In arid ecosystems, biological soil crusts closely interact with microarthropod communities. Together, both communities play one of the most important environmental services: decomposition of organic matter. In a desert scrub in the southern Baja California Peninsula of Mexico, microarthropod communities were correlated to biological soil crusts and the way soil properties influence distribution of the microarthropods. Twenty five soil samples were taken from 3 site types: without crusts (10), with crusts (10), and eroded surfaces (5). Microarthropods were extracted; specimens were identified to family level and feeding groups were identified. Of the 4 682 microarthropods within 40 taxa, Prostigmata had the greatest richness. The lack of plant coverage at eroded sites seems to affect micro- environmental conditions, so that no microarthropods were found at these sites and biological soil crusts were simple in structure. Among desert scrub, biological soil crusts were complex in structure, and edaphic properties were more favorable for microarthropods to thrive. Specific dissimilarities in community structure of microarthropods for each microhabitat were related to feeding preferences of each taxa.
En los ecosistemas áridos, las costras biológicas de suelo junto con las comunidades de microartrópodos dan lugar a uno de los servicios ambientales más importantes: la descomposición de la materia orgánica. La relación entre comunidades de microartrópodos y costras biológicas de suelo, así como la influencia de las propiedades edáficas en la distribución de microartrópodos fueron estudiadas en un matorral desértico en el sur de la península de Baja California. Se tomaron 25 muestras de suelo de 3 sitios: sin costras (10), con costras (10) y superficies erosionadas (5). Se extrajeron los microartrópodos, que fueron identificados hasta familia y se identificaron hábitos alimenticios. Se encontraron 4 682 microartrópodos en 40 taxa, Prostigmata con la mayor riqueza. La falta de cobertura vegetal en los sitios erosionados parece afectar las condiciones micro-ambientales, por lo que no fueron encontrados microartrópodos en estos sitios y las costras biológicas encontradas fueron simples. En el resto del matorral, las costras biológicas eran complejas en su estructura y las propiedades edáficas fueron más favorables para el desarrollo de microartrópodos. Se relacionaron las diferencias específicas dentro de la estructura de la comunidad de microartrópodos para cada microhábitat con los hábitos alimenticios de cada taxa.
Over more than 70% of the surface of the world is arid and semiarid soils, where vascular plants are widely dispersed or absent; there, a highly specialized microorganism community of biological soil crusts (BSC), composed of cyanobacteria, algae, microscopic fungi, lichens, and mosses predominates (Belnap, 2001a). These organisms grow inside or on the surface of the upper layer of the soil; during its development, polysaccharides secreted by cyanobacteria and algae, together with filaments of lichens and moss, adhere to soil particles (Coleman et al., 2004). Consequently, BSC stabilize and reduce the susceptibility of soil to water and wind erosion. Furthermore, BSC supply a source of nitrogen for desert soils, which can support germination and establishment of vascular plants (Belnap, 2001a). Flat and rough types of BSC have been identified from a mountainous range of southern Baja California Peninsula (Maya et al., 2002).
According to Neher et al. (2009), there is a close relationship between the porous structure of BSC and the edaphic fauna; this microhabitat of microarthropods also supplies its main source of food. In turn, microarthropods support the bacteria and fungi by supplying detritus (Palacios-Vargas, 1983), as well as disseminating bacteria and fungi spores, and lichen fragments and soredia (Steinberger, 1991). Hence, microarthropods are important regulators of the bacterial and fungal populations of soil. Since microarthropods are relatively sedentary, they reveal soil conditions better than mobile microfauna (Olfert et al., 2002); hence, they can be used as bio-indicators of the health of desert soils (Sandor and Maxim, 2008).
Unfortunately, from the terrestrial ecosystems, soil comprises one of the less studied resources (Coleman et al., 2004), both in its biodiversity and its internal processes. Of the microarthropods only ∼10% have been examined and probably only 10% of the species described (André et al., 2002). The basis of distribution of the edaphic fauna and the way it interacts and develops are far from being understood. As a result, the importance of the biota in the soil processes are commonly underestimated (Coleman et al., 2004), regardless of the importance of the services provided to humanity and the rest of the biota. Few studies have been made regarding interactions between biological soil crusts and microarthropod communities. It is remarkable that in North America, studies of microarthropods in arid lands have focused on the Chihuahuan and Mojave Deserts and not the Sonoran Desert. The main objective was to analyze the influence of some edaphic parameters in microarthropod communities related to BSC in a desert scrub.
Material and methodsStudy siteThe study was conducted at the Northwest Biological Research Center (CIBNOR) reserve, located on an alluvial plain dominated by desert scrub vegetation; the common shrubs include Jatropha cinerea, J. cuneata, Prosopis articulata, Bursera microphylla, Fouquieria diguetti, Cyrtocarpa edulis and the cardon cactus Pachycereus pringlei. Summers are hot and arid, with occasional tropical storms bringing most of the rain. There is only one rainy season, from August to February with 2 peaks in September and January, with the greatest precipitation in summer; winter storms provide <10% of the annual total (León de la Luz et al., 1996).
