The aim of this study was to describe and compare the inflammatory reaction caused by Gnathostoma spp., in various tissues of wild vertebrates and humans in Mexico. Parasitized tissues were collected from 10 species of vertebrates from 8 states. Samples were fixed in 10% formalin, pH 7.2 and processed with standard histological techniques. The lesions were classified into 4 categories. The advanced third stage larvae in fish, amphibians, and reptiles were associated with inflammatory reaction categories I and II (no injury, discrete or minimum); birds presented lesions classified in categories II, III, and IV (injuries minimal to discrete, moderate to severe; granulomatous); 1 human sample had lesions classified in categories III and IV (injuries moderate to severe; granulomatous). Adult parasites in mammals generated responses in categories III and IV. Inflammatory reactions observed in the different studied vertebrates suggest a complex host response to the presence of the parasite, causing variable tissue changes. The tissue reactions provide basic knowledge on the general principles of the pathogenesis and pathology of gnathostomiasis.
El objetivo de este estudio fue describir y comparar la reacción inflamatoria que causa Gnathostoma spp., en los tejidos de diversos vertebrados silvestres y el humano en México. Se recolectaron tejidos parasitados de 10 especies de vertebrados, de 8 estados de la República Mexicana. Las muestras se fijaron en formalina al 10%, pH 7.2 y se procesaron con la técnica histológica de rutina. Las lesiones se clasificaron en 4 categorías; las larvas de tercer estadio avanzado en los peces, anfibios y reptiles causaron una reacción inflamatoria de las categorías I y II (sin lesión, o lesión mínima a discreta); en las aves, se registraron las categorías II, III y IV (lesión mínima a discreta, moderada a intensa y granulomatosa) y en el humano, categorías III y IV (lesión moderada a intensa y granulomatosa). Los parásitos adultos en los mamíferos generaron respuestas de las categorías III y IV. Las reacciones inflamatorias que causan a los diferentes vertebrados estudiados sugieren una respuesta compleja debido a la presencia del parásito y a la respuesta de los hospederos, causando alteraciones tisulares muy variables. Las reacciones tisulares proporcionan conocimientos básicos sobre los principios generales de la patogenia y patología de la gnatostomiasis.
Gnathostoma is represented in Mexico by 3 species: Gnathostoma binucleatum Almeyda-Artigas, 1991, Gnathostoma lamotheiBertoni-Ruiz, García-Prieto, Osorio-Sarabia and León-Règagnon, 2005, and Gnathostoma turgidum Stossich, 1902; its geographic distribution comprises 15 of the 32 states of the Mexican territory, and it parasitizes 80 host species (Pérez-Álvarez et al., 2008; García-Márquez et al., 2009). Additionally, more than 8 000 cutaneous and 11 ocular human cases have been recorded in this country (Lamothe-Argumedo, 2003, 2006), with G. binucleatum being the only species confirmed as an ethiological agent in Mexican human infections (León-Règagnon et al., 2002).
Histopathology caused by adult worms of this genus has been described in several regions of the world for several host species including dogs (Nayar et al., 1978), cats (Beveridge et al., 1998), weasels (Ashizawa et al., 1979), and pigs and boars (Mei et al., 2001), among others. In contrast, pathogenic changes by advanced third stage larvae (A3L) of Gnathostoma have only been studied in fish-eating birds (García-Márquez et al., 2001). In humans, histological lesions have been described in skin (Ollague and Ollague, 1987), brain (Schmutzhard et al., 1988), eyes (Barua et al., 2007), and intestine (Seguchi et al., 1995). The main objective of this study is to describe and compare the histological lesions caused by A3L and adults of Gnathostoma sp., in several vertebrate host species including humans.
