There are few reports of cloacal mycobiota on wild reptiles, and in particular, fungal presence and function in Caiman latirostris remains unknown. Our objective was to describe the fungal community present in the cloaca of wild female broad-snouted caimans during their reproductive season determine whether the number of fungi has some relationship with the female's corporeal condition. Fungi were found in 9 out of 13 cloacal samples and 14 species of fungi were isolated and identified. Three of the species isolated had the highest occurrence values, and 2 of them are pathogenic. In this case, body condition index had no relationship with fungal frequency; the fungi found in this study may have originated from soil habitat and nest substrate that are in constant contact with the cloaca of the C. latirostris females. The findings in this work support the theory that reptiles are facultative carriers of fungi or their spores.
Existen pocos registros acerca de la micobiota cloacal en reptiles silvestres y en particular, la presencia de hongos y su función en Caiman latirostris es aún desconocida. El objetivo de este trabajo fue describir la comunidad fúngica presente en la cloaca de hembras silvestres de Yacaré Overo durante su época reproductiva y analizar si la cantidad de hongos tiene alguna relación con la condición corporal de la hembra. Las hembras que resultaron positivas para hongos fueron 9 de las 13 muestreadas y fueron 14 las especies de hongos aisladas e identificadas. Del total de especies listadas, 3 tienen los valores de abundancia más altos y 2 de ellas son reconocidas como hongos patógenos. La condición corporal no tuvo relación con la frecuencia de hongos en general. Los hongos identificados pudieron tener su origen en el contacto permanente de la cloaca de la hembra de C. latirostris con el suelo del hábitat y/o el sustrato del nido. Los resultados de este trabajo apoyan la teoría de que los reptiles son transportadores facultativos de hongos y/o sus esporas.
Fungi are considered ubiquitous organisms in nature. Therefore, it is common to isolate fungi from tissues (e.g., skin, intestines, and lungs) that are in contact with water and soil in different reptiles species (Mitchell and Tully, 2009). Although there is some information available for marine turtles (Phillot et al., 2002) and giant lizards (genus Gallotia; Martinez-Silvestre et al., 2003), the cutaneous or intestinal mycobiota of reptiles are poorly known. Some authors suggest that reptiles' mycobiota (both cutaneous and gastrointestinal), could be rich and varied (Austwick and Keymer, 1981; Migaki et al., 1984). Information describing mycobiota in wild crocodilians remains unknown (Mitchell and Tully, 2009). Some cutaneous and gastrointestinal fungi have been isolated from crocodilians in captivity (Thomas et al., 2002; Mitchell and Tully, 2009); these mycobiota were associated with disease, whereas others were incidental findings (Jacobson et al., 2000).
Studies describing and identifying mycobiota in wild animals are the first step to understand how fungi could affect the survival of wild populations (Jacobson, 2007). Only a small proportion of mycotic diseases in animals are regularly communicable or contagious (Jacobson et al., 2000). Most fungi are opportunistic invaders of the integument, respiratory system, and gastrointestinal tract and may become pathogenic with changes in the immunological or ecological status of the animal host (Jacobson, 2007). Previously, some studies showed a negative relationship between body condition and fungal presence in reptiles (Buenviaje et al.,1998; Miller et al., 2004; Gartrell and Hare, 2005). In this sense it should be expected that in caimans, a poor body condition would be related to a higher abundance of fungi.
Mycotic diseases are generally acquired from non-animal sources in the environment; and most mycotic disease agents have other, usually saprobic, ecological roles through which they exert their main ecological impact (Ghirardi et al., 2011). In this context the aim of our study was to describe the fungi found in the cloaca of broad-snouted caiman (C.latirostris) females during their reproductive stage and to determine whether a poor body condition is linked to higher fungal frequencies in the females.
Samples were obtained during the broad-snout caiman reproductive season from January to February in 2011 in Santa Fe Province, Argentina, in the following sites: Fisco (n=8; 30°11'43.56” S, 61°0'38.94” W); Cacique (n=2; 30°38'1.00” S, 60°17'34.50” W); Los Saladillos (n=2; 30°42'59.5” S, 60°17'47.3” W) and Espín (n=1; 29°56'12.94” S, 60° 3'43.85” W). Females (mean total length: 155.7±9.2cm, SVL: 79.3±2.9cm and body mass: 16.5±1.9 gr) were captured next to their nests in their natural habitats (n=13).
