithrum joanae | Mesonauta acora | 89.4 | 8.7 | Igarapé Fortaleza (AP) | Pantoja, Flores, and Tavares-Dias (2015) |
| Sciadicleithrum araguariensis | Crenicichla labrina | 100 | – | Araguari river (AP) | Paschoal et al. (2016) |
| Sciadicleithrum joanae | Mesonauta acora | 85.7 | 25.7 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Sciadicleithrum uncinatum | Cichla ocellaris | – | – | Negro river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum uncinatum | Cichla piquiti | 17.1 | – | Paraná river (PR) | Yamada et al. (2011) |
| Sciadicleithrum tortrix | Uaru amplhiacanthoides | – | – | Negro river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum umbilicum | Cichla ocellaris | – | – | Negro river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum iphthimum | Pterophyllum scalare | – | – | Solimões river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum iphthimum | Pterophyllum scalare | 100 | 13.4 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Gussevia spiralocirra | Pterophyllum scalare | 92.8 | 32.8 | Igarapé Fortaleza (AP) | Pantoja et al. (2015) |
| Sciadicleithrum geophagi | Geophagus surinamensis | – | – | Negro river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum geophagi | Chaetobranchopsis orbicularis | 58.1 | 12.8 | Igarapé Fortaleza (AP) | Bittencourt et al. (2014) |
| Sciadicleithrum variabillum | Symphysodon discus | – | – | Amazon river (AM) | Kritsky et al. (1989) |
| Sciadicleithrum aequidens | Aequidens maroni | – | – | Guiana Inglesa | Kritsky et al. (1989) |
| Sciadicleithrum juruparii | Satanoperca jurupari | – | – | Guamá river (PA) | Melo et al. (2012) |
| Sciadicleithrum edgari | Satanoperca jurupari | 50.0 | – | Araguari river (AP) | Paschoal et al. (2016) |
| Sciadicleithrum cavanaughi | Aequidens maroni | – | – | Guiana Inglesa | Kritsky et al. (1989) |
| Sciadicleithrum sp. | Crenicichla niederleini | 41.5 | 11.0 | Paraná river (PR) | Yamada et al. (2008) |
| Sciadicleithrum sp. | Crenicichla niederleini | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Sciadicleithrum sp. | Satanoperca pappaterra | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Sciadicleithrum sp. | Satanoperca pappaterra | 64.7 | 23.0 | Paraná river (PR) | Yamada et al. (2008) |
| Sciadicleithrum sp. | Crenicichla britskii | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Sciadicleithrum spp. | Geophagus brasiliensis | 40.9 | 20.3 | Parque Ingá lake (PR) | Graça and Machado (2007) |
| Sciadicleithrum spp. | Geophagus proximus | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Cleidodiscus sp. | Satanoperca pappaterra | – | – | Paraná river (PR) | Takemoto et al. (2009) |
| Cleidodiscus sp. | Laetacara sp. | – | – | Paraná river (PR) | Takemoto et al. (2009) |
P, prevalence; MI, mean intensity.
Studies on infections by monogeneans in populations of Amazonian wild cichlids are scarce. For species of economic importance, such as Satanoperca jurupari Heckel, 1840; Satanoperca acuticeps Heckel, 1840 and Geophagus camopiensis Pellegrin, 1903, as well as for fish important for food consumption of riverine populations from Amazon and for ornamental aquaculture information is limited (Soares et al., 2011). In addition, the monogenean fauna is also unknown for Pterophyllum scalare Schultze, 1823, a fish utilized in Amazonian aquaculture and the ornamental industry in Asia, Europe and North America (Tavares-Dias, Lemos, & Martins, 2010). However, some monogenean species have been reported in some cichlids from the Amazon Basin.
In the Amazon region, Gussevia spiralocirraKritsky, Thatcher and Boeger, 1986 (Kritsky et al., 1986), and Sciadicleithrum iphthimumKritsky, Thatcher and Boeger, 1989 were described from P. scalare (Kritsky et al., 1989). Recently, Tripathi, Agrawal, and Sriivastana (2010) found S. iphthimum parasitizing the gills of P. scalare in aquariums in India, due to intercontinental translocation of this ornamental fish from the Amazon. Sciadicleithrum jurupariiMelo, Santos and Portes-Santos, 2012 (Melo et al., 2012), Sciadicleithrum satanopercaeYamada, Takemoto, Bellay and Pavanelli, 2009 (Mendoza-Franco, Scholz, & Rozkošná, 2010) and Sciadicleithrum edgariPaschoal, Scholz, Tavares-Dias & Luque, 2016 (Paschoal et al., 2016) were described from S. jurupari. Therefore, since there are no other studies on parasites of wild P. scalare, S. jurupari, G. camopiensis and S. acuticeps, this study investigated the fauna of monogeneans of these hosts from a tributary of the Amazon River system, in Northern Brazil.