Materials and methodsNine species of wild vertebrates (2 fish, 1 amphibian, 1 reptile, 3 birds, and 2 mammals, all in adult stage) were obtained from 9 localities from Mexico between June 2004 and September 2005 (Table 1). One larva was obtained from a skin biopsy conducted on a human adult patient in the General Hospital from Tepic (Secretaría de Salud del Estado de Nayarit) in 2000. Fish were collected with a seine net, amphibians were collected manually, and reptiles, birds, and mammals were shot by local hunters. All organisms were collected under the scientific collection permit FAUT-0056 issued to VLR. Skeletal muscles of all vertebrates except the mammals were ground individually, compressed between 2 glass plates, and examined with the aid of a magnifying glass to search for A3L of Gnathostoma. The digestive tract and liver of mammals were dissected within 4hr after capture, and reviewed under the microscope. Histologic lesions were evaluated based on 1 larva (alive or degenerating) or 1 live adult in host tissue. The positive tissues were fixed in 10% formaldehyde buffered solution (pH=7.2) during 24hr, and processed following conventional histologic techniques (Prophet et al., 1992). Five cross sections 6μm thick were made for each host species, and 10 microscopic fields were analyzed for each section following Vivar-Díaz (2010). Observations were made with a Carl Zeiss Primo star 07745 microscope (10x and 40x). Classification of the lesions follows the criteria proposed by Vázquez-Núñez et al. (2004) (Table 2). Voucher specimens of representative histological sections were deposited at the Colección Nacional de Helmintos (CNHE), Instituto de Biología, UNAM, Mexico City (CNHE 8739-8743).
Hosts infected with Gnathostoma spp. included in this study
| Host species | Stage | Distribution |
| Fish | ||
| Petenia splendidaa | L3A | Oaxaca: Presa Temascal (18°13'00” N, 96°25'00” W) |
| Gobiomorus dormitora | L3A | Tabasco: Pantanos de Centla (18°28'19” N, 92°39'15” W) |
| Amphibians | ||
| Lithobates forreria | L3A | Chiapas: Laguna Catazajá (17°46'03” N, 92°02'00” W) |
| Reptiles | ||
| Crocodylus acutusa | L3A | Colima: Laguna de Amela (18°50'07” N, 103°45'55” W) |
| Birds | ||
| Ardea albaa | L3A | Sinaloa: Laguna Alhuate (24°31'01” N, 107°20'10” W) |
| Pelecanus erythrorhynchusa | L3A | Sinaloa: Laguna Alhuate (24°31'01” N, 107°20'10” W) |
| Phalacrocorax brasilianusa | L3A | Guerrero: Laguna de Tres Palos (16°47'47” N, 99°44'30” W) |
| Mammals | ||
| Homo sapiensb | L3A | Nayarit: Tepic (21°30'13” N, 104°53'40” W) |
| Didelphis virginianac | Adult | Veracruz: Tlacotalpan (18°38'29” N, 95°41'26” W) |
| Procyon lotorc | Adult | Veracruz: Tlacotalpan (18°38'29” N, 95°41'26” W) |
Histologic category of lesions found in vertebrates infected by Gnathostoma spp. Evaluation was based in all cases on 1 larva or 1 adult in host tissue
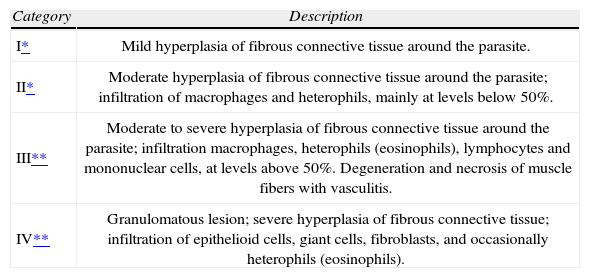
| Category | Description |
| I* | Mild hyperplasia of fibrous connective tissue around the parasite. |
| II* | Moderate hyperplasia of fibrous connective tissue around the parasite; infiltration of macrophages and heterophils, mainly at levels below 50%. |
| III** | Moderate to severe hyperplasia of fibrous connective tissue around the parasite; infiltration macrophages, heterophils (eosinophils), lymphocytes and mononuclear cells, at levels above 50%. Degeneration and necrosis of muscle fibers with vasculitis. |
| IV** | Granulomatous lesion; severe hyperplasia of fibrous connective tissue; infiltration of epithelioid cells, giant cells, fibroblasts, and occasionally heterophils (eosinophils). |
categories described for degenerating larvae and live adults. Mild lesion: histopathological changes present in less than 25% of the ocular field (acute infection); moderate lesion: histopathological changes present in 25–50% of the ocular field (sub-acute infection); severe lesion: histopathological changes present in 50–100% of the ocular field (chronic infection).
