Research on benthic diatom floristics from Mexican littorals is scarce. For the coasts of Nayarit, within the NW region, no formal floristic studies were hitherto available. However, during identification of diatoms found in the gut contents of Crassostrea corteziensis from Nayarit wetlands, 9 benthic diatom taxa that constitute new species records for the Mexican littorals were observed and they are here described and depicted.
Los estudios florísticos de diatomeas bentónicas en los litorales mexicanos son escasos. Para la costa de Nayarit, dentro de la región noroeste, no existían estudios florísticos formales; sin embargo, durante la identificación de diatomeas encontradas en el contenido intestinal de Crassostrea corteziensis en un humedal de Nayarit, fueron observados y descritos 9 taxones de diatomeas bentónicas que constituyen nuevos registros para México.
Most of the benthic marine diatom species from Mexican littorals have been recorded for the northwestern region (Siqueiros-Beltrones, Argumedo-Hernández, & López-Fuerte, 2017), particularly for both coasts of the Baja California peninsula. According to the later authors, the scarce investigation on benthic diatom floristics in Mexican littorals favors the expectations that further surveys for most regions would yield new records of benthic diatom species. For the coasts of Nayarit, located within the NW region, no formal floristic studies on benthic diatoms were hitherto available. However, focusing on the diet of the Cortez oyster Crassostrea corteziensis (Hertlein, 1951), an economically important mollusk in Nayarit, the need to formally identify the diatom species found in the gut contents of specimens of this species was evidenced. In the process, 9 benthic diatom species that constitute new records for the Mexican littorals were observed and are here described and represented iconographically.
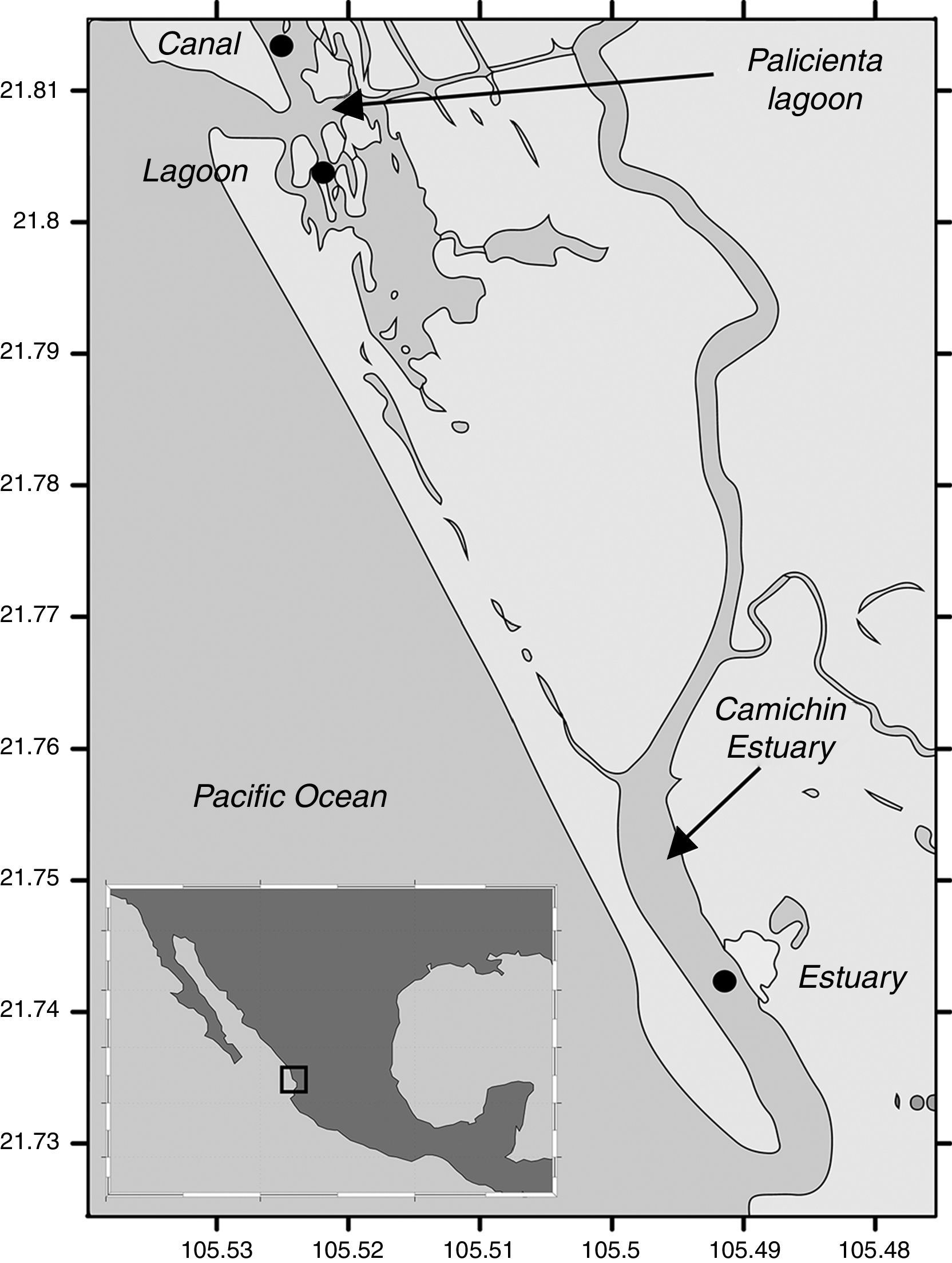
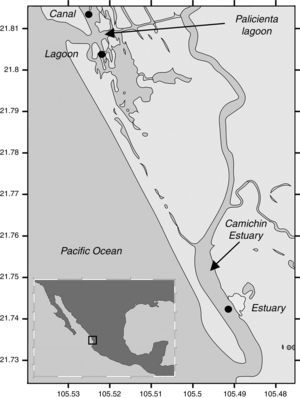
We sampled 3 sites: estuary, canal and lagoon, located within the Biosphere Reserve Marismas Nacionales (Fig. 1), where 130 specimens of C. corteziensis were collected from 3 substrata: culture lines, mangrove roots, and sediments; samples were extracted in November 2013, March and June 2014. The digestive system of all the specimens were extracted and oxidized using Siqueiros-Beltrones and Voltolina (2000) technique to obtain