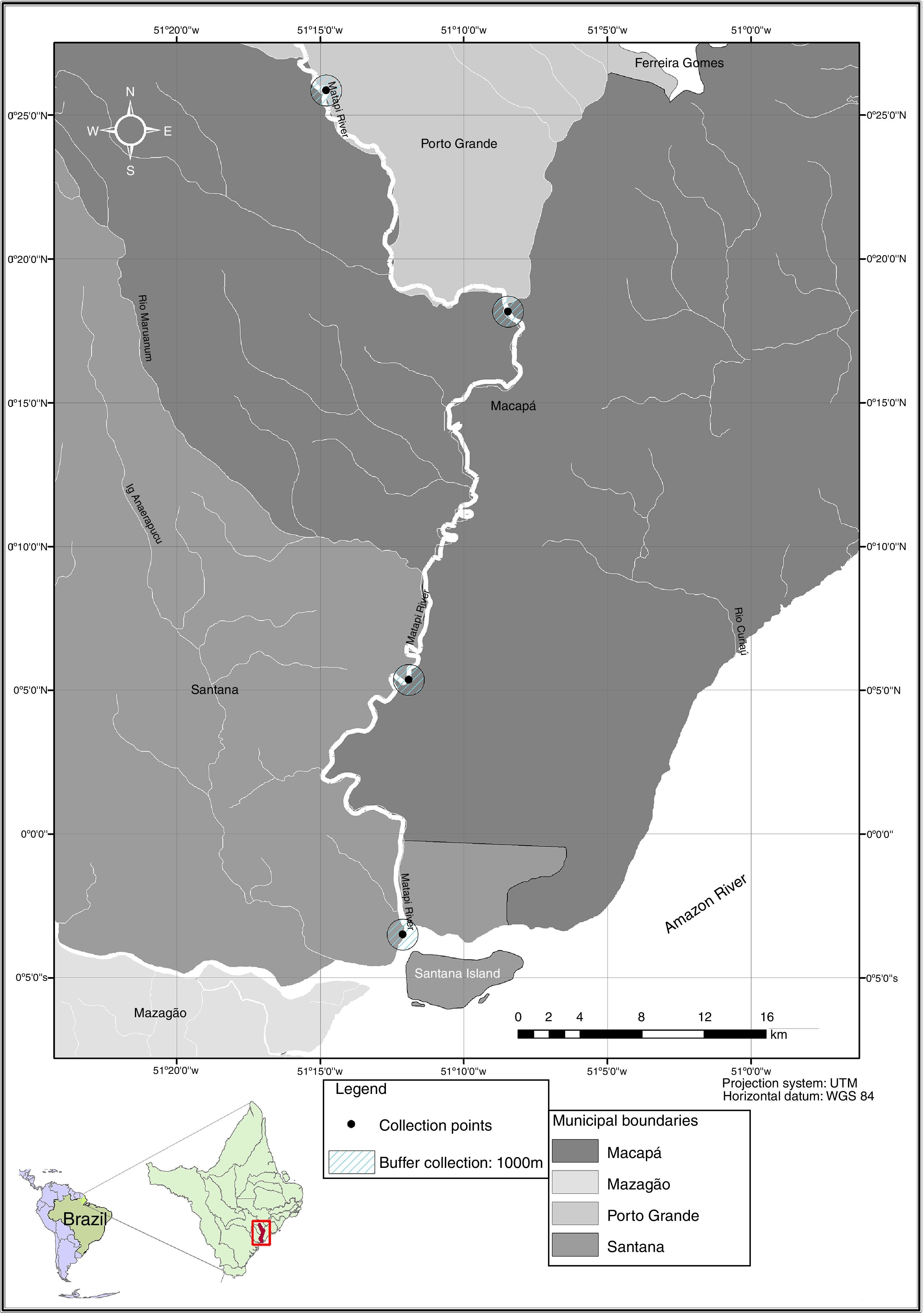
Materials and methodsThe Matapi River basin, with 2,518km2, crosses the city of Porto Grande, flowing into the mouth on the Amazon River, in the municipality of Santana, Amapá State (eastern Amazon, Brazil). This watershed spreads over different cities and has several tributaries, including rivers and streams (Fig. 1), all used by various human riverine communities that earn their living from agriculture, livestock and fisheries. It is strongly influenced by a high rainfall in the Amazon region and also by the daily tides of the Amazon River (Takiyama et al., 2007).
