From April to December of 2010, we performed a cross sectional study in order to collect and identify the species of anoplurans associated with cricetid and heteromyd rodents from montane forests in 5 localities in Guerrero and Oaxaca, Mexico. We analyzed 147 rodents belonging to 10 cricetid species and 1 heteromyd species. A total of 378 sucking lice were collected (189 ♀, 106 ♂, 83 nymphs), distributed in 6 species (Fahrenholzia microcephala, Hoplopleura emphereia, Hoplopleura ferrisi, Hoplopleura reithrodontomydis, Neohaematopinus neotomae, Polyplax auricularis) and 2 families (Hoplopleuridae and Polyplacidae). Lice specimens were processed for morphological and molecular identification, using the mitochondrial gene cytochrome oxidase subunit I. Infestations were characterized based on the prevalence and mean abundance. Five of the 6 species were confirmed by molecular analysis. The highest levels of infestation were recorded for H. emphereia (66.7%; 4.4) on Megadontomys thomasi. All localities represent new records for the species studied.
De abril a diciembre de 2010 desarrollamos un estudio con el objetivo de recolectar e identificar anopluros asociados con roedores cricétidos y heterómidos de bosques montañosos en 5 localidades en Guerrero y Oaxaca, México. Analizamos un total de 147 roedores pertenecientes a 10 especies de cricétidos y una especie de heterómido. Se recolectó un total de 378 piojos (189 ♀, 106 ♂, 83 ninfas), distribuidos en 6 especies (Fahrenholzia microcephala, Hoplopleura emphereia, Hoplopleura ferrisi, Hoplopleura reithrodontomydis, Neohaematopinus neotomae, Polyplax auricularis) y 2 familias (Hoplopleuridae y Polyplacidae). Los piojos fueron procesados para su identificación morfológica y molecular, usando el gen mitocondrial citocromo oxidasa subunidad i. Las infestaciones fueron caracterizadas con base en la prevalencia y la abundancia promedio. Cinco de las 6 especies fueron confirmadas molecularmente. Los más altos niveles de infestación fueron alcanzados por H. emphereia (66.7%; 4.4) sobre Megadontomys thomasi. Todas las localidades representan nuevos registros para las especies estudiadas.
Sucking lice are obligate hematophagous ectoparasites of eutherian mammals. Currently, 550 species of Anoplura distributed in 16 families and 49 genera have been recorded worldwide (Durden & Musser, 1994; Light, Smith, Allen, Durden, & Reed, 2010); two-thirds of these arthropods belong to the families Polyplacidae and Hoplopleuridae, both including species parasites of rodents (Durden, 2002). The inventory of Mexican sucking lice is conformed by 44 species distributed in 8 genera (Antarctophthirus Enderlein, 1906; Enderleinellus Fahrenholz, 1912; Fahrenholzia Kellogg and Ferris, 1919; Hoplopleura Enderlein, 1904; Linognathoides Cummings, 1914; Linognathus Enderlein, 1905; Neohaematopinus Mjöberg, 1910 and Polyplax Enderlein, 1904) and 5 families (Echinophthiriidae, Enderleinellidae, Hoplopleuridae, Linognathidae and Polyplacidae). Forty two of these species (95.5%) have been associated with 61 species of rodents belonging to 4 families (Cricetidae, Heteromyidae, Muridae and Sciuridae), and 21 genera distributed in 28 states of the Mexican Republic (Sánchez-Montes, Guzmán-Cornejo, León-Paniagua, & Rivas, 2013). As part of a project to describe the metazoan fauna associated with cricetid rodents from montane forests of Mexico, we determined the richness and abundance of sucking lice associated with cricetid rodents from forest in the mountains of Guerrero and Oaxaca, Mexico. For this purpose we identified the specimens morphologically and molecularly (using the cytochrome oxidase subunit I [COI] gene) and additionally, we calculated the prevalence and mean abundance for each lice species.
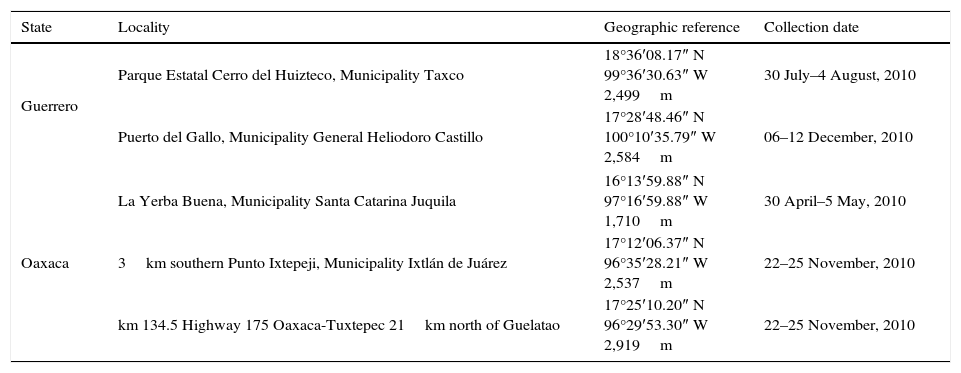
Material and methodsFrom April to December 2010, hosts were collected under permission FAUT-0170 issued by Semarnat, Mexico from 5 localities, 2 in Guerrero and 3 from Oaxaca, Mexico (Table 1). Rodents were captured using 4 transects of 40 Sherman traps (Romero-Almaraz, Sánchez-Hernández, García-Estrada, & Owen, 2007), and sacrificed in compliance with the guidelines of the American Society of Mammalogy for the Use of Wildlife Mammals in Research (Gannon & Sikes, 2007). Lice were recovered from the external surface of hosts, and were fixed and preserved in vials with 96% ethanol. Likewise, each host was brushed on a sheet of white paper to extract additional lice adhering to the fur, and was posteriorly processed in the laboratory. For morphological determination sucking lice were mounted on slides using the modified techniques of Kim, Pratt, and Stojanovich (1986) and Wirth and Marston (1968). Specimens were identified using the specialized keys of Cook and Beer (1959), Ewing (1935), Kim et al. (1986), Pratt and Lane (1951), Stojanovich and Pratt (1961a, 1961b) and Stojanovich and Pratt (1965). Prevalence and mean abundance were calculated according with (Bush, Lafferty, & Lotz, 1997). Additionally micrographs of specimens were taken using a Photomicroscope Olympus Provis AX70. Sucking lice were deposited in the collection of Laboratorio de Acarología, Facultad de Ciencias (LAFC), Universidad Nacional Autónoma de México.
