A total of 3 494 snails from Biomphalaria straminea (Dunker, 1848) were collected from December 2010 to May 2011, in a ricefield in Corrientes province, Argentina, and 5 species of larval trematodes belonging to Strigeidae (Furcocercaria sp. XIV, Furcocercaria sp. XV, Furcocercaria sp. XVI), Diplostomidae (Furcocercaria sp. XVII) and Schistosomatidae (Furcocercaria sp. XVIII) found in 115 snails (3.29%) are described. Prevalence of infection ranged between 0.11% (e.g., Furcocercaria sp. XVIII) and 4.22% (e.g., Furcocercaria sp. XVI) in the snails examined. Furcocercaria sp. XIV, Furcocercaria sp. XV and Furcocercaria sp. XVI were the most common species present in nearly all months of the sampling period, whereas Furcocercaria sp. XVII and Furcocercaria sp. XVIII were rarer species. Infection rates of most larval digeneans were highest in March. The species of furcocercariae in B. straminea from the agricultural habitat described in the present study are now added to the 4 species of furcocercariae already reported for the region from the genus Biomphalaria.
Un total de 3 494 caracoles de Biomphalaria straminea (Dunker, 1848) fueron recolectados desde diciembre de 2010 a mayo de 2011en una arrocera en la provincia de Corrientes, Argentina y 5 especies (Furcocercaria sp. XIV, Furcocercaria sp. XV, Furcocercaria sp. XVI, Furcocercaria sp. XVII y Furcocercaria sp. XVIII) encontradas en 115 caracoles (3.29 %) se describieron. La prevalencia de infección varió entre 0.11 % (Furcocercaria sp. XVIII) y 4.22 % (Furcocercaria sp. XVI) en los caracoles examinados. Furcocercaria sp. XIV, Furcocercaria sp. XV y Furcocercaria sp. XVI fueron las especies más comunes presentes en casi todos los meses del periodo de muestreo, mientras que Furcocercaria sp. XVII y Furcocercaria sp. XVIII fueron las especies más raras. Las tasas de infección de la mayoría de los digeneos larvales fueron altas en marzo. Las especies de furcocercarias descriptas en el presente estudio en B. straminea de un hábitat agrícola, se suman a las 4 especies de furcocercarias reportadas en la región para el género Biomphalaria.
In South America, some snail species of the genus Biomphalaria Preston, 1910, such as B. glabrata (Say, 1818), B. straminea (Dunker, 1848), B. tenagophila (D'Orbigny, 1835), B. peregrina (D'Orbigny, 1835) are of public health importance due to their role as potential vectors of Schistosoma mansoni Sambon, 1913. Brazil is the most affected country in the Americas, with 4–6million infected persons (Lambertucci, 2010). In recent years, the geographical range of the endemic areas of schistosomiasis in this country has been expanding, reaching its southernmost transmission focus in Rio Grande Do Sul State (Graeff-Teixeira et al., 1999, 2004), adjacent to northeastern Argentina. The presence of this parasite has not yet been reported in our country, although 2 of the natural vectors in Brazil, the snails B. tenagophila and B. straminea, are common in areas at risk of infection (Rumi et al., 2008).
Studies about the fauna of larval trematodes in planorbid molluscs (B. occidentalis Paraense, 1981, B. tenagophila,B. orbignyi Paraense, 1975, B. peregrina and B. straminea) have been carried out in natural environments of Corrientes province, Argentina (Ostrowski-de Nóñez et al., 1990, 1991, 1997; Hamann et al., 1991), but there is little information concerning agroecosystems with alternating periods of desiccation and flooding, such as ricefields (Rumi and Hamann, 1990). These environments provide favorable conditions for the development of dense populations of planorbids, which in turn are important from a health perspective for being in direct contact with humans (Rumi, 1986). Additionally, Corrientes province, with more than half of its cultivated area occupied by rice crops, is the main rice producer of Argentina (Aacrea, 2003). Therefore, it is important to obtain information on the larval trematode species that infect snails of the genus Biomphalaria, before the possible introduction of S. mansoni in the area. Furthermore, B. straminea is widely distributed in northeastern Argentina (Rumi, 1991; Rumi et al., 2008). The main goals of this study are to describe the larval trematodes found in B. straminea collected in a ricefield of Corrientes province, Argentina, and to determine the prevalence of larval infection.
Materials and methodsStudy area. The study site was an agricultural area of 25 ha, with 4 cultivated rice parcels connected or associated to the Paraná river basin, located approximately 30km south from Corrientes city, in Corrientes province, Argentina (27°40'23.5” S, 58°48'21.6” W). During the sampling months, December 2010 to May 2011, water depth ranged between 5 and 10cm in the cultivated parcels, and between 10 and 50cm in the irrigation canals. Water temperature ranged between 17°C (May 2011) and 28°C (February 2011). Table 1 shows data of monthly temperature and precipitation for this period.