Biological soil crusts are distributed in patches on the soil surface. Based on appearance, they are designated as “flat” or “rough” crusts; rough crusts contain very conspicuous lichens; in contrast, flat crusts are dominated by cyanobacteria. Flat crusts were found on eroded soils; rough crusts on scrubland soils. Stereoscopic and bright-field microscopy show that filamentous cyanobacteria are dominant in both crusts, most species belonging to Microcoleus, as well as many species of the genera Scytonema and Nostoc, which are nitrogen fixers.
SamplingField work was performed in September and October 2011. From a satellite image of 22 July 2009, we identified 25 sites in this area, of which 10 sites had BSC, 10 sites were without BSC, and 5 sites were eroded. To collect microarthropods, a sample of litter and the upper layer of the soil were excavated (together with the crusts, if present). At 10 sites (5 with and 5 without crusts) a soil sample (0–10cm deep) was collected for analysis. From each sample, a volume of 500mL of soil (or soil + litter) was placed in a box. At each site, the surrounding vegetation and the type of BSC (flat or rough) was described. The following soil properties were measured: pH, electrical conductivity, total dissolved solids, soluble phosphorus, organic matter, calcium, magnesium, total nitrogen, sodium, potassium, soil texture, bulk density, pore space, temperature, and relative humidity (the 2 last in the field). Microarthropod extraction and identification. Microarthropods were extracted from the soil samples using Berlese-Tullgren funnels (Palacios-Vargas and Mejía, 2007). Voucher specimens of each family were mounted in Hoyer's fluid on glass slides for initial identification and subsequently archived (Palacios-Vargas and Mejía, 2007). Only colembolans (Christiansen and Bellinger, 1998) and mites (Balogh and Balogh, 1988; Kethley, 1990; Walter et al., 2009) were identified to family. Feeding groups were assigned to each Acari family based on the feeding behavior (McDaniel, 1979; Neher et al., 2009; Walter et al., 2009). The groups were: predators (nematode and other microarthropods), phytophages, microphytophages (fungi and algae eaters), and saprophytes.
Data analysisKruskall-Wallis analysis was used to determine differences between the edaphic parameters in both conditions (“soil with crusts” and “soil without crusts”). For each condition, the diversity, evenness, and dominance were estimated. For diversity, the Shannon index (H') was used; evenness was obtained by dividing the value of Shannon's diversity index by the logarithm of the number of taxa, and dominance was obtained with the Berger-Parker index (Magurran, 1988). To assess differences in richness between crust types, Whittaker's similarity index was used (Arellano and Halffter, 2003).