Sampling sites of specimens collected in this study.
| State | Locality | Geographic reference | Collection date |
|---|---|---|---|
| Guerrero | Parque Estatal Cerro del Huizteco, Municipality Taxco | 18°36′08.17″ N 99°36′30.63″ W 2,499m | 30 July–4 August, 2010 |
| Puerto del Gallo, Municipality General Heliodoro Castillo | 17°28′48.46″ N 100°10′35.79″ W 2,584m | 06–12 December, 2010 | |
| Oaxaca | La Yerba Buena, Municipality Santa Catarina Juquila | 16°13′59.88″ N 97°16′59.88″ W 1,710m | 30 April–5 May, 2010 |
| 3km southern Punto Ixtepeji, Municipality Ixtlán de Juárez | 17°12′06.37″ N 96°35′28.21″ W 2,537m | 22–25 November, 2010 | |
| km 134.5 Highway 175 Oaxaca-Tuxtepec 21km north of Guelatao | 17°25′10.20″ N 96°29′53.30″ W 2,919m | 22–25 November, 2010 | |
DNA extraction was performed using the DNeasy Blood & Tissue Kit (QIAGEN Ltd., UK). Amplification of a partial segment of ≈620 of COI was done using primers Jerry 5′-CAACATTTATTTTGATTTTTTGG-3′ and PatII 5′-TCCATTACATATAATCTGCCATATTAG-3′ (Marsico et al., 2010).
The reaction mixture consisted of 2μl of primers (10μM, 1μl each), 0.4μl (1.25units) of Taq DNA Axygen®, 2.0μL of 10× Promega reaction buffer, 2μL of 25mM MgCl2, 0.8μL of 10mM mix dNTPs, 12.3μL nuclease-free water and 5ng DNA in a final volume of 19.5μL. PCR conditions were those used by Marsico et al. (2010). The PCR products were analyzed by electrophoresis on 1.5% agarose gels, using a 100bp and 1kb molecular weight marker (nucleic acid markers, Axygen) in 1× TBE buffer.
Purified amplification products were submitted for sequencing to Unidad de Síntesis y Secuenciación de DNA (USSDNA), Instituto de Biotecnología and Laboratorio de Biología Molecular y de la Salud, Instituto de Biología, Universidad Nacional Autónoma de México. Sequences were compared with other sequences of sucking lice available in GenBank using the basic local alignment search tool [BLAST] (Altschul, Gish, Miller, Myers, & Lipman, 1990). The sequences obtained were submitted to GenBank.
Additionally to our sequences, we obtained another 25 from GenBank belonging to the families Hoplopleuridae and Polyplacidae, and 1 Ischnoceran (Columbicola columbae), which was used as an outgroup in accordance with the proposal of Light et al. (2010), with the following accession numbers: AF385003, AF545717, DQ324548, DQ324549, DQ324564, DQ324578, EU162163, EU375771, HM171425, HM171426, HM171427, HM171428, HM171429, HM171430, HM171431, HM171432, HM171433, HM171442, HM171443, HM171444, HM171445, HQ542195, HQ542196.
Sequences were edited and analyzed in Mega 5.1 software (Tamura, Peterson, Peterson, Stecher, Nei, & Kumar, 2011), all were aligned using Clustal W (Thompson, Higgins, & Gibson, 1994). Mega 5.1 was used to select the best nucleotide substitution model. A Neighbor-joining phylogenetic tree was generated using the Tamura 3 parameter distance model. Additionally, uncorrected pairwise ‘p’ divergences were calculated for comparative purposes.
ResultsA total of 147 hosts pertaining to 10 species and 5 genera of Cricetidae (Habromys schmidly, Megadonthomys thomasi, Neotoma mexicana, Peromyscus aztecus, Peromyscus beatae, Peromyscus megalops, Peromyscus melanurus, Reithrodontomys bakeri, Reithrodontomys sumichrasti, Reithrodontomys mexicanus) and 1 Heteromyidae (Liomys pictus) were reviewed. These hosts were infested by 378 sucking lice (189 ♀, 106 ♂, 83 nymphs), distributed in 6 species belonging to 2 families (Hoplopleuridae and Polyplacidae) and 4 genera (Fahrenholzia, Hoplopleura, Neohaematopinus and Polyplax). The heteromids were included since they were collected during the collection of rodents in Cerro del Huizteco, Guerrero, and also because they were parasitized by sucking lice.
Rodent species distributed in 2 or more localities were infected by the same lice species, excepting P. aztecus which was parasitized by a different species in the sites where it was collected (Table 2). Almost all rodent species harbored only 1 species of sucking lice, excepting P. megalops, which was co-infested by H. emphereia and P. auricularis in Puerto del Gallo, Guerrero. Hoplopleura reithrodontomydis showed the highest geographic distribution, being found in 4 of the 5 sampled localities. These sucking lice were the most generalist species and were found in association with 4 cricetids species (H. schmidly, R. bakeri, R. mexicanus, and R. sumichrasti). On the other hand, N. neotoma and F. microcephala were found in only 1 host species at a single locality. The number of host species collected among localities varied from 1 to 6; the highest specific richness was recorded in Puerto del Gallo, Guerrero with 4 species of lice associated with 6 species of cricetids; in contrast, Parque Estatal Cerro del Huizteco exhibited the lowest species richness, as only 2 species were collected infesting 2 host species. Considering only populations of rodents represented by 10 or more specimens, prevalence ranged from 50 to 66.7%, while mean abundance varies from 4.4 to 5.1; among these populations, the highest levels of prevalence and mean abundance were reached by H. emphereia in M. thomasi in Puerto del Gallo and P. megalops in 3km southern Punto Ixtepeji, respectively (Table 2). Below, we present previous geographic distribution and the new records obtained in this study for each species of sucking lice recovered.