Temperature (°C): minimum-maximum (mean ± standard deviation) and mean precipitation (mm) ± SD during a rice cultivation cycle in a ricefield of Corrientes province, Argentina
| December | January | February | March | May | |
|---|---|---|---|---|---|
| Temperature | 19.7–31.4 (25.4±4.9) | 22.1–33.3 (27.1±4.1) | 21.0–30.3 (25.4±3.4) | 19.5–30.5 (24.3±4.5) | 13.8–23.4 (18.1±4.4) |
| Precipitation | 12.9±13.4 | 16.1±16.4 | 17.9±24.2 | 7.9±12.2 | 6.2±7.8 |
In the initial phase of flooding, no vegetation was observed in the irrigation canals; later on, the predominant hydrophilic vegetation consisted of Sagittaria montevidensis Cham. and Schlecht, Ludwigia peploides (Kunt) P. H. Raven, Hydrocotyle ranunculoides L. f., and Limnobium sp. During the months of sampling several waterfowl species were observed: Egretta sp., Nomonyx dominicus Linnaeus, 1766, Jacana jacana Linnaeus, 1766, Vanellus chilensis Molina, 1782, Himantopus mexicanus Vieillot, 1817, Aramus guarauna Linnaeus, 1766, Mycteria americana Linnaeus, 1758, Tringa flavipes Gmelin, 1789 and Plegadis chihi Vieillot, 1817.
Sampling and laboratory procedure. Snails were collected during the flooding period, from the time of sowing to soon after harvesting of the rice, between December 2010 and May 2011. The samples were taken manually by 2 persons who sampled during 1.5 hours, from the cultivated parcels and irrigation canals, or using simple mesh nets, locally named “copos” (25cm frame diameter). In the laboratory the snails were kept individually in vials with 20ml of tap water, and were observed for the emergence of cercariae. Apparently uninfected snails were dissected to check for other larval intramolluscan stages (e.g. immature infections and metacercariae). Cercariae were studied alive, with and without vital dyes. Drawings were made using a camera lucida attached to a Carl Zeiss Jena microscope. Cercariae fixed in hot 4% formalin were preserved in vials with 70% ethanol, and deposited in the Helminthological Collection of the Centro de Ecología Aplicada del Litoral (CECOAL), Corrientes, Argentina. Photographs were taken with an Olympus DH10 camera mounted on an Olympus BH2 interference contrast microscope, and a Leica DFC 295 camera mounted on a Leica DM 2500 microscope. Specimens studied by scanning electron microscopy (SEM) were dehydrated in an ethanol series, dried using the critical point technique, coated with gold-palladium and examined with a Jeol 5800 LV Scanning Electron Microscope. Measurements of heat-killed and formalin-fixed specimens are expressed in micrometers (μm), with range followed by the mean in parentheses. The “open nomenclature” recommended by Odening (1971) was adopted for new species of cercariae.
To determine the second intermediate hosts, laboratory-reared Cnesterodon sp. and specimens of Cheirodon piaba Lütken, 1874 collected from an artificial tank were exposed to the emerged cercariae. The fish were maintained in small aquaria under controlled conditions until dissection, which was carried out 24–36 hours post-exposure (PE). Prevalence of infection was calculated according to Bush et al. (1997).
ResultsFurcocercaria sp. XIV (Figs. 1a-c).