Multiple regression tests identified the association between edaphic parameters and abundances of each taxon. For these correlations, only taxa that were present in at least 3 of the sample sites, each with more than 3 individuals were used. Kruskall-Wallis analysis and multiple regression tests were processed with software (Statistica 6.0, StatSoft, 1995). Indexes were obtained with additional software (PAST 2.04; Hammer et al., 2001).
ResultsDescription of the areaThe total annual precipitation in 2009 was 215.9mm, while a total of 0.15mm was recorded until the sampling period in September-October 2011, the time of sampling. Dryness contributed to the very low quantity of litter found, around 1cm thick, when most abundant. The vegetation was leafless and non-flowering.
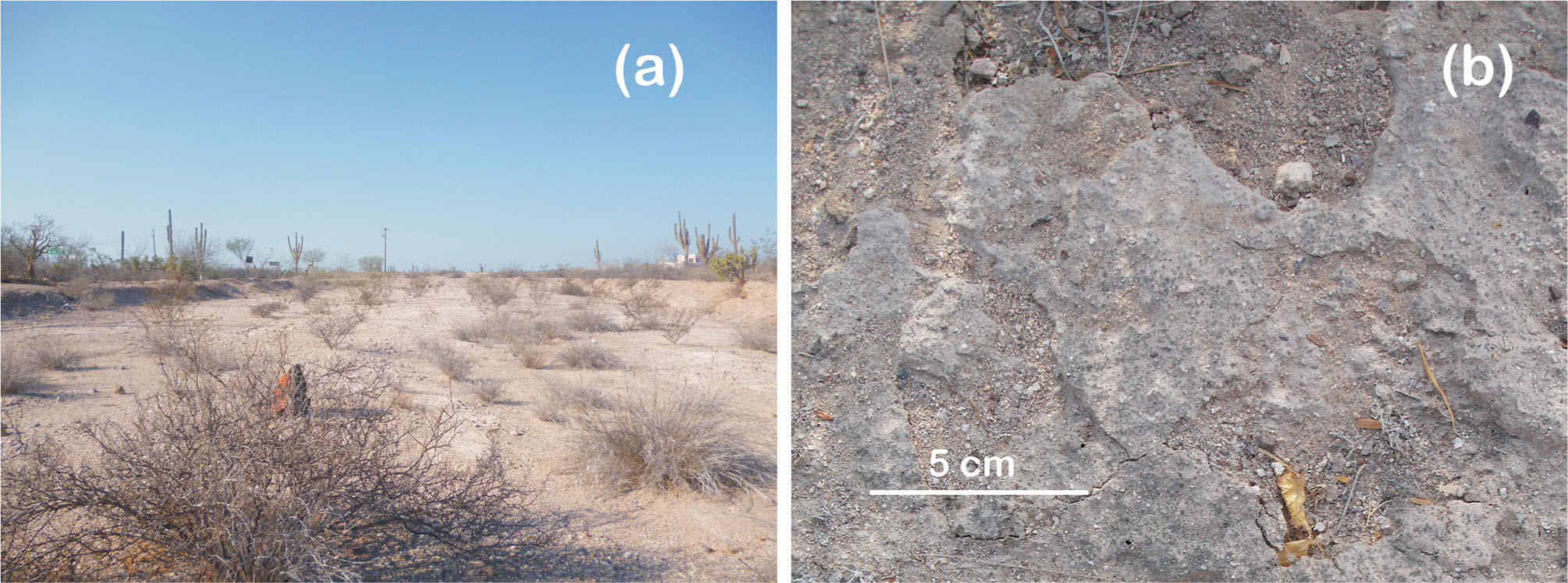
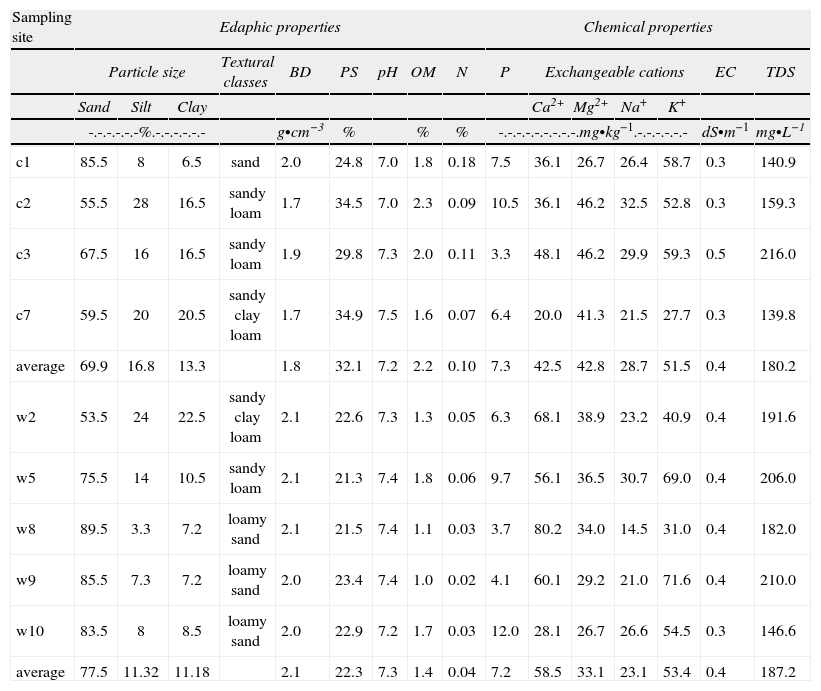
Most of the study area had well developed fleshy-stemmed scrub, but eroded sites lacked native plant cover, with scattered introduced buffel grass (Cenchrus ciliaris) as the dominant species (Fig. 1). Flat BSC was found underneath the buffel grass, but no microarthropods were captured from samples collected at eroded sites. In the remaining study area, where desert scrub prevailed, rough BSC were common (Fig. 2), but less abundant where the plant coverage was dense, probably as a consequence of the reduction of radiation.
No significant differences were found regarding average temperature (26.4°C) and (75.07%) relative humidity at sites with rough crusts. At sites without crusts, the averages were 24.26°C and 72.85% RH. No significant differences were found between textural classes at both kinds of sites. Some slight differences occurred in the frequency of soil particle sizes. At sites without crusts, sand was more common than silt and clay, compared with sites with rough crusts (Table 1). At the latter sites, bulk density was less (1.8g•cm−3 vs. 2.0g•cm−3) and pore space was greater (32.1% vs. 22.3%). These results were significantly different (H=10, df=1, p=0.0016). Regarding chemical parameters, nitrogen content was the only parameter that had significant differences between site types, with higher values from sites with rough crusts (0.10% vs. 0.04%, H=10, df=1, p=0.0016). There were no significant differences between the values of OM and pH; however, the results suggest some variance that could be related to the presence of biological crusts (Table 1).