From March 2012 to March 2013, 75 specimens of G. camopiensis (12.5±3.1cm and 62.0±43.0g), 38 specimens of P. scalare (4.5±0.8cm and 6.0±4.4g), 15 specimens of S. jurupari (11.8±1.6cm and 58.5±10.4g) and 12 specimens of S. acuticeps (11.6±1.1cm and 50.0±14.5g) were collected along the Matapi River (Fig. 1). Fish were caught with cast nets, matapi, longlines, handlines and gillnets (20, 30, 40 and 50mm between nodes) to study monogeneans from gills.
For G. camopiensis, the most captured host species, 39 specimens were collected during the rainy period and 36 during the drought period aiming to study the effects of seasonality in levels of infection. Seasonality was based on rainy and dry seasons, as the region is a tropical forest characterized by a rainy season that runs from December to May (summer and fall) and a dry season that runs from June to November (autumn and winter) (Souza & Cunha, 2010).
For each fish, standard length (cm) and body weight (g) were obtained. The gills were collected and fixed in 5% formalin to collect monogeneans, which were then quantified and preserved in 70% alcohol. To analyze the internal morphology of monogeneans, GAP (picric acid and glycerin) and Hoyer methods were used to study the sclerotized structures. Some parasites were also stained with Masson trichrome (Boeger & Viana, 2006).
The ecological terms used are those recommended by Bush, Lafferty, Lotz, and Shostak (1997). The index of dispersion (ID) and index of discrepancy (D) were calculated using the Quantitative Parasitology 3.0 software to detect the distribution pattern for each infracommunity of parasites (Rózsa, Reiczigel, & Majoros, 2000) in species with a prevalence ≥10%. The significance of ID for each parasite species was tested using the d-statistics (Ludwig & Reynolds, 1988).
To study seasonality, the prevalence of parasites was compared between seasons using the Chi-square (χ2) test, and the abundance was calculated using the Mann–Whitney (U) test. Spearman correlation coefficient (rs) was used to determine possible correlations between abundance of parasites and length and body weight of hosts (Zar, 2010).
During fish collection, in each sampling site along the Matapi River, the pH, water temperature and dissolved oxygen were measured using the appropriate digital devices for each purpose. The mean rainfall was obtained from the Center for Hydrometeorology and Renewable Energy (NHMET) of the Institute of Scientific and Technological Research of Amapá State (IEPA).
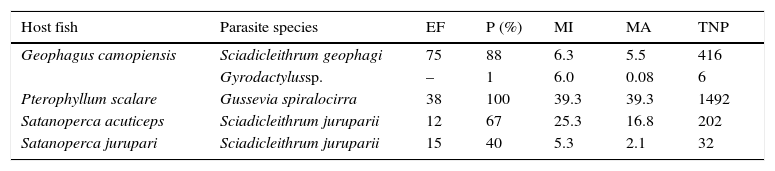
ResultsFrom a total of 140 fish, 84.3% were parasitized by Sciadicleithrum geophagiKritsky et al., 1989, Gussevia spiralocirra Kohn & Paperna 1964, S. juruparii and/or Gyrodactylus sp., and 2,148 parasites were collected. Satanoperca jurupari was the host with the lowest level of infection, and only G. camopiensis was parasitized by more than 1 parasite species. Gussevia spiralocirra was the monogenean species with the highest level of infection, followed by S. geophagi and S. juruparii, which infected more than 1 host (Table 2).
Infection by monogeneans in the gills of cichlid species from eastern Amazon, Brazil.
| Host fish | Parasite species | EF | P (%) | MI | MA | TNP |
|---|---|---|---|---|---|---|
| Geophagus camopiensis | Sciadicleithrum geophagi | 75 | 88 | 6.3 | 5.5 | 416 |
| Gyrodactylussp. | – | 1 | 6.0 | 0.08 | 6 | |
| Pterophyllum scalare | Gussevia spiralocirra | 38 | 100 | 39.3 | 39.3 | 1492 |
| Satanoperca acuticeps | Sciadicleithrum juruparii | 12 | 67 | 25.3 | 16.8 | 202 |
| Satanoperca jurupari | Sciadicleithrum juruparii | 15 | 40 | 5.3 | 2.1 | 32 |
EF, examined fish; P, prevalence; MI, mean intensity; MA, mean abundance; TNP, total number of parasites.