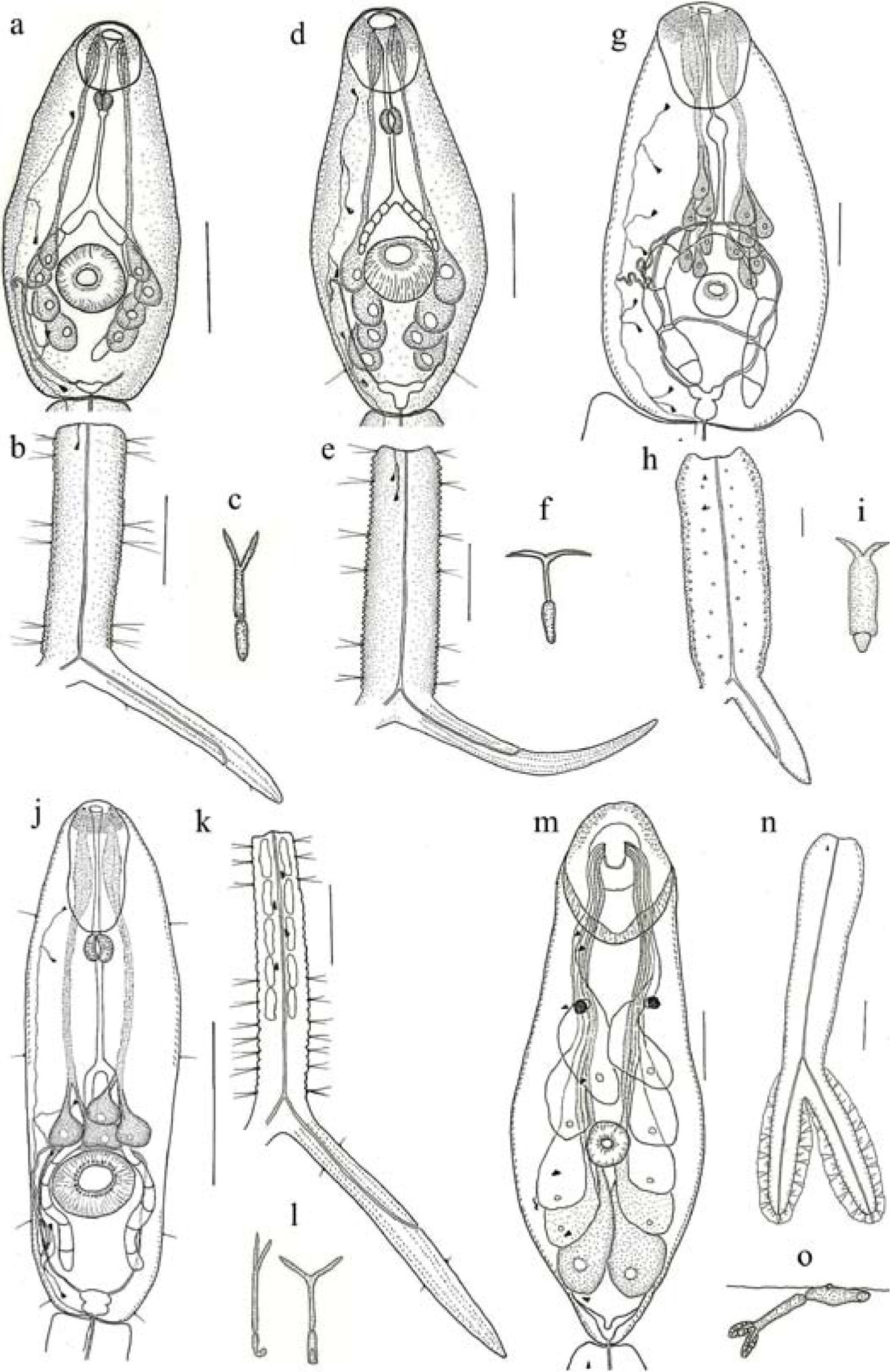
Furcocercaria sp. XIV: a, cercarial body; b, tail; c, resting position. Furcocercaria sp. XV: d, cercarial body; e, tail; f, resting position. Furcocercaria sp. XVI: g, cercarial body; h, tail; i, resting position. Furocercaria sp. XVII: j, cercarial body; k, tail; l, resting position. Furocercaria sp. XVIII: m, cercarial body; n, tail; o, resting position. Sacale bars= 50μm.
Measurements based on 10 specimens. Body 103–140 (122) long by 34–57 (48) wide, and anterior organ 2734 (30) long by 23–32 (34) wide, covered with minute spines. Sensory hairs on body not observed. Ventral sucker post-equatorial, 20–25 (22) long by 20–25 (23) wide, with a circle of irregularly arranged spines surrounding its opening. Prepharynx absent, pharynx small, esophagus long, bifurcating short distance anterior to ventral sucker. Intestinal ceca long, septated into 5 cells, reaching near excretory vesicle. Four pairs of coarsely granulated penetration glands, situated one behind the other, latero-posterior to ventral sucker. Excretory system with small excretory vesicle, 4 pairs of flame cells in body and one pair in tail stem. Flame cell formula: 2([1+1] + [1+1]+1)=10. Excretory duct passing through tail stem, bifurcating at caudal fork, opening on each furca near its end. Tail stem 119–142 (126) long by 32–46 (39) wide, without caudal bodies, but with tegumental spines, and 6 pairs of sensory hairs: 2 pairs on each margin near body, 2 pairs in the middle, and 2 pairs near furcae. Furcae 128–158 (141) long, each with 2 rows of minute spines and 2 short marginal sensory hairs, one on proximal and one on distal end.
At resting position, the body and tail stem are kept straight, with the furcae spread at a small angle. Cercariae emerge from sporocysts. In some cases metacercariae of normal Tetracotyle form were observed encysted in their own sporocysts; cysts round-oval of 182–210 (192)/ 130–143 (134) with thick double wall.
Taxonomic summary
Prevalence: 1.45, December 2010; 0.44, January; 0.11, March 2011.
Accession number: CECOAL 11020201.