The swab samples were taken from the edge inside the cloaca(ca. 2cm deep) from each animal by sampling the lining of the cavity where the clitoris is laying (Nuñez-Otaño et al., 2010). Striations were performed with the swabs in Petri dishes with agar containing streptomycin (5mg/ml) and chloramphenicol (2.5mg/ml) to avoid excessive bacterial growth. The plates were maintained under laboratory temperature and photoperiod conditions and were examined macroscopically (Wild Heerbrugg - Plan 1x) and microscopically (Leitz-Dialux 20 EB) for at least a 7 day period. Taxonomic identifications were made by direct comparison of specimens and by the use of taxonomic keys, descriptions, and illustrations. We considered an individual as 1 fungal CFU (colony forming unit). Cultures were labeled as LPSC 1103 through LPSC 1120 in the culture collection of the Institute Carlos Spegazzini (La Plata, Buenos Aires, Argentina). Nutritional modes have been used as a means of delimitating econutritional categories of behavior according to whether fungi were biotrophic-pathogens or biotrophic-saprotrophs (Cooke and Whipps, 1993). Body condition index (BCI) was considered as the residuals of the linear regression between bodies size (SVL in our case) and body mass, as commonly used (Litzgus et al., 2008). Analyses for SVL and body mass were conducted on log transformed data using the program INFOSTAT (InfoStat version 2011; Di Rienzo et al., 2011). Individuals with positive residuals are considered to be in a better condition than individuals with negative residuals (Litzgus et al., 2008). The relationship between BCI and abundance of fungi (CFU) was analyzed with linear regression.
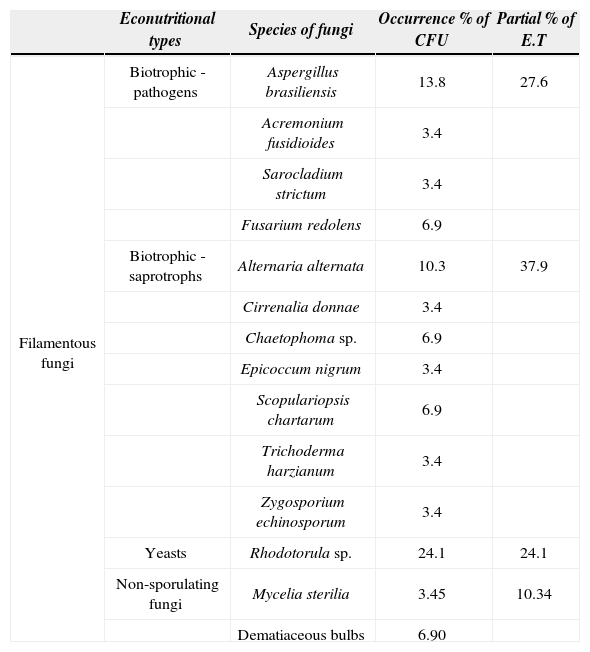
Fungi were found in 9 out of 13 cloacal samples (69.2%), 5 females out of 9 (55.6%) were positive for only 1 species of fungus, the others (4 out of 9=44.4%) resulted positive for more than 1 species. The occurrence of multiple species of isolated fungi within a single host was uncommon, being detected in only 1 sampled female (1 out of 13). Distributions of fungal occurrence were found inpositive animals (Table 1) as follows: filamentous fungi (n=19 CFU), yeasts (n=7 CFU) and non-sporulating fungi (n=3 CFU).
Mycobiota obtained from wild females of Caiman latirostris (swabbed positive for fungi). List of fungal species, occurrence percentage of CFU(colonies forming-units) ([niCFU/total CFU sampled]*100) and partial percentage of econutritional types (E.T) of each fungal species identified
| Econutritional types | Species of fungi | Occurrence % of CFU | Partial % of E.T | |
|---|---|---|---|---|
| Filamentous fungi | Biotrophic - pathogens | Aspergillus brasiliensis | 13.8 | 27.6 |
| Acremonium fusidioides | 3.4 | |||
| Sarocladium strictum | 3.4 | |||
| Fusarium redolens | 6.9 | |||
| Biotrophic - saprotrophs | Alternaria alternata | 10.3 | 37.9 | |
| Cirrenalia donnae | 3.4 | |||
| Chaetophoma sp. | 6.9 | |||
| Epicoccum nigrum | 3.4 | |||
| Scopulariopsis chartarum | 6.9 | |||
| Trichoderma harzianum | 3.4 | |||
| Zygosporium echinosporum | 3.4 | |||
| Yeasts | Rhodotorula sp. | 24.1 | 24.1 | |
| Non-sporulating fungi | Mycelia sterilia | 3.45 | 10.34 | |
| Dematiaceous bulbs | 6.90 |
Altogether, 11 genera, 14 species (including yeast and non sporulating fungi) and 29 CFU of fungi were isolated. Aspergillus brasiliensis (= Aspergillus niger Tiegh. 1867) (13.8%; n=4 CFU; 3 out of 13 sampled females) was the most commonly isolated organism followed by Alternaria alternata (10.3%; n=3 CFU). Fusarium redolens, Chaetophoma sp. and Scopulariopsis chartarum were represented by 6.9% (n=2 CFU) each; while Acremonium fusidioides, Sarocladium strictum (= Acremonium strictum W. Gams 1971), Cirrenalia donnae, Epicoccum nigrum (= E. purpurascens Ehrenb. 1818), Trichoderma harzianum, and Zygosporium echinosporum were represented by 1 CFU each (3.4%; n=1 CFU). Other organisms, including hyaline Mycelia sterilia and dematiaceous bulbs were isolated.