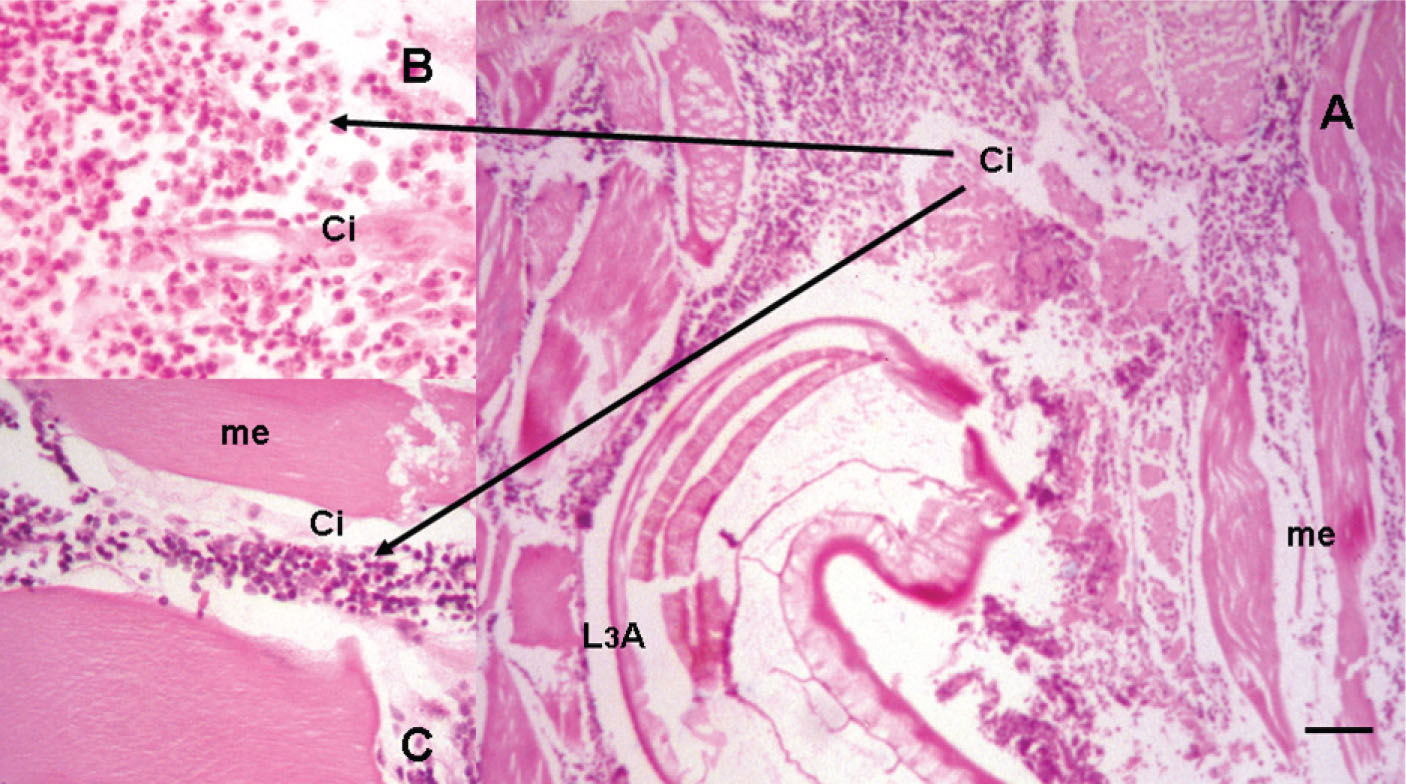
The presence of live larvae encysted in skeletal muscle of fishes, amphibians, and reptiles caused histological lesions ranging from mild hyperplasia of fibrous connective tissue around the parasite (lesions category I) to moderate hyperplasia of this tissue with infiltration of macrophages and heterophils in the interstitial zone, at levels below 50% (category II) (Fig. 1). In birds, inflammatory response in muscle tissue included moderate to severe hyperplasia of fibrous connective tissue around the parasite, as well as infiltration of macrophages, heterophils (eosinophils), lymphocytes and mononuclear cells, at levels above 50% causing degeneration and necrosis of muscle fibers with vasculitis (categories II, III) (Fig. 2). In the most severe cases, granulomatous lesions were recorded, with degeneration of the parasite, severe hyperplasia of fibrous connective tissue, and infiltration of epithelioid cells, giant cells, fibroblasts, and occasionally heterophils (eosinophils) (category IV). Inflammatory reaction in human skin was characterized by a moderate to severe hyperplasia of fibrous connective tissue surrounding the parasite, infiltration of macrophages, heterophils (eosinophils), lymphocytes and mononuclear cells, at levels above 50%, as well as degeneration and necrosis of muscle fibers with vasculitis. At the epidermic level, necrosis, acanthosis and hyperkeratosis were present, while in the dermis, infiltration of lymphocytes, eosinophils, macrophages, and plasmatic cells was recorded, producing an eosinophilic migratory panniculits (categories III and IV), without encysting of the A3L of Gnathostoma spp. (Fig. 3).