Remarks. Furcocercaria sp. XIV shows characteristics typical of the genus Strigea regarding the number and position of penetration glands, the flame-cell formula, and the morphology of cysts (Tetracotyle). It is similar to Furcocercaria sp. XIII (aff. Strigea sp.) Ostrowski-de Nóñez et al., 1997 from B. tenagophila and B. orbingyi of San Roque, Corrientes, Argentina, and to Furcocercaria sp. V Ostrowski-de Nóñez, 1977 from B. peregrina of Luján River, Buenos Aires, Argentina, regarding the number and position of penetration glands, flame cell formula, and the presence of encysted metacercariae in their own sporocysts; but they differ by having sensory hairs on the body, larger body size, smaller penetration glands and different resting position. Furthermore, Furcocercaria sp. XIII has unpigmented eyespots and fewer sensory hairs on the tail stem, and in Furcocercaria sp. V, supernumerary flame cells were observed; these features were not present in Furcocercaria sp. XIV.
Furcocercaria sp. XV (Figs. 1d-f).
Measurements based on 15 specimens. Body 110–152 (130) long by 53–82 (66) wide, and anterior organ 35–45 (41) long by 24–40 (33) wide, covered with minute spines; one pair of sensory hairs on lateral margins of body at level of excretory vesicle. Ventral sucker 28–31 (29) long by 30–36 (32) wide, situated somewhat posterior to middle of body, with more than 2 alternating circles of spines surrounding its opening. Prepharynx present, pharynx small, esophagus long. Intestinal ceca small, septated into 5 cells, extending until anterior border of ventral sucker. Four pairs of large, coarsely granulated penetration glands, situated one behind the other, latero-posterior to ventral sucker. Excretory system with small Y-shaped excretory vesicle, 5 pairs of flame cells in the body, and 2 pairs in the tail stem. Flame cell formula: 2([2+1] + [2] + 2)= 14. Excretory duct passing through tail stem, bifurcating at caudal fork, opening on each furca about midway down its length. Tail stem 143–259 (163) long by 39–53 (49) wide, without spines nor caudal bodies, and with 6 pairs of sensory hairs on lateral margins, 2 pairs near body, 2 pairs in the middle, and 2 pairs near furcae. Furcae 163–202 (183) long, with 4 rows of minute spines.
At resting position body and tail stem are maintained straight, and furcae spread at an angle of more than 90°.
Taxonomic summary
Prevalence: 1.94, December 2010; 0.89, January; 0.65, February; 2.85, March; 0.17, May 2011.
Accession number: CECOAL 11020202.
Remarks. This cercaria is similar to those of the genus Strigea by position and number of penetration glands but differs from them by the flame-cell formula, which in turn resembles that of Alaria (Paralaria) (flame cell formula 5+2=14). Furcocercaria sp. VIII wóñez et al., 1997 from B. orbignyi and B. peregrina of San Roque, Corrientes, Argentina, and Furcocercaria sp. I Ostrowski-de Nóñez, 1972 from B. peregrina of Laguna de Monte, Buenos Aires, Argentina have the same number and position of penetration glands as the present cercaria, but differ by the presence of caudal bodies in the tail stem, unpigmented eyespots and fewer flame cells in the body, although 2 pairs of flame cells in the tail stem have been mentioned for Furcocercaria sp. I. Further, Furcocercaria sp. VIII is similar in body size, but possesses a higher number of sensory hairs on the body, a different resting position, and supernumerary flame cells.
Furcocercaria sp. XVI (Figs. 1g-i; 2a, d, e).