Physical and chemical properties of soil samples
| Sampling site | Edaphic properties | Chemical properties | ||||||||||||||
| Particle size | Textural classes | BD | PS | pH | OM | N | P | Exchangeable cations | EC | TDS | ||||||
| Sand | Silt | Clay | Ca2+ | Mg2+ | Na+ | K+ | ||||||||||
| -.-.-.-.-.-%.-.-.-.-.-.- | g•cm−3 | % | % | % | -.-.-.-.-.-.-.-.-.mg•kg−1.-.-.-.-.-.- | dS•m−1 | mg•L−1 | |||||||||
| c1 | 85.5 | 8 | 6.5 | sand | 2.0 | 24.8 | 7.0 | 1.8 | 0.18 | 7.5 | 36.1 | 26.7 | 26.4 | 58.7 | 0.3 | 140.9 |
| c2 | 55.5 | 28 | 16.5 | sandy loam | 1.7 | 34.5 | 7.0 | 2.3 | 0.09 | 10.5 | 36.1 | 46.2 | 32.5 | 52.8 | 0.3 | 159.3 |
| c3 | 67.5 | 16 | 16.5 | sandy loam | 1.9 | 29.8 | 7.3 | 2.0 | 0.11 | 3.3 | 48.1 | 46.2 | 29.9 | 59.3 | 0.5 | 216.0 |
| c7 | 59.5 | 20 | 20.5 | sandy clay loam | 1.7 | 34.9 | 7.5 | 1.6 | 0.07 | 6.4 | 20.0 | 41.3 | 21.5 | 27.7 | 0.3 | 139.8 |
| average | 69.9 | 16.8 | 13.3 | 1.8 | 32.1 | 7.2 | 2.2 | 0.10 | 7.3 | 42.5 | 42.8 | 28.7 | 51.5 | 0.4 | 180.2 | |
| w2 | 53.5 | 24 | 22.5 | sandy clay loam | 2.1 | 22.6 | 7.3 | 1.3 | 0.05 | 6.3 | 68.1 | 38.9 | 23.2 | 40.9 | 0.4 | 191.6 |
| w5 | 75.5 | 14 | 10.5 | sandy loam | 2.1 | 21.3 | 7.4 | 1.8 | 0.06 | 9.7 | 56.1 | 36.5 | 30.7 | 69.0 | 0.4 | 206.0 |
| w8 | 89.5 | 3.3 | 7.2 | loamy sand | 2.1 | 21.5 | 7.4 | 1.1 | 0.03 | 3.7 | 80.2 | 34.0 | 14.5 | 31.0 | 0.4 | 182.0 |
| w9 | 85.5 | 7.3 | 7.2 | loamy sand | 2.0 | 23.4 | 7.4 | 1.0 | 0.02 | 4.1 | 60.1 | 29.2 | 21.0 | 71.6 | 0.4 | 210.0 |
| w10 | 83.5 | 8 | 8.5 | loamy sand | 2.0 | 22.9 | 7.2 | 1.7 | 0.03 | 12.0 | 28.1 | 26.7 | 26.6 | 54.5 | 0.3 | 146.6 |
| average | 77.5 | 11.32 | 11.18 | 2.1 | 22.3 | 7.3 | 1.4 | 0.04 | 7.2 | 58.5 | 33.1 | 23.1 | 53.4 | 0.4 | 187.2 | |
c= rough biological soil crusts; w= without crusts; BD= bulk density; PS= pore space; OM= organic matter; EC= electrical conductivity; TDS= total dissolved solids.
The 4 628 microarthropods were placed in 40 taxa, 23 families belonged to Acari, 3 to Collembola, and 12 to a miscellaneous group that contains specimens in Insecta and Aracnida (Table 2). Within Acari, the order with higher richness was Prostigmata with 20 families (1 306 specimens). In Oribatida, there were 4 families (1 020 specimens), and for Acaridae, there were 1 510 specimens in Astigmata; this family represented 32.6% of the specimens.
Results of abundance (number of individuals), density (ind-area−1), richness (number of taxa), and percentage of edaphic microfauna. Only the feeding habits of the families belonging to Acari were included
| With crust | Without crust | Abundance/family | |||||||
| Abd | Density in 339.09cm2 | Density in 1m2 | % | Abd | Density in 339.09cm2 | Density in 1m2 | % | ||
| Prostigmata | |||||||||
| 1Adamystidae | 3 | 0.01 | 0.26 | 0.11 | 13 | 0.04 | 1.13 | 0.67 | 16 |
| 1Bdellidae | 90 | 0.27 | 7.83 | 3.33 | 83 | 0.24 | 7.22 | 4.31 | 173 |
| 1, 2Caeculidae | 16 | 0.05 | 1.39 | 0.59 | 11 | 0.03 | 0.96 | 0.57 | 27 |
| 1Caligonellidae | 13 | 0.04 | 1.13 | 0.48 | 27 | 0.08 | 2.35 | 1.40 | 40 |
| 1, 2Camerobiidae | 1 | 0.00 | 0.09 | 0.04 | — | — | — | — | 1 |
| 1Cheyletidae | — | — | — | — | 1 | 0.00 | 0.09 | 0.05 | 1 |
| 1,2Cunaxidae | 7 | 0.02 | 0.61 | 0.26 | 2 | 0.01 | 0.17 | 0.10 | 9 |
| 2Dolichocybidae | 4 | 0.01 | 0.35 | 0.15 | 1 | 0.00 | 0.09 | 0.05 | 5 |
| *Eutrombidiidae | 1 | 0.00 | 0.09 | 0.04 | 1 | 0.00 | 0.09 | 0.05 | 2 |
| 4Linotetranidae | 51 | 0.15 | 4.44 | 1.89 | 13 | 0.04 | 1.13 | 0.67 | 64 |
| 4Nanorchestidae | 6 | 0.02 | 0.52 | 0.22 | — | — | — | — | 6 |
