Microsatellite loci were isolated and characterized for the endemic fish Chirostoma humboldtianum using an enrichment procedure. Eight polymorphic microsatellites were genotyped for 32 - 48 individuals from Tepuxtepec Dam, Michoacán. The number of alleles per locus ranged from 3 to 11 and the average observed and expected heterozygosities were 0.61 and 0.63, respectively. All loci deviated significantly from Hardy-Weinberg expectations, which might be related to small population sizes associated to human disturbances and habitat loss. These are the first loci described for the species and the genus and could be useful in studies of population genetics, conservation and management of the species.
Se aislaron y caracterizaron los loci microsatélites para el pez endémico Chirostoma humboldtianum a partir de una genoteca enriquecida. Se genotipificaron 8 microsatélites polimórficos para 32 a 48 individuos de la presa Tepuxtec, Michoacán. El número de alelos por locus varió de 3 a 11 y la heterocigosidad observada y esperada promedio fue de 0.61 y 0.63, respectivamente. Todos los loci presentaron desviaciones significativas del equilibrio de Hardy-Weinberg, lo cual puede deberse al tamaño poblacional pequeño asociado a disturbios humanos y pérdida del hábitat. Éstos son los primeros loci descritos para la especie y el género y podrían ser útiles en estudios de genética de poblaciones, conservación y manejo de la especie.
The Charal de Xochimilco Chirostoma humboldtianum (Atherinopsidae: Menidiinae) is an endemic species from Central Mexico. In the last 6 decades their populations have been reduced or extirpated from some of their natural habitats as a result of habitat alteration (Barbour, 1973). However, the population size of this species has not been determined, and the population genetic structure remains unknown, thus, microsatellites markers can be useful addressing related questions. We isolated and characterized 8 polymorphic microsatellite loci for this species in order to increase our knowledge on the population structure and to test hypothesis about diversification and speciation that aid in defining strategies for the conservation and maintenance of the species.
Enrichment procedures were utilized to develop microsatellite genetic markers (Glenn and Schable, 2005). Out of 165- screened clones, 29 possessed repetitive elements that allow designing primers for 12 loci according to MSATCOMMANDER (Faircloth, 2008), the remaining 17 clones had flanking region that did not allow primer design. PCR reactions were carried out using15μl reactions containing 50 to 100ng DNA, 10 pmol each primer, 0.5U Taq DNA polymerase (Promega), 200μM of each dNTP, 2.5μmM MgCl2 and 1X PCR buffer (15μmM MgCl2, 200μmMTris-HCl, pH 8.5,75μmM (NH4)2SO4). PCR amplifications were performed in a BIO-RAD thermocycler as follows: 95° C for 5min or 10min, followed by 30 cycles of 15sec at 94° C, 45sec at 50° C to 60° C depending on the locus and 15sec at 72° C, with a final extension at 70° C for 5min. The PCR products were analyzed by capillary electrophoresis in the automatic sequencer ABI Prism 3100 Avant at Laboratorio Divisional de Biología Molecular (LDBM) of the UAM- Iztapalapa. Allele sizes were determined using LIZ-500 as size standard (Applied Biosystems) and GeneMarker 2.4.0 software.
Polymorphism for each microsatellite loci was characterized by screening a sample of 32 to 48 individuals of C. humboldtianum from Tepuxtepec Dam, Michoacán (19°59'42” N, 100°13'33” W). The total number of alleles per locus, observed (H0) and expected (HE) heterozygosities were calculated. All loci were tested for Hardy-Weinberg equilibrium (HWE), and all pairwise combinations of loci were tested for linkage disequilibrium (LD). All these parameters and tests were computed in Arlequin version 3.5.1.2 (Excoffier and Lischer, 2010). Microchecker version 2.2.3 (Van Oosterhout et al., 2004) was used to estimate the frequency of null alleles in the microsatellites markers. The genotypes of the loci that showed null alleles were corrected using FreeNA (Chapui and Estoup, 2007). Finally, the C. humboldtianum population was evaluated in order to detect the occurrence of genetic bottleneck using TPM (two phases mutation) model and the Wilcoxon rank test as implemented in Bottleneck ver. 1.2.1 (Cornuet and Luikart 1996).