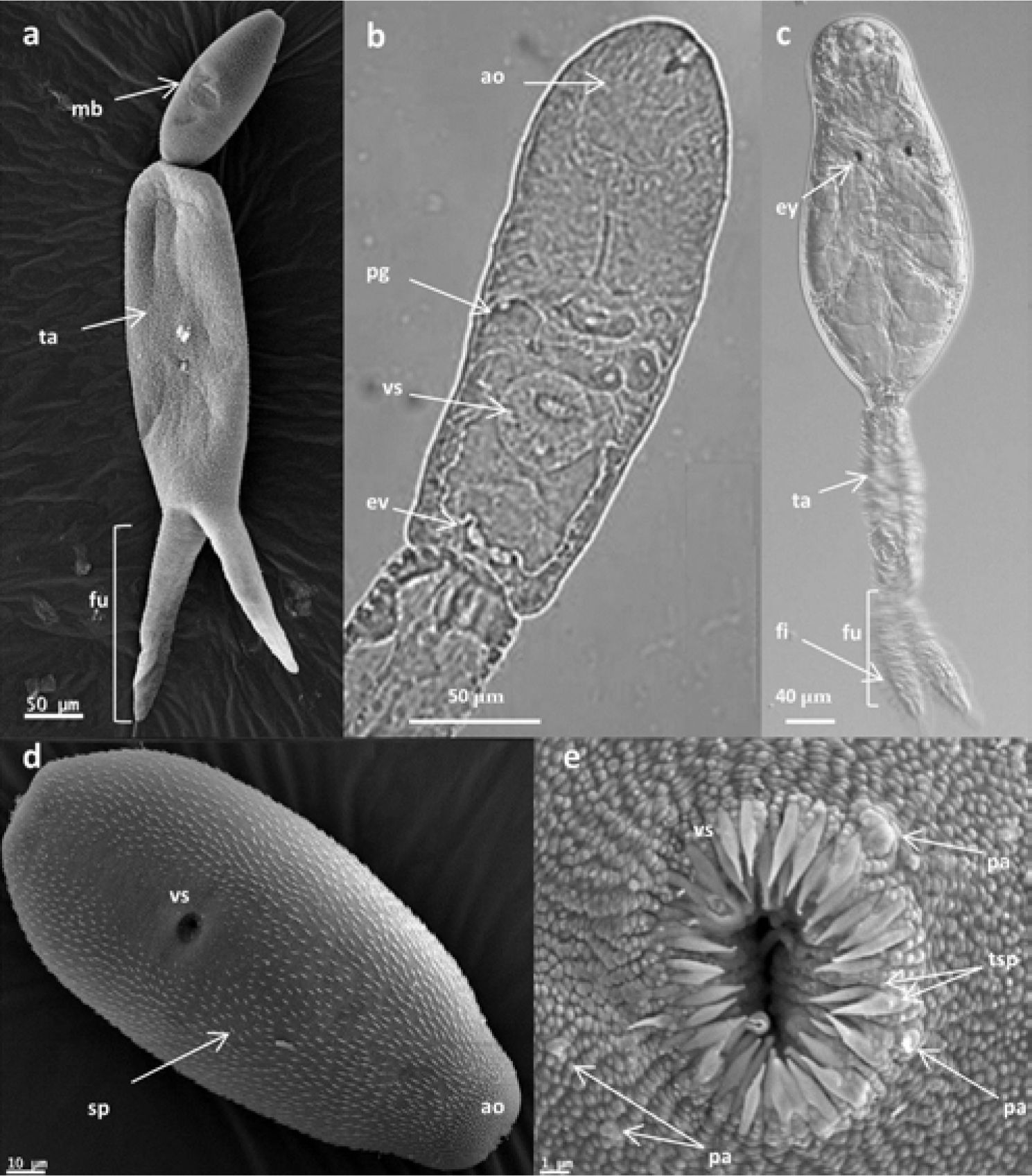
Scanning electron micrographs Furcocercaria sp. XVI: a), entire cercaría; d), cercarial body covered with larger spines; e), detail of the ventral sucker with 2 circles of spines surrounding its opening. Light micrographs Furcocercaria sp. XVII: b), cercarial body. Light micrographs using Nomarski interference-contrast (NIC) optics Furcocercaria sp. XVIII: c), entire cercaria. Abreviations: mb, main body; ta, tail stem; fu, furcae; ao, anterior organ; pg, penetration glands; vs, ventral sucker; ev, excretory vesicle; ey, eyespots; fi, finfolds; sp, spines; tsp, 2 rows of spines; pa, papillae.
Measurements based on 10 specimens. Body 174–222 (200) long by 90–120 (111) wide, tegument covered with large spines, 3–5 (4) long, except on anterior and posterior region of ventral sucker. Sensory hairs on body not observed. Anterior organ 46–57 (48) long by 34–44 (40) wide, densely covered with small spines. Ventral sucker small, post- equatorial, 21–25 (22) long by 21–23 (22) wide, with 2 alternating circles of spines surrounding its opening. Some sensorial papillae could be observed anteriorly and posteriorly to ventral sucker. Pharynx inconspicuous, non-muscular. Esophagus long, bifurcation of intestine nearly mid-way between pharynx and ventral sucker. Intestinal ceca septated into 5 cells with yellow-orange content. Penetration glands difficult to distinguish, 6 pairs grouped in front of ventral sucker. Excretory system with small Y-shaped excretory vesicle, 8 pairs of flame cells in body, and 2 pairs in tail stem. Flame cell formula: 2([2+2]+[2+2]+2)=20. Two transverse commissures present, one anterior and one posterior to ventral sucker.
Excretory ducts passing through tail stem, opening on each furca somewhat posterior to mid-way down its length. Tail stem broader, 360–420 (388) long by 138–222 (173) wide, with spines, but without sensory hairs. Furcae small, 168–282 (218) long, with small spines.
At resting position the cercarial body is retracted into the proximal part of straight tail stem, the furcae spread at an angle of approximately 90°. Cercariae emerge from sporocysts preferentially between 7 am. and 15 pm.