| Paratydeidae | |||||||||
| 1Morphospecies 1 | 57 | 0.17 | 4.96 | 2.11 | 234 | 0.69 | 20.35 | 12.15 | 291 |
| 1Morphospecies 2 | 51 | 0.15 | 4.44 | 1.89 | 32 | 0.09 | 2.78 | 1.66 | 83 |
| 4Penthaleidae | — | — | — | — | 1 | 0.00 | 0.09 | 0.05 | 1 |
| Pyemotidae | |||||||||
| 2Pyemotes sp. | 1 | 0.00 | 0.09 | 0.04 | — | — | — | — | 1 |
| 2Pygmephoridae | 262 | 0.77 | 22.79 | 9.70 | 288 | 0.85 | 25.05 | 14.95 | 550 |
| 1Smarididae | 1 | 0.00 | 0.09 | 0.04 | 2 | 0.01 | 0.17 | 0.10 | 3 |
| Tarsonemidae | |||||||||
| 2Tarsonemus sp. | — | — | — | — | 1 | 0.00 | 0.09 | 0.05 | 1 |
| Teneriffiidae | |||||||||
| 4Parateneriffia sp. | 3 | 0.01 | 0.26 | 0.11 | — | — | — | — | 3 |
| 4Tetranychidae | — | — | — | — | 19 | 0.06 | 1.65 | 0.99 | 19 |
| 1Tydeidae | 1 | 0.00 | 0.09 | 0.04 | 9 | 0.03 | 0.78 | 0.47 | 10 |
| Richness | 17 | 17 | S21 | ||||||
| Total | 568 | 1.68 | 49.40 | 21.03 | 738 | 2.18 | 64.18 | 38.32 | 1,306 |
| Oribatida | |||||||||
| Aphelacaridae | |||||||||
| 2Aphelacarus acarinus | 588 | 1.73 | 51.14 | 21.77 | 292 | 0.86 | 25.40 | 15.16 | 880 |
| Cymbaeremaeidae | — | — | — | — | 1 | 0.00 | 0.09 | 0.05 | 1 |
| 2Scapheremaeus sp. | |||||||||
| 2Gymnodamaeidae | — | — | — | — | 2 | 0.01 | 0.17 | 0.10 | 2 |
| Haplochthoniidae | 40 | 0.12 | 3.48 | 1.48 | 97 | 0.29 | 8.44 | 5.04 | 137 |
| 2Haplochthonius sp. | |||||||||
| Richness | 2 | 4 | S4 | ||||||
| Total | 628 | 1.85 | 54.62 | 23.25 | 392 | 1.16 | 34.09 | 20.35 | 1,020 |
| Astigmata | |||||||||
| 2,3Acaridae | 1,098 | 3.24 | 95.49 | 40.65 | 412 | 1.22 | 35.83 | 21.39 | 1,510 |
| Richness | 1 | 1 | S1 | ||||||
| Total | 1,098 | 3.24 | 95.49 | 40.65 | 412 | 1.22 | 35.98 | 21.39 | 1,510 |
| Collembola | |||||||||
| Brachystomellidae | 3 | 0.01 | 0.26 | 0.11 | 6 | 0.02 | 0.52 | 0.31 | 9 |
| Brachystomella sp. | |||||||||
| Entomobryidae | 2 | 0.01 | 0.17 | 0.07 | — | — | — | — | 2 |
| Isotomidae | |||||||||
| Folsomides sp. | 19 | 0.06 | 1.65 | 0.70 | 8 | 0.02 | 0.70 | 0.42 | 27 |
| Richness | 3 | 2 | S3 | ||||||
| Total | 24 | 0.07 | 2.09 | 0.89 | 14 | 0.04 | 1.22 | 0.73 | 38 |
| Thysanura | 13 | 0.04 | 1.13 | 0.48 | 16 | 0.05 | 1.39 | 0.83 | 29 |
| Araneae | 1 | 0.00 | 0.09 | 0.04 | 1 | 0.00 | 0.09 | 0.05 | 2 |
| Pseudoescorpionida | 3 | 0.01 | 0.26 | 0.11 | 22 | 0.06 | 1.91 | 1.14 | 25 |
| Coleoptera | 10 | 0.03 | 0.87 | 0.37 | 5 | 0.01 | 0.43 | 0.26 | 15 |
| Diptera | 24 | 0.07 | 2.09 | 0.89 | 20 | 0.06 | 1.74 | 1.04 | 44 |
| Embioptera | 3 | 0.01 | 0.26 | 0.11 | — | — | — | — | 3 |
| Hemiptera | — | — | — | — | 2 | 0.01 | 0.17 | 0.10 | 2 |
| Homoptera | 158 | 0.47 | 13.74 | 5.85 | 28 | 0.08 | 2.44 | 1.45 | 186 |
| Hymenoptera | 3 | 0.01 | 0.26 | 0.11 | 4 | 0.01 | 0.35 | 0.21 | 7 |
| Isoptera | 7 | 0.02 | 0.61 | 0.26 | 51 | 0.15 | 4.44 | 2.65 | 58 |
| Neuroptera | 1 | 0.00 | 0.09 | 0.04 | — | — | — | — | 1 |
| Psocoptera | 162 | 0.48 | 14.09 | 6.00 | 220 | 0.65 | 19.13 | 11.42 | 382 |
| Richness | 11 | 10 | S12 | ||||||
| Total | 385 | 1.14 | 33.48 | 14.25 | 369 | 1.09 | 32.09 | 19.16 | 754 |
| Abundance per site type | 2 703 | 1 925 | 4 628 | ||||||
Among Collembola, the most abundant family was Isotomidae, with 27 specimens of a new species of Folsomides; it is remarkable that these specimens belonged to a new species (Palacios-Vargas and Villarreal-Rosas, 2013). It is worth mentioning that the 3 families of Collembola represent only 0.82% in all specimens. Amongst the remaining taxa, the orders with greatest abundance was Psocoptera (382 specimens), and Homoptera (186 specimens) (Table 2).