Prevalence and mean abundance of sucking lice collected from rodents in 5 localities in the neotropical region of Mexico.
| Locality/host species | n | HP | Sucking lice species | TSL | % | A |
|---|---|---|---|---|---|---|
| Guerrero | ||||||
| Parque Estatal Cerro del Huizteco | ||||||
| Habromys schmidly Romo-Vázquez et al., 2005 | 6 | 3 | H. reithrodontomydis | 12 | 50.0 | 2.0 |
| Liomys pictus Thomas, 1893 | 5 | 2 | F. microcephala | 15 | 40.0 | 3.0 |
| Puerto del Gallo | ||||||
| Megadontomys thomasi Merriam, 1898 | 15 | 10 | H. emphereia | 66 | 66.7 | 4.4 |
| Neotoma mexicana Baird 1855 | 2 | 2 | N. neotomae | 9 | 100.0 | 4.5 |
| Peromyscus beatae Thomas, 1903 | 10 | 5 | P. auricularis | 7 | 50.0 | 0.7 |
| Peromyscus megalops Merriam, 1898 | 15 | 9 | H. emphereia | 62 | 13.3 | 1.0 |
| 2 | P. auricularis | 15 | 60.0 | 4.1 | ||
| Reithodontomys bakeri Bradley, Mendez-Harclerode, Hamilton and Ceballos, 2003 | 1 | 1 | H. reithrodontomydis | 3 | 100.0 | 3.0 |
| Reithrodontomys sumichrasti Saussure, 1861 | 5 | 3 | H. reithrodontomydis | 5 | 60.0 | 1.0 |
| Oaxaca | ||||||
| La Yerba Buena | ||||||
| Peromyscus aztecus Saussure, 1860 | 15 | 2 | H. ferrisi | 6 | 13.3 | 0.4 |
| Peromyscus melanurus Osgood, 1909 | 35 | 4 | H. ferrisi | 19 | 11.4 | 0.5 |
| Reithrodontomys mexicanus Saussure 1860 | 1 | 1 | H. reithrodontomydis | 1 | 100.0 | 1.0 |
| 3km al Sur del Punto Ixtepeji | ||||||
| Peromyscus beatae | 4 | 1 | P. auricularis | 3 | 25.0 | 0.8 |
| Peromyscus megalops | 26 | 12 | H. emphereia | 133 | 46.2 | 5.1 |
| Oaxaca-Tuxtepec | ||||||
| Km 134.5 de la Carretera 175 | ||||||
| Peromyscus aztecus | 2 | 1 | H. emphereia | 2 | 50.0 | 1.0 |
| Peromyscus beatae | 3 | 1 | P. auricularis | 2 | 33.3 | 0.7 |