Taxonomic summary
Prevalence: 1.45, February; 4.22, March; 1.32, May.
Accession number: CECOAL 11022103.
Remarks. These furcocercariae were exposed to Cnesterodon sp. and their ingestion was observed. The fish were dissected 36 hours PE, and larvae were found free in the body cavity, not yet encysted. The life cycle of a species with a similar cercaria, Apharyngostrigea simplex, was described by Ostrowski-de Nóñez (1989), starting with adults from naturally infected Egretta thula. The cercariae were obtained by exposure of B. straminea to eggs present in the faeces of the infected bird; exposure of fish to these cercariae resulted in infection of the former with different free developmental stages and finally encysted metacercariae of the “tetracotyle” form in the body cavity, within 3 weeks. Furcocercaria sp. XVI differs from the cercariae of A. simplex by the absence of sensory hairs in the body and tail stem, and by its larger body, tail, furca and sucker.
Furcocercaria sp. XVII (Figs. 1 j-l; 2 b).
Measurements based on 10 specimens. Body 154–172 (163) long by 25–41 (31) wide, covered with spines, except space between bifurcation of esophagus and ventral sucker. Four pairs of sensory hairs on lateral margins of body: one at level of anterior organ, one at cecal bifurcation, one at level posterior to ventral sucker and one at level of excretory vesicle. Anterior organ 34–41 (39) long by 16–21 (19) wide, with 7–9 irregular rows of spines. Ventral sucker post-equatorial, 18–21 (19) long by 18–21 (19) wide, with one circle of 21–27 hooks surrounding its opening. Prepharynx absent; pharynx muscular; esophagus long, bifurcating at mid-body. Intestinal ceca long, septated into 5 to 6 cells. Four pairs of finely granulated penetration glands, lying immediately in front of ventral sucker: 2 overlapping dorso-ventrally between the bifurcation of esophagus and ventral sucker, the other 2 lying one on either side of the former. Excretory system with small excretory vesicle, 6 pairs of flame cells in body and 2 pairs in tail stem. Flame cell formula: 2([2+2]+[2]+2)=16. Two pairs of ciliary patches in common collecting ducts. Transverse commissure anterior to ventral sucker. Excretory ducts opening on the margin of each furca about midway down its length. Tail stem 166–195 (180) long by 23–32 (28) wide, with 5 pairs of caudal bodies, and 10 pairs of sensory hairs on each side: 3 pairs near body, and 7 pairs near furcae. Furcae 177–209 (195) long, with 4 rows of minute spines and 2 pairs of short marginal sensory hairs.
At resting position the body is kept bent and the tail stem straight, with the furcae spread at an angle of about 90°.
Taxonomic summary
Prevalence: 1.02, March; 0.09, May.
Accession number: CECOAL 11031404.
Remarks. This cercaria penetrated experimentally in Cheirodon piaba, where it was visible by the sanguinolent traces in different parts of the body, especially intense in the head of the fish; 17–24 hours post-exposure the fish were dissected, showing many young metacercariae free in the brain and eyes. These metacercariae retained many features of the cercaria, such as similar size, presence of ventral sucker, penetration glands, intestinal ceca and excretory system.
The morphology of the present cercaria is similar to those of the genera Tylodelphys and Austrodiplostomum, e.g. Furcocercaria sp. A (cf. Tylodelphys sp.) Ostrowski-de Nóñez and Quaggiotto, 1995, from Chilina sp. of Río Negro province, Argentina, regarding the flame cell formula, position and number of penetration glands, number of hooks in the ventral sucker, body spination, and resting position in the water, among other characteristics, but differs by having smaller body size, more numerous sensory hairs on the body, and in that its first intermediate host is a planorbid. Furcocercaria sp. XVII is also similar to the cercariae of Austrodiplostomum mordax Szidat and Nani, 1951 from B. peregrina, and of A. compactum (Lutz, 1928) from B. prona (Martens, 1873), but differs by having smaller body size, fewer sensory hairs on the body and the tail stem, and 21–27 vs 39–46 and 26–29 hooks, respectively, on the ventral sucker (Ostrowski-de Nóñez, 1982). As well as Furcocercaria A, Furcocercaria sp. XVII penetrated experimentally in fishes, migrating to the same locations, the brain and eyes. Similarly, A. mordax metacercariae are found in the brain, but never in the eyes, and A. compactum metacercaria occur in the eyes, and more rarely in the brain (Ostrowski-de Nóñez, 1982).
Furcocercaria sp. XVIII (1 m-o; 2 c).