During the rainy season, the mean water temperature was 28.4±0.9°C, pH 5.7±0.5, dissolved oxygen 5.4±2.7mg/L and mean rainfall 357.2±88.5mm. In the dry season, the mean water temperature was 28.4±1.1°C, pH 5.5±0.07, dissolved oxygen 3.5±1.6mg/L and mean rainfall 43.4±47.8mm.
In G. camopiensis, the prevalence of S. geophagi was 87% in the rainy season and 89% during the dry season, thus indicating no seasonal differences (χ2=0.052, p=0.820). The mean abundance of S. geophagi in the rainy season was 5.4 and 5.7 in the dry season; therefore, there were no differences (U=652.0, p=0.595) between these 2 seasons.
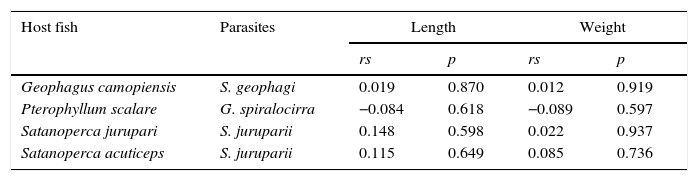
The helminths S. geophagi and G. spiralocirra, showed an aggregated dispersion pattern (Table 3). No significant correlation between the abundance of monogenean species and length and weight of hosts was found (Table 4).
Dispersion index (DI), index of discrepancy (D) and d-statistic for infracommunities of monogeneans in cichlid species of the eastern Amazon, Brazil.
| Host fish | Parasite species | DI | d | D |
|---|---|---|---|---|
| Geophagus camopiensis | Sciadicleithrum geophagi | 2.542 | 7.3 | 0.43 |
| Pterophyllum scalare | Gussevia spiralocirra | 1.577 | 2.3 | 0.31 |
Spearman correlation coefficient (rs) between the abundance of monogenean species and the length and weight of cichlid species of the eastern Amazon, Brazil.
| Host fish | Parasites | Length | Weight | ||
|---|---|---|---|---|---|
| rs | p | rs | p | ||
| Geophagus camopiensis | S. geophagi | 0.019 | 0.870 | 0.012 | 0.919 |
| Pterophyllum scalare | G. spiralocirra | −0.084 | 0.618 | −0.089 | 0.597 |
| Satanoperca jurupari | S. juruparii | 0.148 | 0.598 | 0.022 | 0.937 |
| Satanoperca acuticeps | S. juruparii | 0.115 | 0.649 | 0.085 | 0.736 |
p, probability.
Thirty-one monogenean species for native cichlids from South America are listed, and 80.6% of these parasites are known to infect cichlids from Brazil (Cohen, Just, & Kohn, 2013). In wild populations of G. camopiensis, P. scalare, S. acuticeps and S. jurupari, the monogenean fauna was composed by S. geophagi, S. juruparii, G. spiralocirra, and Gyrodactylus sp., parasite species known to parasitize cichlid species. Only G. camopiensis was parasitized by more than 1 monogenean, S. geophagi and Gyrodactylus sp. Although, S. acuticeps and S. jurupari, are probably phylogenetically close hosts, were parasitized by S. juruparii. The richness of monogeneans may vary among different cichlids, since some ancirocephalid species of South America and Africa have a low host specificity, infecting different hosts (Pariselle et al., 2011), while others infect only a few host species (Boeger & Viana, 2006; Braga et al., 2014; Cohen et al., 2013), as occurred in the present study. Nevertheless, monogeneans seem to infect potentially available hosts when both have experienced a co-evolution (Braga et al., 2014; Poulin, 1992).
The monogenean S. iphthimum, from the gills of P. scalare of central Amazon, was not found in this host in the present study. Anyhow, S. geophagi, described from gills of Geophagus surinamensis Bloch, 1791 from Solimões River (Table 1), was found to parasitize G. camopiensis for the first time. It was also found that only 1 individual of G. camopiensis had 6 specimens of Gyrodactylus von Nordmann, 1932, thereby precluding identification of the species. In South America, few species of Gyrodactylus are known to parasitize cichlid species. Gyrodactylus cichlidarum Paperna, 1968 has been reported in Oreocromis niloticus Linnaeus, 1758 from Colombia and Ecuador (García-Vásquez et al., 2010), and Gyrodactylus geophagensis Boeger & Popazoglo, 1995 in G. brasiliensis from Brazil (Cohen et al., 2013). However, the Gyrodactylus specimens found in G. camopiensis present no resemblance to G. geophagensis. This is the first report of the monogenean S.juruparii in S. acuticeps.