| Reithrodontomys mexicanus | 2 | 1 | H. reithrodontomydis | 26 | 50.0 | 13.0 |
Host collected: HP, host parasitized; TSL, total of sucking lice; %, prevalence; A, mean abundance.
Family Hoplopleuridae
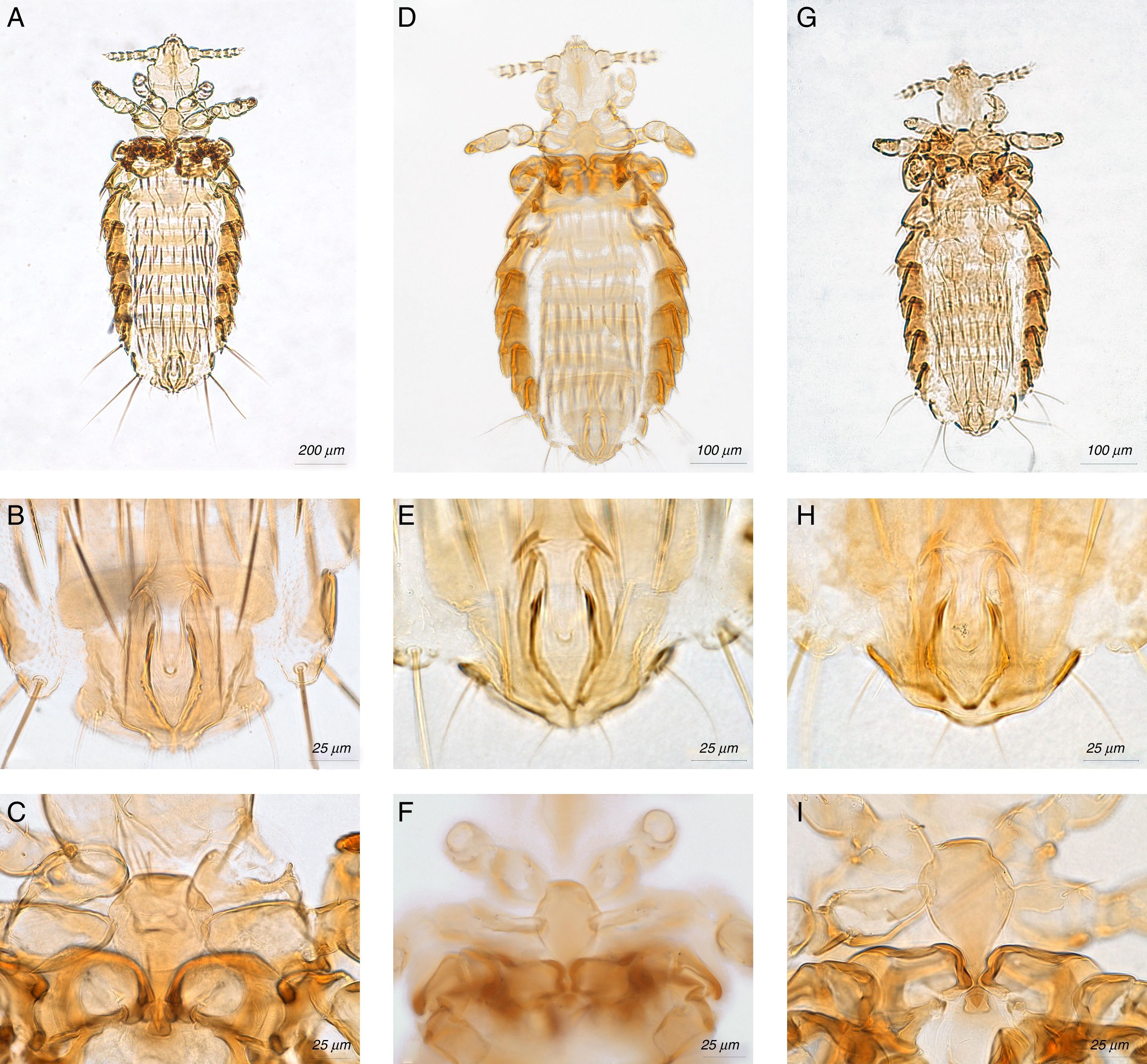
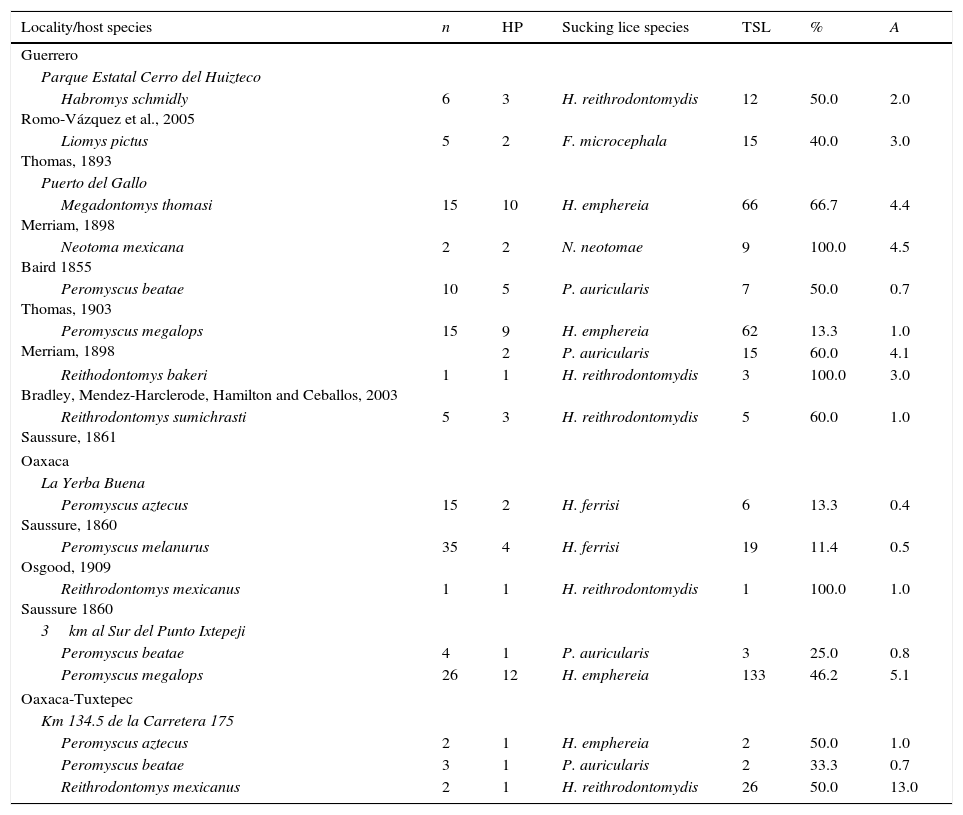
Hoplopleura emphereia Kim, 1965(Fig. 1A–C)
Material studied
4♂, 7♀, 3km southern Punto Ixtepeji, Municipality de Ixtlán de Juárez, Oaxaca, ex P. megalops; 4♂, 7♀, 1N, Puerto del Gallo, Municipality General Heliodoro García, Guerrero, ex M. thomasi; 2♂, 6♀, 3N, Puerto del Gallo, Municipality General Heliodoro García, Guerrero, ex P. megalops.
Distribution
Guatemala, Mexico, Nicaragua and Panama (Castro & González, 1997).
Hoplopleura ferrisi Cook & Beer, 1959(Fig. 1D–F)
Material studied
2♂, 4♀, La Yerba Buena, Municipality Santa Catarina Juquila, Oaxaca, ex P. aztecus; 5♂, 21♀, 1N, La Yerba Buena, Municipality Santa Catarina Juquila, Oaxaca, ex P. melanurus. 2♀, Km 134.5 de la Carretera 175 Oaxaca-Tuxtepec a 21 Km al Norte de Guelatao, Oaxaca, ex P. aztecus.
Distribution
Southeastern of United States (Arizona, Nuevo Mexico) to Mexico (Kim et al., 1986).
Hoplopleura reithrodontomydis Ferris, 1951 (Fig. 1G–I)
Material studied
3♂, 3♀, Parque Estatal Cerro del Huizteco, Municipality Taxco, Guerrero, ex H. schmidly. 1♂, La Yerba Buena, Municipality Santa Catarina Juquila, Oaxaca, ex R. mexicanus. 1♂, 1♀, 7N, 3km al sur del Punto Ixtepeji, Municipality Ixtlán de Juárez, Oaxaca, ex R. mexicanus. 1♂, 1♀, 1N, Puerto del Gallo, Municipality General Heliodoro Castillo, Guerrero, ex R. sumichrasti.
Distribution
Southeastern of United States, Mexico to Central America (Kim et al., 1986).