A), skeletal muscle (me) of bird (Ardea alba) with unencysted larva (A3L) of Gnathostoma sp., exhibiting moderate to severe mixed inflammatory infiltration (Ci); B-C), inflammatory cells, mainly eosinophils, neutrophils and macrophages, muscledegeneration and necrosis (me) (categories II-III lesions). Hematoxylin -eosin staining. Scale bar 100μm.
A), human skin with unencysted larva (A3L) of Gnathostoma sp. in the dermis, exhibiting severe to granulomatous mixed inflammatory infiltration (Ci); B), inflammatory cells eosinophils (Eo) macrophages and lymphocytes; C), Giantcell (Cg) forming granuloma (Categories III-IV lesions). Masson's trichrome staining. Scale bar 100μm.
Adults collected in the stomachs of opossums and raccoons caused lesions of categories III and IV; these specimens also exhibited congestion, hemorrhages, and mineralization (Figs. 4, 5).
Gastric mucosa of Didelphis virginiana. A), adult female of Gnathostoma turgidum on the submucosa with inflammatory infiltration; B), foreign body giant cells; C), proliferation of fibrous connective tissue on the submucosa; D), necrosis and dystrophic mineralization with fibroplasia (categories III-IV lesions). Hematoxylin -eosin staining. Scale bar 100μm.
In this study, we analyzed and compared the inflammatory reaction caused by larvae and adults of the 3 species of Gnathostoma distributed in Mexico, i.e., G. binucleatum (adult in Leopardus pardalis), G. lamothei (adult in Procyon lotor hernandezi), and G. turgidum (adults in Didelphis spp., and Philander opossum). The specific identity of larvae found in fish, amphibians, reptiles, and birds could not be determined due to lack of morphometric traits in histological cross sections.