Measurements based on 12 specimens. Body 264–312 (288) long by 114–132 (123) wide, and anterior organ 92–113 (103) long by 62–80 (74) wide, covered with minute spines. Posterior region of penetration organ muscular. Ventral sucker post-equatorial, 25–32 (29) long by 25–39 (33) wide, without spines surrounding its opening. Pigmented eyespots with lens present. Five pairs of large penetration glands, grouped into 2 anterior pairs, very finely granulated, and 3 posterior pairs, more coarsely granulated, relative to ventral sucker. Excretory vesicle small, 7 pairs of flame cells in the body, and one pair in the tail stem. Flame cell formula: 2 ([4]+[3]+1)=16. A pair of ciliary patches in common collecting ducts. Caudal duct of excretory system opens on tip of each furca. Tail stem 192–258 (220) long by 36–48 (40) wide, with spines. Furcae 96–144 (123) long, with spines and undulating dorso-ventral finfold, extending along whole length of furca.
Cercariae emerge from sporocysts, preferentially between 7 am. and 7 pm. The cercariae have little movement; at resting position the body remains attached to the water surface by the ventral sucker, with the tail stem and furcae kept at approximately 45°.
Taxonomic summary
Prevalence: 0.11%, March.
Accession number: CECOAL 11031405.
Remarks. The pharynx, esophagus and intestinal ceca were not observed in the present cercaria; the intestinal tract is often poorly developed in this type of cercariae, and was possibly overlooked. Regarding the morphological characters (pigmented eyespots, anterior organ with a posterior muscular part, 5 pairs of penetration glands, small ventral sucker, spines on the body, dorso-ventral finfolds on the furcae) the present cercaria is similar to those of the family Schistosomatidae. Two schistosome cercariae have been described for Argentina that use planorbid snails as intermediate hosts: a), Cercaria quequeniSzidat, 1951 from B. peregrina of Quequen River, Buenos Aires province, Argentina, differs from the present cercaria by having non-pigmented eyespots, fewer flame cells (4+1), the 2 anterior pairs of penetration glands are more coarsely granulated, while the 3 posterior pairs are finely granulated, the ventral sucker is nearly equatorial, spines are only present in the anterior region of the tail stem, it has a smaller body and larger tail stem, and a different resting position; b), Cercaria planorbicolaSzidat and C. de Szidat, 1960 from B. peregrina of Quequen River, Buenos Aires province, Argentina, is similar to Furcocercaria sp. XVIII by having pigmented eyespots with lens, the 3 anterior pairs of penetration glands more finely and the posterior glands more coarsely granulated, and a similar resting position, but differs by the absence of spines in the body, smaller body and furcae sizes and larger tail stem, it is noted that flame cells were not observed in this cercaria.
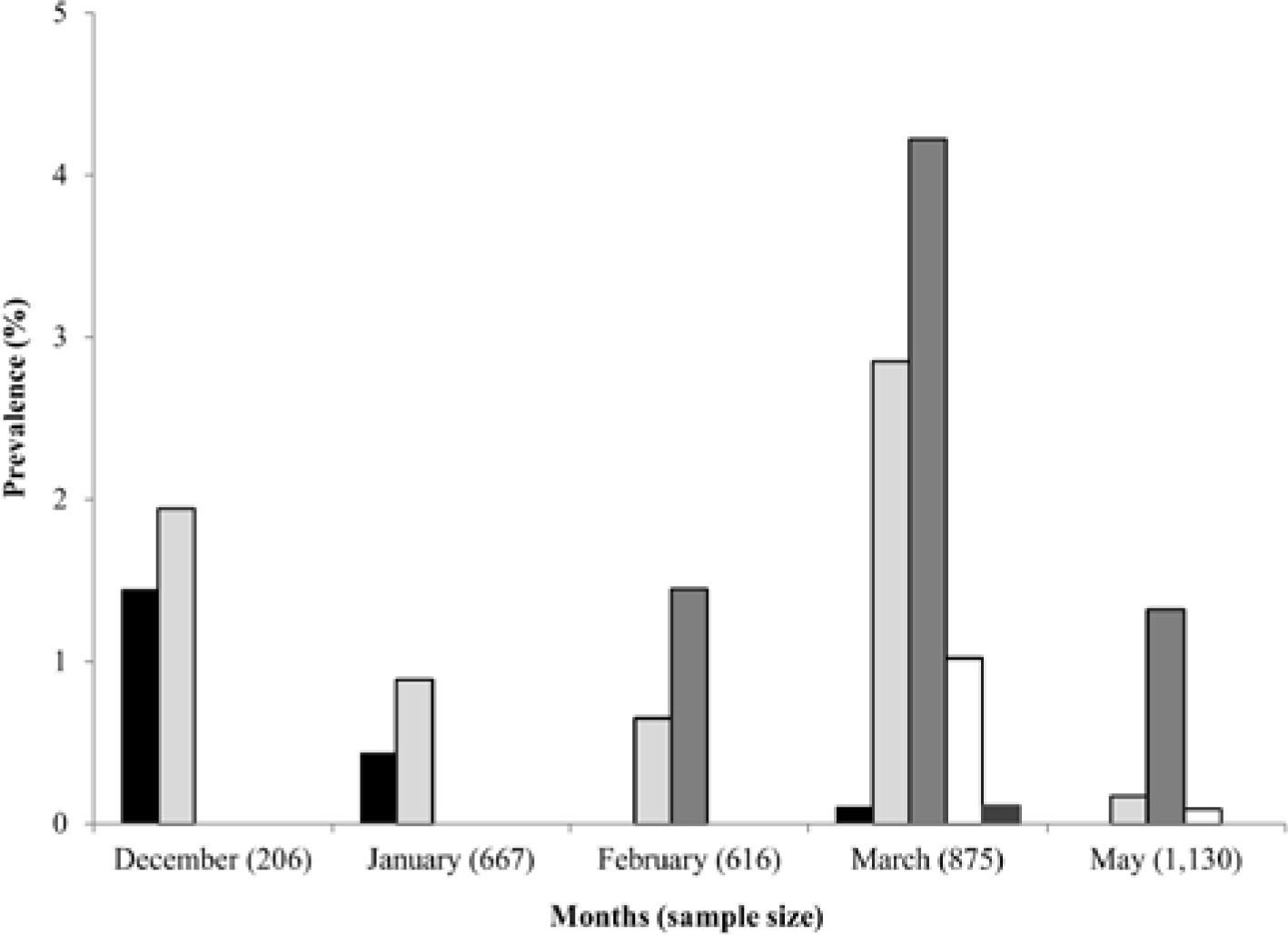
Prevalence of larval trematode infections