Family Polyplacidae
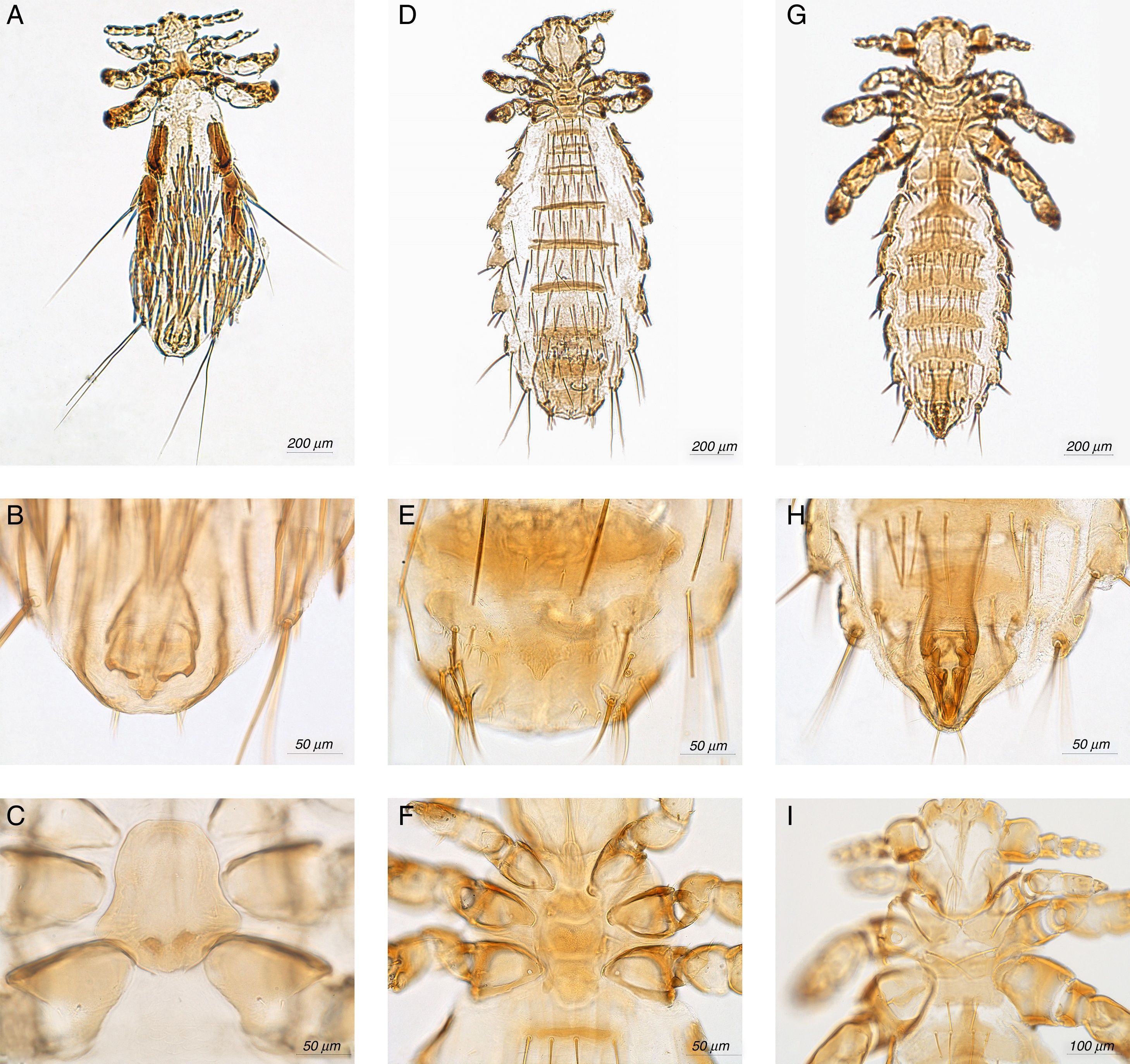
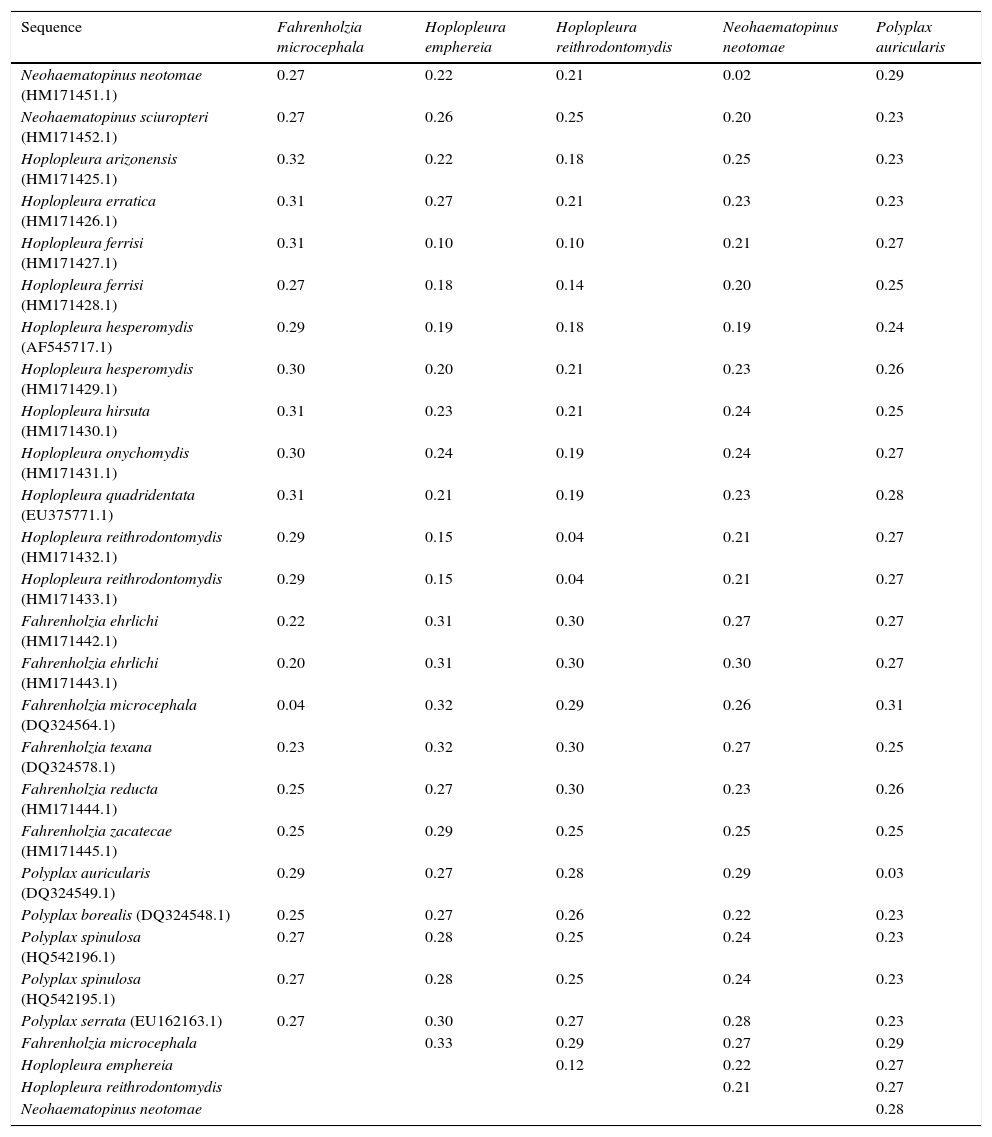
Fahrenholzia microcephala Ferris, 1922 (Fig. 2A–C)
Material studied
3♂, 2♀, 2N, Parque Estatal Cerro del Huizteco, Municipality Taxco, Guerrero, ex L. pictus.