The inflammatory reaction and lesions caused by A3L and adults of Gnathostoma spp., in all of the 10 vertebrate host species studied, including 1 human, are a complex response to the presence of the parasite, resulting in very different tissue alterations (categories I-IV, see Table 2). The A3L of Gnathostoma sp. found in fish, amphibians, and reptiles causes a minimal response in these 3 hosts groups, because of the encystment of the A3L in the skeletal muscle; this suggests an acute infection without tissue reaction by the host, allowing parasite survival and thus, continuation of its life cycle (Lamothe-Argumedo, 1997). In the bird species studied, the more frequent lesion categories correspond to II, III and IV, which agree with the findings of García-Márquez et al. (2001) in the same group of hosts. This inflammatory reaction is due to the number of larvae involved in the infection and to the role of birds as paratenic hosts of Gnathostoma larvae, accumulating a large number of nematodes through time (León-Règagnon et al., 2005).
The initial inflammatory and immunological response caused by the parasite is acute; it is characterized by cellular infiltration of eosinophils and neutrophils (heterophils in birds, fishes, and reptiles) with phagocytic action and release of cytosine, leukotrienes, prostaglandins, etc. The release of chemical substances activates the cellular and humoral (IgE antibodies) immunological response. Finally, the chronic condition of the infection is distinguished by a granulomatous lesion (Mitchell et al., 2006).
The human inflammatory reaction to the presence of A3L of G. binucleatum in the skin is an eosinophilic granulomatose panniculitis; this is caused by the erratic migration of the larvae through the dermis; similar results have been reported in human biopsies in Mexico (Magaña et al., 2004) and Ecuador (Ollague and Ollague, 1987), and by other Gnathostoma species in several Asian countries (e.g., Grobusch et al., 2000). In some cases, larvae can migrate to other organs such as brain, eyes, and liver, causing meningitis (Schmutzhard et al., 1988), retinopathies (Rao et al., 1999), and eosinophilic hepatitis (Seguchi et al., 1995), respectively.
The presence of adults of G. turgidum and G. lamothei infecting the stomach of opossum and raccoon, respectively, led to a granulomatous eosinophilic gastritis, (Categories III-IV); this condition was caused by the parasite migration to the stomach fundic region, to form an intraluminal nodule on the mucosa. This pathology has been observed in several definitive hosts such as domestic and feral cats (Mei et al., 2001; Beveridge et al., 1998), domestic dogs (Álvarez-Guerrero et al., 2012), raccoons (Almeyda-Artigas et al., 1994), Asiatic lions (Patel et al., 2000), domestic swine (Mei et al., 2001), wild boar (Ishiwata et al., 1998), raccoons (Bertoni-Ruiz et al., 2005), and opossums (García-Márquez, 2005).
The histological damage produced by these nematodes is the result of 2 main parasitic mechanisms: 1) migration through tissues of A3L; 2) enzymatic release which causes tissue degeneration, necrosis, and granulomas. The parasitized host reacts with a cellular immune response with production of cytokines such as interleukin (IL)-type IL-4, IL-5, IL-10, and IL-13, as well as the colony stimulating granulocyte and monocyte factor (GM-CSF), which activate and stimulate the proliferation of cells, such as eosinophils, macrophages, monocytes, lymphocytes, plasmatic cells, epithelioid cells, giant cells, and fibroblasts (Cox and Liew, 1992). This condition promotes a tissue response and granuloma formation, originating a chronic infection due to the host immune response against the parasite or their secretions or excretions (Cox and Liew, 1992; Díaz-Camacho et al., 1998).
Host-parasite interaction determines the type of inflammatory reaction; the characterization of the different levels (categories) of this response, proposed by Vázquez-Núñez et al. (2004), is a useful guide to analyze microscopic features of the lesions caused by parasites as Gnathostoma spp. and provides a basic understanding of the general principles of the pathogenesis and pathology of gnathostomiasis.
This study was partially funded by Trust fund Ramón Álvarez Bullya Aldana, University of Colima to LJGM, and proj. PAPIIT-UNAM 221307 to VLR and RLA. Special thanks to Luis Antonio Morales Arreola for technical assistance and Dr. Messina-Robles, from the Departmento de Dermatología, Hospital General de Tepic, Secretaría de Salud de Nayarit, for the donation of parasitized human tissue.