A total of 3 494 snails were examined, of which 115 (3.29 %) were infected with furcocercariae. Prevalence of infection of the different cercariae, and sample size for each month are shown in figure 3. Prevalence of infection ranged between 0.11% (Furcocercaria sp. XVIII) and 4.22% (Furcocercaria sp. XVI). Furcocercaria sp. XIV, Furcocercaria sp. XV and Furcocercaria sp. XVI were the most common species present in nearly all months sampled, while Furcocercaria sp. XVII and Furcocercaria sp. XVIII were rarer species. Occurrence of all the species described was recorded in March, when peaks in infection prevalence were also recorded for most samples (Furcocercaria sp. XV, Furcocercaria sp. XVI and Furcocercaria sp. XVII); only Furcocercaria sp. XIV peaked in December.
DiscussionPrevious studies of furcocercariae in different planorbid snails have been performed in non-agricultural habitats of northeastern Argentina (Ostrowski-de Nóñez et al., 1990, 1991, 1997; Hamann et al. 1991, 1993). The present study described 5 furcocercariae parasitizing B. straminea in an agricultural habitat, which can now be added to the 4 species of furcocercariae reported for the genus Biomphalaria in the region. Furthermore, Furcocercaria sp. XVIII is the third species of Schistosomatidae reported to be using planorbid snails as intermediate hosts in Argentina, along with Cercaria quequeni and Cercaria planorbicola. These species are particularly noteworthy as they are potential producers of cercarial dermatitis after recurrent exposures to cercariae (Ostrowski-de Nóñez, 1978).
The prevalence of the different furcocercariae was generally low (0.11–4.22%); this result agrees with those reported in several studies of the prevalence of cercariae from field collections (Anderson and May, 1979; Brockelman et al., 1986; Lawmbo, 1988; Ostrowski-de Nóñez et al., 1990, 1991, 1997; Väyrynen, 2000). In turn, the prevalence level seems to be related with the size of the samples: when the number of snails collected is high, the prevalence of infection is relatively low, and vice versa (Ewers, 1964; Ostrowski-de Nóñez et al., 1991). In the present study the number of snails collected was generally high, increasing with each month sampled, while the prevalence did not exceed 5% for each furcocercaria.
The life cycles and definitive hosts of the cercariae described herein are not known. The definitive hosts for Furcocercaria sp. XV, Furcocercaria sp. XVI, and Furcocercaria sp. XVIII are possibly birds, according to similar furcocercariae for which life cycles are known. In the case of Furcocercaria sp. XIV, mammals may also act as definitive hosts (Ostrowski-de Nuñez, 1977; Ostrowskide Nóñez, 1989; Yamaguti, 1975). Additionally, rice fields provide an excellent habitat for aquatic birds, which was reflected in the number of species recorded during the sampling.
Due to the importance of B. straminea as an intermediate host of S. mansoni, the study of larval trematodes in agricultural habitats should be encouraged in order to obtain further information about species that may affect the interaction between S. mansoni and its host (Spatz et al., 2012).