Regarding diversity, H' was 1.97 for the sites with crusts and 2.40 for the sites without crusts (t=−12.27, df=4537.6, p<0.05). Diversity was higher at the sites without crusts despite the fact that the number of taxa was 34 in both site types. At sites without crusts, the number of individuals was lower (1 925 vs. 2 703). At sites without crusts, the communities have greater evenness, with values of 0.68 vs. 0.59. The results for the Berger-Parker index strengthen the finding that at the sites with crusts, there are fewer taxa with more individuals in each taxa, with values of 0.40 vs. 0.21. The taxa that may be influencing this result are: Aphelacarus acarinus (588 specimens), Acaridae (1 098 specimens), and Homoptera (158 specimens). The taxa richness per order is similar at sites with crusts and without crusts, but not abundances. Prostigmata was more abundant at sites without crusts (738 vs. 568). Oribatida, Astigmata, Collembola, and the miscellaneous group were more abundant at sites with crusts (Table 2).
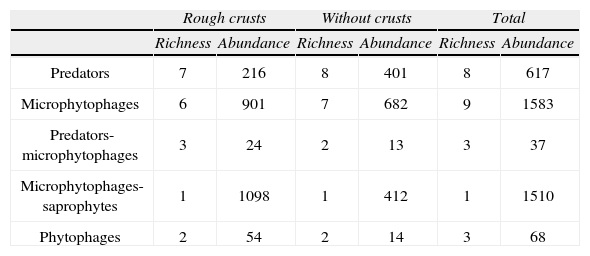
The similarity value between the communities of both types of sites, as estimated by the Whittaker index was 0.20, which implies that the communities are very similar, although only in the number of taxa. As stated earlier, both types of communities differ mainly in the number of individuals per taxa. Related to this, the feeding habits of the taxa appear to follow this pattern (Table 3), with the number of taxa in each habit similar, but with differences in abundance.
Richness and abundance of the recorded taxa according to the feeding behavior in which were classified
| Rough crusts | Without crusts | Total | ||||
| Richness | Abundance | Richness | Abundance | Richness | Abundance | |
| Predators | 7 | 216 | 8 | 401 | 8 | 617 |
| Microphytophages | 6 | 901 | 7 | 682 | 9 | 1583 |
| Predators-microphytophages | 3 | 24 | 2 | 13 | 3 | 37 |
| Microphytophages-saprophytes | 1 | 1098 | 1 | 412 | 1 | 1510 |
| Phytophages | 2 | 54 | 2 | 14 | 3 | 68 |
The families of predators were more abundant at sites without crusts (401 vs. 216). The microphytophages and phytophages were more abundant at sites with crusts (901 vs. 682 and 54 vs. 14, respectively). There were families containing 2 groups, predators and microphytophages, which were more abundant at sites with crusts (24 vs. 13).
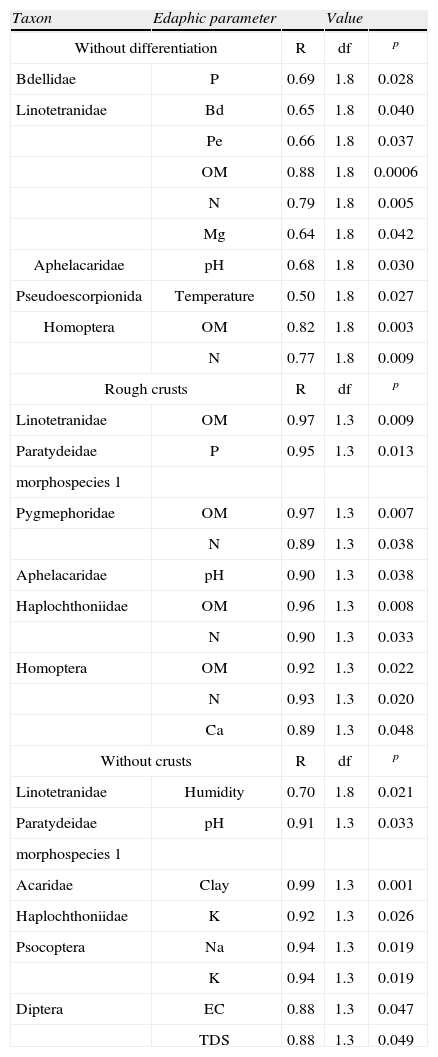
Correlation analysisOnly Linotetranidae, Bdellidae, and Pseudoescorpionida showed a significant correlation with a specific parameter (pore space, phosphorus, and temperature, respectively), when the total abundance (without discrimination between site types) was considered. Aphelacaridae, Linotetranidae, and Homoptera were positively correlated with pH and organic matter (Table 4) when total abundance was taken into account and when only abundances recorded at the sites with rough crusts were considered. In contrast, they were not positively correlated when only abundance at the sites without crusts were considered.