The lentic environment, preferred by G. camopiensis, P. scalare, S. acuticeps and S. jurupari, favors dispersal and reproduction of monogeneans, ectoparasites with free-living stages during some phases of its lifecycle (Bittencourt, Pinheiro, Cárdenas, Fernandes, & Tavares-Dias, 2014; Dogiel, 1961; Neves et al., 2013). Pterophyllum scalare and S. acuticeps were the hosts with the highest level of infection when compared to G. camopiensis and S. jurupari. However, a low number of S. acuticeps and S. jurupari, were examined over an annual cycle, due to low population densities. Nevertheless, the prevalence of S. juruparii in these 2 hosts does not seem to be underestimated. Marques and Cabral (2007) reported that, for helminths with an aggregated dispersion pattern, as in the present study, the mean intensity and mean abundance may be under or overestimated when a small sample of host fish are examined, while prevalence is not affected. In the gills of S. jurupari, the intensity of S. juruparii ranged from 1 to 12 parasites per host, but in S. acuticeps it ranged from 3 to 152, demonstrating a pattern of differentiated infection. However, a lower intensity of S. juruparii in the gills of S. jurupari from Guamá River, Brazil (Table 1) was reported. Sciadicleithrum satanopercae also parasitized the gills of S. jurupari farmed in the Peruvian Amazon (Mendoza-Franco et al., 2010), but infection levels were not studied. Therefore, these results suggest that S. jurupari is host to both species of monogeneans depending on their geographic location.
In G. camopiensis infection levels by S. geophagi are higher than in Chaetobranchopsis orbicularis Steindachner, 1875 (Table 1), and were not influenced by seasonality due to the low environmental variation during the rainy and dry seasons. In contrast, due to seasonal environmental changes that occurred during the Amazon dry season, high levels of infection by Gussevia asotaKritsky et al., 1989, Gussevia astronotiKritsky et al., 1989 and Gussevia rogersiKritsky et al., 1989 in Astronotus ocellatus Agassiz in Spix and Agassiz, 1831 (Neves et al., 2013), and Gussevia alioidesKritsky, Thatcher & Boeger, 1986 and Gussevia disparoidesKritsky, Thatcher & Boeger, 1986 in Aequidens tetramerus Heckel, 1840 (Tavares-Dias et al., 2014) were reported. An aggregated dispersion for S. geophagi and G. spiralocirra was observed, a distribution pattern also reported for other species of dactylogirids from the gills of A. ocellatus (Neves et al., 2013) and A. tetramerus (Tavares-Dias et al., 2014), both cichlids from the Amazon. This aggregated pattern is common in fish and it can be related to the reproductive strategy of monogeneans (Scott, 1987), as well as to differences in susceptibility to infection caused by the genetic and immunological heterogeneity of hosts and environmental characteristics.
In the gills of G. camopiensis, P. scalare, S. acuticeps and S. jurupari, the high abundance of monogeneans was not influenced by host size. For S. acuticeps and S. jurupari, this lack of correlation was due to sampling of hosts with a reduced size variation. Furthermore, most specimens of G. camopiensis (5.3–17.5cm) examined were adults with a low abundance of parasites. However, the abundance of Sciadicleithrum frequens Bellay, Takemoto, Yamada & Pavanelli, 2008 was positively correlated with the length of hosts in Geophagus brasiliensis Quoy & Gaimard, 1824, reflecting an accumulation of these parasites throughout fish growth (Bellay et al., 2012). On the other hand, the abundance of G. alioides and G. disparoides was negatively correlated with the length of A. tetramerus (Tavares-Dias et al., 2014). Therefore, different infracommunities of monogeneans may have different responses to host size, thus determining different levels of infection.
In summary, this is the first report on infection levels by monogeneans in wild populations of S. geophagi. For G. camopiensis and S. juruparii in S. acuticeps we found that parasitism was not influenced by host size. In G. camopiensis, the infection of S. geophagi also had a constant annual pattern, indicating that in these Amazonian hosts, other factors are involved in the parasitism levels of monogenean species. S. juruparii was found in 2 congeneric hosts and most likely may infect other Satanoperca species, but this needs to be further researched.
The present work was conducted according to the principles adopted by the Brazilian College of Animal Experiments (COBEA) and under the authorization from ICMBio (# 23276-1). M. Tavares-Dias was supported by a Research fellowship from Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq, Brazil).
Peer Review under the responsibility of Universidad Nacional Autónoma de México.