From the total species found (n=14) when grouped by their econutritional type, the results showed that biotrophic-saprotrophic fungi had higher percentage abundances than biotrophic-pathogenic fungi, yeasts, and non-sporulating fungi, respectively (Table 1). The total fungal abundance and the groups of pathogen sand saprotrophs were not related with a poor body condition of C. latirostris females (p >0.77).
As was observed in this study, Aspergillus brasiliensis also was isolated from skin and intestinal contents of Crocodylus porosus, Osteolaemus tetraspis, and Alligator mississippiensis (Buenviaje et al., 1994; Hibberd et al., 1996); while A. alternata was isolated from A. Mississippiensis skin and as a normal constituent of cutaneous mycobiota in other reptiles (Paré et al., 2003). Pathogens like A. Brasiliensis and some Fusarium species have worldwide distributions and could be found on different types of substrates (Domschet al., 1993) and have been implicated repeatedly in crocodilian severe mycotic pneumonias (Frelier et al., 1985; Ladds, 2009). We can account F. redolens as a constituent of cloacal mycobiota on 2 of the females captured, and it is widespread in the temperate zone and the tropical region (Domsch et al., 1993). Some species of the genus Fusarium contain elaborate toxins (Richard, 1990) and were recorded in egg membranes of C. latirostris (unpublished data). Future studies could evaluate the effects of these fungi on some aspects of reproductive traits (e.g., hatching success and hatchlings survival rate) and mycosis affecting animal health.
Our results also show the appearance of Scopulariopsis chartarum. Most species in this genus are soil fungi, and a few species become human-pathogenic (Domsch et al., 1993). Chaetophoma sp. is a coelomycete known mainly from tropical and temperate regions (Sutton, 1973); it has been found in stems and roots of C. latirostris nests (unpublished data). Other fungi isolated were Epicoccum nigrum, Curvularia lunata, Sarocladium strictum, A. Fusidioides and Trichoderma harzianum; these were found in previous works on skin and intestinal contents of O. tetraspis and A. mississippiensis (Huchzermeyer et al., 2003) and as an etiologic agent of a fatal diffuse granulomatous pneumonia and focal necrotizing hepatitis in spectacled caimans (Caiman crocodilus) (Trevino, 1972). Trichoderma is generally regarded as a low-grade opportunistic pathogen (Jacobson, 2007). This study is the first to report hyaline Mycelia sterilia and dematiaceous bulbs on crocodilians.
There is no record for Zygosporium echinosporum and Cirrenalia donnae on crocodiles and/or alligators. With our results, we added these biotrophic-saprotrophic fungi as new records for Crocodilian and extend the known distribution of these fungi to Argentina. Z. echinosporum was cited for Brazil (Gusmão et al., 2001) and Peru (Matsushima, 1993) and C. donnae was found for first time in Canada (Sutton, 1973), and there are some works recording this species in China (Zhao and Liu, 2005). The yeast (Rhodotorula sp.) found here was also isolated from lungs, liver, and kidneys of turtles, lizards, and snakes, and are highly prevalent in the gut of all reptiles (Enweani et al., 1997; Reavill et al., 2004).
These fungi which are not specific to crocodilians can be isolated from a wide range of animals with and without signs of disease (Mitchell and Tully, 2009). The species of fungi/frequency in the females sampled were not related to their BCI. We cannot use these individual values to explain the 10 genera of fungi found on one of these females and/or if the number of species/fungal load could be a consequence of a good or a poor animal body condition. Probably, C. latirostris females acquired cloacae mycobiota by contact with feces of other contaminated animals, or during defecation by contact with contaminated substrate that was accumulated on the exterior of the cloaca and/or during oviposition by contact with nest substrate (Phillot et al., 2002). Another unexplored means for acquiring fungal propagules between vertebrates could be by venereal transmission. It would be interesting to evaluate this means of transmission in caimans by examining the cloacal mycobiota of adult males in future studies. The distribution of the same fungal isolates in different females hosts suggest that reptiles may act as facultative animal carriers for fungi and yeast in their cloacae (Nardoni et al., 2008); and the presence of biotrophic-pathogenic isolates could be used as a tool to identify potential fungal pathogens that could exert a negative effect on wild caimans.
We would like to thank Instituto Botánica Spegazzini, ProyectoYacaré, and CICyTTP-CONICET for logistical support and Agencia Nacional de Promoción Científica y Tecnológica (through the PICT 2008 N404 and PICT 2008 N220) for the financial support. Also thanks to Ana Maria Buczinsky for fungal isolations; Ferrero B. and Blanco Torres A., for their comments on the preliminary draft.