Values obtained for the correlations between the edaphic parameters and the taxa. The following combination of variables was considered: 1), every edaphic parameter with every taxon, considering abundance in both site types; 2), each edaphic parameter with each taxon, setting aside abundance of each site type. No positive correlations were obtained when estimating each edaphic parameter with the total number of taxa.
| Taxon | Edaphic parameter | Value | ||
| Without differentiation | R | df | p | |
| Bdellidae | P | 0.69 | 1.8 | 0.028 |
| Linotetranidae | Bd | 0.65 | 1.8 | 0.040 |
| Pe | 0.66 | 1.8 | 0.037 | |
| OM | 0.88 | 1.8 | 0.0006 | |
| N | 0.79 | 1.8 | 0.005 | |
| Mg | 0.64 | 1.8 | 0.042 | |
| Aphelacaridae | pH | 0.68 | 1.8 | 0.030 |
| Pseudoescorpionida | Temperature | 0.50 | 1.8 | 0.027 |
| Homoptera | OM | 0.82 | 1.8 | 0.003 |
| N | 0.77 | 1.8 | 0.009 | |
| Rough crusts | R | df | p | |
| Linotetranidae | OM | 0.97 | 1.3 | 0.009 |
| Paratydeidae | P | 0.95 | 1.3 | 0.013 |
| morphospecies 1 | ||||
| Pygmephoridae | OM | 0.97 | 1.3 | 0.007 |
| N | 0.89 | 1.3 | 0.038 | |
| Aphelacaridae | pH | 0.90 | 1.3 | 0.038 |
| Haplochthoniidae | OM | 0.96 | 1.3 | 0.008 |
| N | 0.90 | 1.3 | 0.033 | |
| Homoptera | OM | 0.92 | 1.3 | 0.022 |
| N | 0.93 | 1.3 | 0.020 | |
| Ca | 0.89 | 1.3 | 0.048 | |
| Without crusts | R | df | p | |
| Linotetranidae | Humidity | 0.70 | 1.8 | 0.021 |
| Paratydeidae | pH | 0.91 | 1.3 | 0.033 |
| morphospecies 1 | ||||
| Acaridae | Clay | 0.99 | 1.3 | 0.001 |
| Haplochthoniidae | K | 0.92 | 1.3 | 0.026 |
| Psocoptera | Na | 0.94 | 1.3 | 0.019 |
| K | 0.94 | 1.3 | 0.019 | |
| Diptera | EC | 0.88 | 1.3 | 0.047 |
| TDS | 0.88 | 1.3 | 0.049 | |
Organic matter was the parameter with the highest number of positive correlations with abundance recorded at the sites with rough crusts, in which Linotetranidae, Pygmephoridae, Haplochtoniidae, and Homoptera had a p<0.05. The second parameter with positive correlations was nitrogen content, with the same taxa and also at the sites with rough crusts (Table 4).
DiscussionDescription of the sampling sitesDuring September and October, the scrub is usually phenologically very active (León de la Luz et al., 1996; Maya and Arriaga, 1996). However, this was not the case during the sampling period. The lack of foliage, flowers, and fruits, as well as small quantities of litter was a consequence of a prolonged drought.
All the environmental factors may affect the abundance of microarthropods. Arriaga and Maya (2007) describe the importance of large plants as shade providers, a factor that favors fungal activity in the litter of J. cuneata and F. diguetti and modulate ground temperatures during the day. In summer, temperature could fluctuate on bare ground more than 22°C. At eroded sites, where the crusts were flat, there was not enough vegetation to provide moderate temperatures; here, microarthropods were not found. This type of crust has less diversity of cyanobacterial morphotypes, characteristics related with earlier successional stages. Furthermore, flat BSC have less organic matter and are found in disturbed areas (Belnap, 2001b).
The differences found between the site types “rough crusts” and “without crusts” regarding the soil properties may be related precisely to the presence or absence of BSC. Broadly speaking, the sites with a rough crust had more favorable soil conditions for microarthropod community development. The fungi hyphae and filamentous cyanobacteria, which are components of a rough crust, interweave the soil particles (Kieft, 1991; Maya et al., 2002); thus a higher nitrogen content derives from cyanobacteria that fix atmospheric nitrogen (Belnap, 2001a; Maya et al., 2002; Rivera Aguilar et al., 2004). Steinberger (1991) states that feces from microfauna may be a source of nitrogen in these crusts. Together, the lower bulk density results from greater pore space and higher N and OM content. Hence, sites with crusts have better soil structure. Although the soil crusts have higher levels of potassium and phosphorus (Rivera-Aguilar et al., 2004), significant differences in these elements were not found, nor in exchangeable cations. The absence of significant differences in these elements may be tied to the OM content as it serves as a substrate for retention of these cations (Knight, 1991).
Edaphic fauna descriptionAt a broad level, there is a similarity among microarthropods in arid lands of North America, Chile, Africa, and Australia (Noble et al., 1996). Our survey found 7 Acari families in common with the 10 reported by Elkins and Whitford (1984). Other taxa were recorded by Santos and Whitford (1983), Cepeda and Whitford (1990), Noble et al. (1996), Kay et al. (1999). Most families collected are associated with arid environments.