cleaned diatoms. Diatoms were identified under a Carl Zeiss GmBh® Axiolab.A1 phase contrast microscope at 1000× (with digital camera) following Cleve-Euler (1953), Desikachary (1989), Foged (1975, 1978), Krammer and Lange-Bertalot (1997), Park et al. (2012), Ricard (1987) and Witkowski, Lange-Bertalot, and Metzeltin (2000).
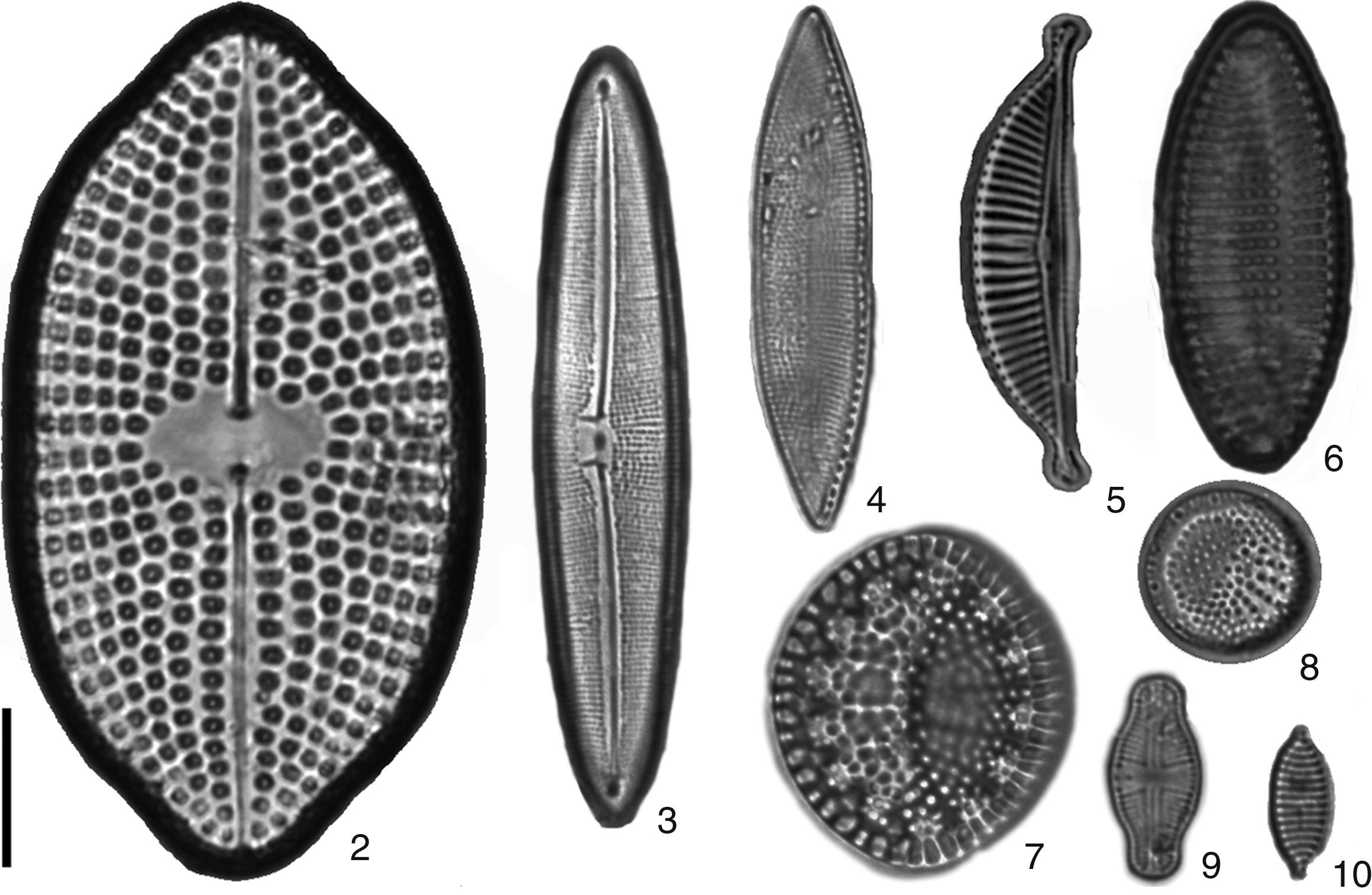
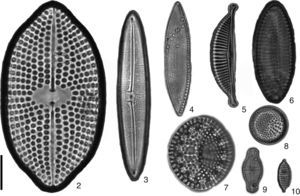
Achnanthes bergiiCleve-Euler, 1953 (Fig. 9).
New records of benthic diatom from Nayarit litoral. 2) Petroneis arabica, 3) Frustulia weinholdii, 4) Nitzschia ligowskii, 5) Amphora ayensuensis, 6) Achnantes separata, 7) Tryblioptychus cocconeiformis, 8) Cymatotheca minima, 9) Achnanthes bergii, 10) Nitzschia vexans. Scale bar 10μm.
Dimensions: L=12.6μm, W=5.8μm; 26–28striae/10μm. Reference: Cleve-Euler (1953, p. 36, fig. 545a). Occurrence: rare, June. Other distribution: Finnland, Stockholm (Europe). Habitat: freshwater.
Achnanthes separata Hustedt, 1958 (Fig. 6).
Dimensions: L=27.6μm, W at center=10μm, at apices=5μm; 10striae/10μm. Reference: Witkowski et al. (2000, pl. 43, p. 528, fig. 9). Occurrence: rare, June. Other distribution: shatt-al-Arab Delta (Asia). Habitat: freshwater.