Distribution
South-eastern of United States to Mexico (Kim et al., 1986).
Neohaematopinus neotomae Ferris, 1942 (Fig. 2D–F)
Material studied
1♂, 1♀, Puerto del Gallo, Municipality General Heliodoro Castillo, Guerrero, ex N. mexicana.
Distribution
South-eastern of United States to Mexico (Kim et al., 1986).
Polyplax auricularis Kellogg & Ferris, 1915 (Fig. 2G–I)
Material studied
3♂, 2♀, km 134.5 on Highway 175 Oaxaca-Tuxtepec, 21km northern Guelatao, Oaxaca, ex P. beatae; 28♂, 46♀, 17N,km 134.5 on Highway 175 Oaxaca-Tuxtepec, 21km northern Guelatao, Oaxaca, ex P. beatae; 1♂, 2♀ Puerto del Gallo, Municipality General Heliodoro Castillo, Guerrero, ex P. beatae; 2♂, 2♀, 2N Puerto del Gallo, Municipality General Heliodoro Castillo, Guerrero, ex P. megalops.
Distribution
From Alaska to Southern United States, Mexico, Costa Rica and Venezuela (Kim et al., 1986).
Molecular characterizationDNA sequences of the COI were obtained for 5 of the 6 species analyzed, F. microcephala (GenBank accession number KT151124); H. emphereia (GenBank accession number KT151125), H. reithrodontomydis (GenBank accession number KT151126), N. neotomae (GenBank accession number KT151127) and P. auricularis (GenBank accession number KT151128). No DNA sequences were obtained for H. ferrisi.
The intraspecific uncorrected pairwise p-distances between COI sequences generated in this study and those obtained for GenBank ranged from 2% to 4%: N. neotomae (2%), P. auricularis (3%), and F. microcephala and H. reithrodontomydis (4%); no sequence for pair comparison was available for H. emphereia on databases. On the other hand, the interspecific variation ranged from 10% (H. reithrodontomydis vs H. ferrisi) to 27% (H. emphereia vs H. erratica) in Hoplopleura; from 20% (F. ehrlichi vs F. microcephala) to 25% (F. microcephala vs F. zacatecae and F. reducta) in Fahrenzolzia; 23% in Polyplax (P. auricularis vs P. borealis, P. spinulosa and P. serrata); and 20% in Neohaematopinus (N. neotomae vs N. sciuropteri (Osborn, 1891)) (Table 3).
Matrix of uncorrected pairwise ‘p’ distances.
| Sequence | Fahrenholzia microcephala | Hoplopleura emphereia | Hoplopleura reithrodontomydis | Neohaematopinus neotomae | Polyplax auricularis |
|---|---|---|---|---|---|
| Neohaematopinus neotomae (HM171451.1) | 0.27 | 0.22 | 0.21 | 0.02 | 0.29 |
| Neohaematopinus sciuropteri (HM171452.1) | 0.27 | 0.26 | 0.25 | 0.20 | 0.23 |