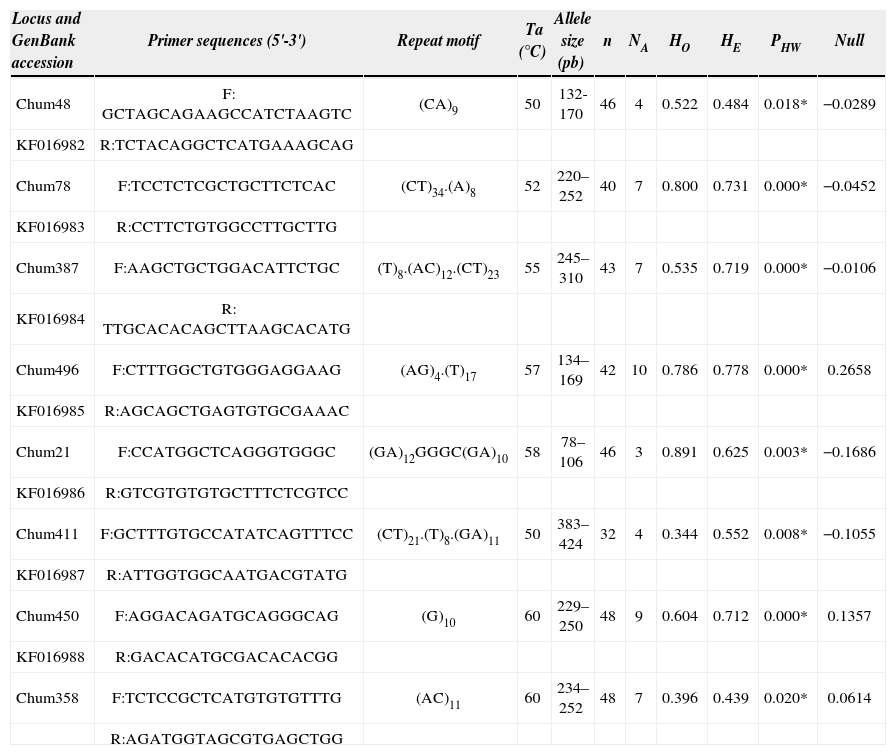
From the 12 amplified microsatellite loci, 4, Chum422, 443, 490 and 363 were monomorphic in all the individuals analyzed. The number of alleles per locus in the remaining 8 loci ranged from 3 (Chum21) to 10 (Chum496). Expected and observed heterozygosities ranged from 0.44 to 0.78 and 0.34 to 0.89, respectively (Table 1). No LD was detected between each pair of loci. All loci exhibited HWE departure after the sequential Bonferroni correction (α=0.05, k=8). Micro-Checker (Van Oosterhout et al., 2004) suggested that this phenomenon might be due to the presence of null alleles that were present in 3 of the 8 loci (Chum496, Chum450 and Chum358). However, the HWE departures were still observed after the genotyping correction using FreeNA. Deviation from HWE may be outcome of heterozygote deficiency found in some loci (Chum387, Chum411, Chum450, Chum358), which in turn might be caused by endogamy as has been suggested in other species of the genus (Barriga-Sosa et al., 2004). On the other hand, an excess of heterozygotes were detected in the remaining 4 loci. Heterozygosity excess has been associated with population bottleneck events (Cornuet and Luikart, 1996), and has also been a phenomenon suggested in other freshwater fish species inhabiting central Mexico (Domínguez-Domínguez et al., 2008). The bottleneck event could be associated to disturbances and habitat loss caused by human activities, which in turn might give result to small population sizes causing deviations from HWE. The TPM under Wilcoxon test did not show significant recent bottleneck (heterozygosity excess) (p=0.187). Likewise, decrease in observed heterozygosity suggests nonrandom mating and genetic drift (Loew et al., 2005), due that genetic drift causes random fixation and loss of alleles within population. In contrast, heterozygote excess appears to be due to the presence of hybrids within the sample (Pitchar et al., 2007) as has been earlier suggested for species of the genus (Barriga-Sosa et al., 2001). Translocations among populations and hybridization have been reported for this and others species of the genus (Alaye, 1996; Barriga-Sosa et al., 2001), however, this issues needs to be punctually addressed in further studies. These 8 microsatellite loci are the first developed for C. humboldtianum and will be useful in investigating population genetics, conservation, and management of this and closely related species.