A general pattern in arid lands is the dominance of Prostigmata over Oribatida (Cepeda and Whitford, 1990; Noble et al., 1996; Neher et al., 2009). Oribatid mites are commonly referred to as moss mites or beetle mites because they ingest litter and fungi directly and are commonly more abundant in soils rich in organic matter. In soils with little organic matter, as in arid lands, oribatid mites are replaced by prostigmatid mites, which generally feed by drilling and sucking the cytoplasm of plants, fungi, bacteria, or microfaunal cells.
Among Oribatida, Neher et al. (2009) found 23 families; only 4 were found in our study area. This low diversity could be attributed to the scant litter in the scrub, which is the main source of food for the oribatida. A family that seems to be well adapted to the arid conditions is Aphelacaridae, specifically Aphelacarus sp., which was abundant or common in several reports (Wallwork et al., 1986; Cepeda and Whitford, 1990; Noble et al., 1996; Neher et al., 2009).
Another common feature in these micro-environments is the low diversity of Collembola, a group that is usually found in moister conditions (Palacios-Vargas, 1991; Hopkin, 1997); however, some families in this group are adapted to arid conditions. One of these are Isotomidae (Suhardjono and Greenslade, 1994). This was the only family found in the Chihuahuan Desert by Neher et al. (2009) and the colembolan family that had the most individuals, of the 3 families of colembolans found in our study.
Most of the mite families recorded in this study were microphytophages and microphytophages-saprophytes, similar to the findings of Neher et al. (2009). Mites with this preference were more abundant at sites with rough crusts. At sites without crusts, predator families were more abundant. This result suggests that the abundance of microarthropods is closely related to the resources provided in the rough crusts, either protective or feeding. Neher et al. (2009) state that species belonging to Aphelacaridae are strict micro-phytophages. This family was the most abundant in their study within the first 10cm of soil, where the biomass of fungi and algae was higher. In our study, A. acarinus and Linotetranidae, which are strict phytophages, were more abundant at the sites with rough crusts.
Among the miscellaneous group, all orders are important in the equilibrium of the soil ecosystem, some of them containing genera that are known to be specific for arid lands. For example, some species of Thysanura survive in habitats with low water content since they absorb atmospheric moisture (McGavin, 2002).
Correlation analysisIn arid ecosystems, where biological processes are mainly controlled by physical and chemical factors more than biological interactions, it is believed that there is less species diversity (Noy-Meir, 1985). Similarly, Schnürer et al. (1985) mentions that the biomass of microorganisms in the soil is proportional to the organic matter content; hence, the mineral soils of arid regions generally support fewer microorganisms. This explains the low microarthropod abundance in the study area in comparison to other environments. Despite the low absolute densities of edaphic microarthropods in arid lands, desert soils can exhibit microarthropod communities that are relatively rich in species, that are, surprisingly well adapted to the stringency of the climate (Wallwork et al., 1984, 1986; Cepeda-Pizzaro and Whitford, 1989). The short length of the body of most of the specimens we captured and their particular shape suggest that some of them are ecomorphic, a phenomenon that implies changes in the morphological and anatomical features adapted to environmental conditions (Palacios-Vargas, 1980). Folsomides californicus (Palacios-Vargas and Villarreal-Rosas, 2013), the new species of collembolan found in this area, can dehydrate and enter an inactive phase called cryptobiosis. This adaptation is not common in terrestrial invertebrates; however, it has been well described in Folsomides angularis (Belgnaoui and Barra, 1989).
Even if there are no positive correlations between edaphic properties and the taxa, Jenny (1980) states that biological activity depends on soil characteristics that positively influence water retention. The latter seems to occur at the sites with rough crusts, where total abundances are higher than at the sites without crusts. Some correlations suggest that the dynamics between edaphic parameters and abundances of taxa are different in each site type, even more so, since there were significant differences between each site type in 3 parameters: nitrogen content, density, and pore space. Indeed, the positive correlations were distinctly different between the 2 types of sites.
The relationship between the quantity of litter and organic matter for abundance and diversity of mites has been established by other authors, mainly in the Chihuahuan Desert (Santos and Whitford, 1983; Kamil et al., 1985; Steinberger and Whitford, 1985; Cepeda and Whitford, 1989; Noble et al., 1996). This relationship is supported in our study by the majority of positive correlations between litter abundance at the sites with rough crusts with the organic matter and nitrogen content. The importance of litter, together with other factors, is confirmed by the absence of microarthropods at eroded sites.
To determine if abiotic factors are influencing the structure of the microarthropod communities or if the feeding habits of the taxa are more important, a long term study covering several years on a monthly basis is recommended. In this way, the trigger effect of rainfall on soil biota could also be assessed.
We thank C. Silva Bejarano for field assistance, M. Trasviña Castro for soil laboratory assistance, M. L. Jiménez for allowing the use of laboratory facilities, and I. Fogel for essential editorial services; all are at CIBNOR. Blanca Mejía (UNAM) helped mounting techniques for microarthropods. Part of this project was funded by the Conacyt grant CB-2007-80431-R.