| Hoplopleura arizonensis (HM171425.1) | 0.32 | 0.22 | 0.18 | 0.25 | 0.23 |
| Hoplopleura erratica (HM171426.1) | 0.31 | 0.27 | 0.21 | 0.23 | 0.23 |
| Hoplopleura ferrisi (HM171427.1) | 0.31 | 0.10 | 0.10 | 0.21 | 0.27 |
| Hoplopleura ferrisi (HM171428.1) | 0.27 | 0.18 | 0.14 | 0.20 | 0.25 |
| Hoplopleura hesperomydis (AF545717.1) | 0.29 | 0.19 | 0.18 | 0.19 | 0.24 |
| Hoplopleura hesperomydis (HM171429.1) | 0.30 | 0.20 | 0.21 | 0.23 | 0.26 |
| Hoplopleura hirsuta (HM171430.1) | 0.31 | 0.23 | 0.21 | 0.24 | 0.25 |
| Hoplopleura onychomydis (HM171431.1) | 0.30 | 0.24 | 0.19 | 0.24 | 0.27 |
| Hoplopleura quadridentata (EU375771.1) | 0.31 | 0.21 | 0.19 | 0.23 | 0.28 |
| Hoplopleura reithrodontomydis (HM171432.1) | 0.29 | 0.15 | 0.04 | 0.21 | 0.27 |
| Hoplopleura reithrodontomydis (HM171433.1) | 0.29 | 0.15 | 0.04 | 0.21 | 0.27 |
| Fahrenholzia ehrlichi (HM171442.1) | 0.22 | 0.31 | 0.30 | 0.27 | 0.27 |
| Fahrenholzia ehrlichi (HM171443.1) | 0.20 | 0.31 | 0.30 | 0.30 | 0.27 |
| Fahrenholzia microcephala (DQ324564.1) | 0.04 | 0.32 | 0.29 | 0.26 | 0.31 |
| Fahrenholzia texana (DQ324578.1) | 0.23 | 0.32 | 0.30 | 0.27 | 0.25 |
| Fahrenholzia reducta (HM171444.1) | 0.25 | 0.27 | 0.30 | 0.23 | 0.26 |
| Fahrenholzia zacatecae (HM171445.1) | 0.25 | 0.29 | 0.25 | 0.25 | 0.25 |
| Polyplax auricularis (DQ324549.1) | 0.29 | 0.27 | 0.28 | 0.29 | 0.03 |
| Polyplax borealis (DQ324548.1) | 0.25 | 0.27 | 0.26 | 0.22 | 0.23 |
| Polyplax spinulosa (HQ542196.1) | 0.27 | 0.28 | 0.25 | 0.24 | 0.23 |
| Polyplax spinulosa (HQ542195.1) | 0.27 | 0.28 | 0.25 | 0.24 | 0.23 |
| Polyplax serrata (EU162163.1) | 0.27 | 0.30 | 0.27 | 0.28 | 0.23 |
| Fahrenholzia microcephala | 0.33 | 0.29 | 0.27 | 0.29 | |
| Hoplopleura emphereia | 0.12 | 0.22 | 0.27 | ||
| Hoplopleura reithrodontomydis | 0.21 | 0.27 | |||
| Neohaematopinus neotomae | 0.28 |
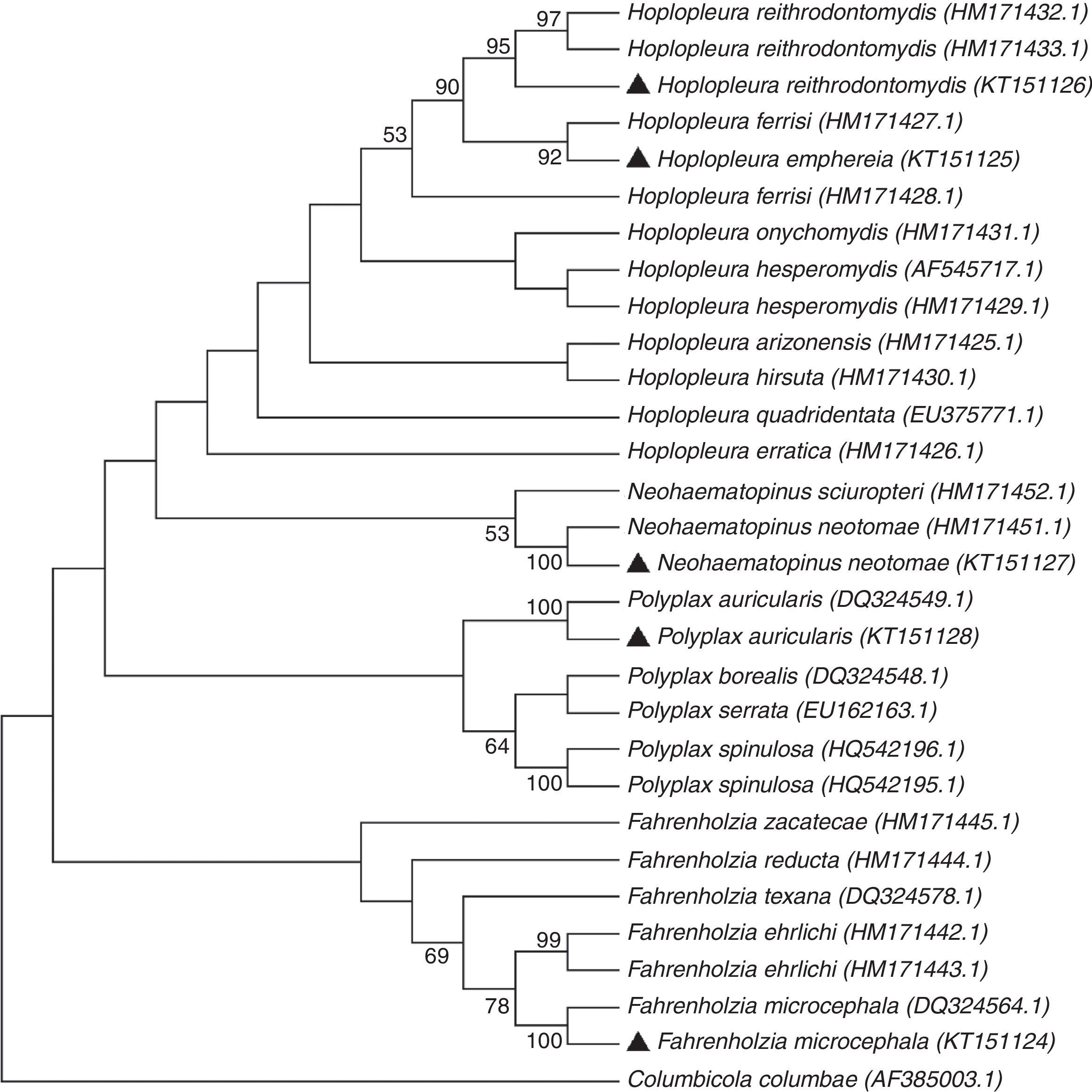
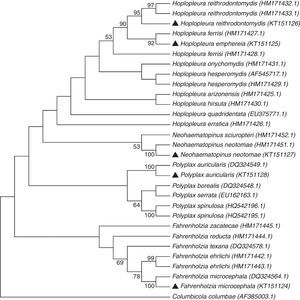
Four major groups can be recognized in the dendrogram constructed using the neighbor-joining method (Fig. 3). Each group contains species pertaining to the same genus (Fahrenholzia, Polyplax, Neohaematopinus, and Hoplopleura). The identity of 4 of the 5 lice species was confirmed by molecular analysis, since the sequences generated in this study joined with the respective sequence for each species obtained from GenBank, except for that of H. emphereia which was grouped with that of H. ferrisi (Fig. 3).