Primer sequences and microsatellite repeat motifs for 8 microsatellite loci on Chirostoma humboldtianum. Annealing temperature (Ta), number of individuals (n), number of alleles (NA), observed and expected heterozygosities (Ho and He, respectively), p value for test of Hardy-Weinberg equilibrium (PHW), expected null allele frequency (Null) and GenBank accession numbers
| Locus and GenBank accession | Primer sequences (5'-3') | Repeat motif | Ta (°C) | Allele size (pb) | n | NA | HO | HE | PHW | Null |
|---|---|---|---|---|---|---|---|---|---|---|
| Chum48 | F: GCTAGCAGAAGCCATCTAAGTC | (CA)9 | 50 | 132-170 | 46 | 4 | 0.522 | 0.484 | 0.018* | −0.0289 |
| KF016982 | R:TCTACAGGCTCATGAAAGCAG | |||||||||
| Chum78 | F:TCCTCTCGCTGCTTCTCAC | (CT)34.(A)8 | 52 | 220–252 | 40 | 7 | 0.800 | 0.731 | 0.000* | −0.0452 |
| KF016983 | R:CCTTCTGTGGCCTTGCTTG | |||||||||
| Chum387 | F:AAGCTGCTGGACATTCTGC | (T)8.(AC)12.(CT)23 | 55 | 245–310 | 43 | 7 | 0.535 | 0.719 | 0.000* | −0.0106 |
| KF016984 | R: TTGCACACAGCTTAAGCACATG | |||||||||
| Chum496 | F:CTTTGGCTGTGGGAGGAAG | (AG)4.(T)17 | 57 | 134–169 | 42 | 10 | 0.786 | 0.778 | 0.000* | 0.2658 |
| KF016985 | R:AGCAGCTGAGTGTGCGAAAC | |||||||||
| Chum21 | F:CCATGGCTCAGGGTGGGC | (GA)12GGGC(GA)10 | 58 | 78–106 | 46 | 3 | 0.891 | 0.625 | 0.003* | −0.1686 |
| KF016986 | R:GTCGTGTGTGCTTTCTCGTCC | |||||||||
| Chum411 | F:GCTTTGTGCCATATCAGTTTCC | (CT)21.(T)8.(GA)11 | 50 | 383–424 | 32 | 4 | 0.344 | 0.552 | 0.008* | −0.1055 |
| KF016987 | R:ATTGGTGGCAATGACGTATG | |||||||||
| Chum450 | F:AGGACAGATGCAGGGCAG | (G)10 | 60 | 229–250 | 48 | 9 | 0.604 | 0.712 | 0.000* | 0.1357 |
| KF016988 | R:GACACATGCGACACACGG | |||||||||
| Chum358 | F:TCTCCGCTCATGTGTGTTTG | (AC)11 | 60 | 234–252 | 48 | 7 | 0.396 | 0.439 | 0.020* | 0.0614 |
| R:AGATGGTAGCGTGAGCTGG |
p<0.05 after sequential Bonferroni corrections
This study is part of the doctoral research of Rosa María García Martínez at Doctorado en Ciencias Biológicas y de la Salud at Universidad Autónoma Metropolitana- Iztapalapa, Mexico City; with fellowship Conacyt-224707. We thank PhD Rubén Valles and PhD Miguel Correa for laboratory assistance at CIBNOR and Biol. Ramón Cisneros for collecting specimens. Funding for this research was provided by Conacyt-CB-2009-01-130220 and UAM.147.07.03/147.09.01 granted to IDLABS. Mexican government kindly issued permit number DG0PA.07343.310810.4128 to conduct this research.