Amphora ayensuensis Foged, 1966 (Fig. 5).
Dimensions: L=29.4μm, W=6μm; 13striae/10μm. Reference: Foged (1978, pl. 36, p. 218, fig. 6). Occurrence: rare, June. Other distribution: Australia, New Zealand, Africa. Habitat: freshwater.
Cymatotheca minima Voigt, 1960 (Fig. 8).
Dimensions: L=10.2μm, W=10μm; 8striae/10μm; 10areolae/10μm. Reference: Ricard (1987, p. 162, fig. 151). Occurrence: abundant, November, March, June. Other distribution: China, Taiwan (Asia). Habitat: marine.
Frustulia weinholdii Husted, 1937 (Fig. 3).
Dimensions: L=48μm, W=9.6μm; 29striae/10μm. Reference: Krammer and Lange-Bertalot (1997, p. 634, fig. 12–14). Occurrence: rare, June. Other distribution: Europe, Japan, South Africa, North America, South America, Australia and New Zealand. Habitat: freshwater.
Nitzschia ligowskii Lange-Bertalot, Kociolek et Brzezinska in Witkowski et al. (2004) (Fig. 4).
Dimensions: L=32.7μm, W at center=7.1μm, at apex=4.2μm; 14fibulae/10μm, 25striae/10μm. Reference: Park et al. (2012, p. 120, fig. 7T). Occurrence: abundant, March. Other distribution: Europe, Canada (Arctic), Atlantic Islands, North America, South Africa, Galápagos Islands, Antarctic, subantarctic islands, Ariake Sea, Japan (Asia). Habitat: marine.
Nitzschia vexans Grunow in Van Heurck, 1881 (Fig. 10).
Dimensions: L=9μm, W=3.5μm; 16striae/10μm. Reference: Witkowski et al. (2000, pl. 184, p. 810, fig. 5–8). Occurrence: rare, November. Other distribution: Netherlands (Europe); Great Lakes, United States of America; Australia and New Zealand. Habitat: freshwater.
Petroneis arabica (Grunow) D. G. Mann, 1990 (Fig. 2).
Dimensions: L=63.5–68μm, W=34.4–35μm; 6areolae/10μm. Reference: Foged (1975, pl. 21, p. 106, fig. 6). Occurrence: rare, March, June. Other distribution: West Indies (North Atlantic Ocean); Florida; Port Jackson, Uruguay; China (Asia). Habitat: marine.
Tryblioptychus cocconeiformis (Grunow) Hendey, 1958 (Fig. 7).
Dimensions: L=21μm, W=18μm; 4striae/1 fascicle. Reference: Desikachary (1989, pl. 809, fig. 1–8). Occurrence: rare, November, March, June. Other distribution: Ariake Sea, Japan, China; South-east Asia: Singapore; Argentina, Brazil; Atlantic Islands. Habitat: marine.
In this report, we add 9 new diatoms species to the Mexican Benthic Diatom Species Checklist by López-Fuerte and Siqueiros-Beltrones (2016). Also, this confirms the Siqueiros-Beltrones et al. (2017) statement that further surveys would yield new species records for the Mexican littorals. The influence of freshwater in the area is evidenced with the presence of 5 taxa from freshwater habitats. Certainly, the convergence of marine and continental waters is expected to influence diatom species diversity. Much floristic work is needed if ecological and biogeographical models of distribution are to be constructed; the above is particularly true for countries like México, where biodiversity related studies are still required for certain taxonomic groups such as benthic diatoms either fresh-water or marine (Novelo & Tavera, 2011).
We acknowledge Conacyt for the support of the Retention project 203630, and the scholarship granted to the first author (CVU 623974). The Laboratory of Environmental Pollution and Toxicology of the Nayarit State University and to CICIMAR-IPN, for providing the facilities and equipment necessary for the development of this study. To the anonymous reviewers whose comments helped to improve this work. We are grateful to all people who participated in the collection of the material. DASB is COFAA and EDI fellow of the IPN.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.