DiscussionAs a result of this study, the geographic distribution of the 6 species recorded is increased since we present 13 new locality records. Nine of the 10 cricetid rodents sampled (H. schmidly, N. mexicana, P. aztecus, P. beatae, P. megalops (for P. auricularis), P. melanurus, R. bakeri, R. sumichrasti, R. mexicanus) represent new host records for their associated sucking lice species (Sánchez-Montes et al., 2013).
Among the recorded sucking lice, H. reithrodontomydis was the most widely distributed species. This species has been recorded infesting only arboricolous and semi-arboricolous rodents of the genus Reithrodontomys (Ceballos & Oliva, 2005), which suggests a possible phenomenon of horizontal transmission between the arboreal host species. The finding of H. reithrodontomydis on H. schmidly is the first record for a host species other than from Reithrodontomys spp.; nonetheless, its presence on H. schmidly could be explained by its arboreal habits (Romo-Vázquez, León-Paniagua, & Sánchez, 2005) co-existing with 2 Reithrodontomys microdon Merriam, 1901; however, the latter host species was not infested by sucking lice.
In accordance with Durden (2002), sucking lice diversity is correlated with host diversity, due to the high specificity exhibited by this arthropod group toward their mammal hosts. In this context, our results fit with this statement, since Puerto del Gallo the locality with the greatest rodent richness also exhibited the greatest sucking lice richness (6 species). In contrast, in Parque Estatal Cerro del Huizteco only 2 species of rodents and sucking lice were collected. Puerto del Gallo has been referred to as a well preserved area (Navarro, 1998); based on personal observations, we agree with Navarro (1998), while Cerro del Huizteco seems to be a more disturbed site. In this context, Saavedra-Millán (2009), mentioned that Cerro del Huizteco is an area with low floral richness, due to the removal of herbaceous strata as a result of the introduction of infrastructure for ecotourism. This probably could explain the differential rodent richness in both localities.
Although most rodent species were parasitized by only 1 lice species, we recorded the co-infestation of H. emphereia and P. auricularis on P. megalops; this particular association had been previously recorded on Reithrodontomys creper Bangs 1902 in Panama (Johnson, 1972). The heterogeneous sample sizes obtained in our study preclude any conclusion about the factors involved in the infection levels recorded; however, variation on ecological parameters has been attributed to different factors such as, host sex, age, immune response, gregarious habits (Durden, 2002). In most sucking lice species infestation levels are less than 15 lice per parasitized host, being an extreme case H. emphereia associated with P. megalops in 3 Km southern Punto Ixtepeji, whose range was 1–46 lice.
Morphological identification of sucking lice is complex as most of the descriptions are based on a single specimen, lacking information about intraspecific variation, an aspect that should be analyzed. Three of the 6 species studied are well characterized morphologically; however, there is controversy about the validity of 3 other species (H. emphereia, H. ferrisi and H. reithrodontomydis) included in the Hoplepleura hesperomydis complex by Kim (1965). This author mentioned that H. emphereia shares morphological characters with H. ferrisi; later, Johnson (1972) postulated that H. emphereia and H. reithrodontomydis could be the same species upon comparison of nymphal stages. In this work we identified the 3 species morphologically; H. reithrodontomydis differs of H. emphereia and H. ferrisi in the shape of abdominal paraterguite 8, which presents a triangular shape, while in the other 2 species is rectangular. Likewise, H. emphereia males can be distinguished from H. ferrisi by the morphology of its thoracic sternal plate which have a posterior process abruptly pointed and paramers abruptly tapering posteriorly. In contrast, H. ferrisi males present thoracic sternal plate with posterior process gradually acute and paramers gradually tapering posteriorly (Johnson, 1972; Kim, 1965).
Intraspecific divergence values among DNA sequences of F. microcephala, P. auricularis, N. neotomae and H. reithrodontomydis (2–4%) obtained in this study and those from GeneBank, allowed us to consider them as valid taxa, since some authors have cited higher ranges for different species, e.g., 13% within Hoplopleura tiptoniJohnson, 1972 and Hoplopleura rimaeJohnson, 1972, 14% for Hoplopleura aitkeniJohnson, 1972, and 18% for Hoplopleura brasiliensis Werneck, 1932 (Smith, Light, & Durden, 2008). For H. ferrisi no COI sequences were obtained and for H. emphereia no sequences for comparative purposes were available in GenBank.
On the other hand, the similarity between the COI sequence of H. emphereia from Mexico and 1 of the 2 sequences of H. ferrisi obtained from GenBank (associated to Peromyscus difficilis (Allen, 1891) from Puebla) showed a genetic divergence of 10%; this value suggests the misidentification of 1 of the 2 specimens involved; however, this situation can only be solved through more sampling of specimens of both taxa (Fig. 3).
In spite of the amount of information generated during the 20th century, the inventory of the sucking lice in Mexico remains scarce and fragmentary. To date, 88% of the mammals distributed in Mexico have been neglected as hosts of this arthropod group. Particularly for rodents, completing the inventory of sucking lice is a major challenge, as these mammals constituted the main group of hosts. Only by increasing the sampling of this group of vertebrates in Mexico, through systematic studies, and avoiding partial analysis of a particular group of ectoparasites, this host-parasite association will be understood.
This research was supported by the grant PAPIIT IN225410 from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, DGAPA-UNAM. We thank Luis García Prieto who kindly reviewed our manuscript and provided a number of valuable comments; Ana Isabel Bieler Antolín for obtaining micrographs of specimens; Laura Del Castillo-Martínez for slide-mounting few louse specimens; Laura Margarita Márquez Valdelamar for her assistance in sequencing PCR products. We also thank Griselda Montiel, Rosario Chavarría, Mirna Hernández, Diego Barrales, Luis Darcy, Christina Lynggaard, Ricardo Paredes, Héctor Olguín, Giovani Canchola, Mónica Rodríguez, Pablo Colunga, Tania Marines, Deborah Veranea and Jesús Lugo for field assistance.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.