Crepis japonica (L.) Benth., Porophyllum ruderale (Jacq.) Cass. and Tridax procumbens L. are weedy species that grow in cultivated fields, roadsides, abandoned fields and open, disturbed spaces in Maringa, Parana state, Brazil. The ontogeny of the fruits and seeds of the 3 Asteraceae species was carried out. The flowers and developing fruits were prepared according to resin inclusion techniques for histochemical tests and scanning electron microscopy. During maturation of the pericarp, processes such as trichome differentiation, tissue sclerification, phytomelanin deposition and breakdown of tissues can be observed. The fruit of C. japonica is entirely lacking in phytomelanin. The seed is only exotestal in C. japonica. Comparative analysis of the pappus and cypsela characters demonstrated that they are efficient in separating species and tribes. The seed coat and embryo may also be useful in the characterization of tribes.
Crepis japonica (L.) Benth., Porophyllum ruderale (Jacq.) Cass. y Tridax procumbens L. son especies dañinas que crecen en los campos cultivados, en las orillas del camino, campos abandonados y áreas perturbadas en Maringá, Paraná, Brasil. Se analizó la ontogenia de los frutos y semillas de las 3 especies de Asteraceae. Las flores y los frutos en desarrollo fueron procesados según las técnicas de inclusiones de la resina, de pruebas histoquímicos y de microscopía electrónica. Durante la maduración del pericarpio puede observarse a menudo procesos como la diferenciación de tricomas, esclerosis de tejidos, deposición de fitomelano y descomposición de tejidos. El fruto de C. japonica es completamente desprovisto de fitomelano. La semilla es sólo exotestal en C. japonica. El análisis comparativo de los caracteres del papus y de la cipsela demostró que son eficaces en la separación de las especies y tribus. La cubierta de la semilla y embrión también pueden ser útiles en la caracterización de tribus.
The fruits of Asteraceae are very distinct from fruits of other families but present great morphological similarity among species. However, the names given to the fruits within the family are variable and include, apparently indiscriminately, the terms achene and cypsela (Marzinek et al., 2008). The cypsela has been regarded as the name for a fruit of Asteraceae which differed from the achene by an additional layer (perianth) over the pericarp due to the inferior position of the ovary; however, many botanists have ignored this distinction, continuing to use the term achene (Spjut, 1994). Barroso et al. (1999) and Judd et al. (2002) have adopted the term achene for Asteraceae. Marzinek et al. (2008) adopt the term cypsela, and Spjut (1994) employed achene and cypsela for Asteraceae species.
Morphological features that are taxonomically important at the tribal level in Asteraceae include many floral characters but they also include characteristics such as pappus form, and anatomical and morphological features of the achenes (Spjut, 1994). Studies of the Asteraceae fruits were performed by Pandey and Singh (1980), Pandey et al. (1983), Bruhl and Quinn (1990), Puttock (1994), Martins and Oliveira (2007), Herman (2008), Zarembo and Boyko (2008), Julio and Oliveira (2009), Galastri and Oliveira (2010), Marzinek and Oliveira (2010).
The species selected for study were Crepis japonica (L.) Benth., tribe Lactuceae, Porophyllum ruderale (Jacq.) Cass., tribe Helenieae, and Tridax procumbens L., tribe Heliantheae, which all grow in cultivated fields, roadsides, abandoned fields and open, and disturbed spaces. In this work, as part of a study of the importance of reproductive organs in the biology of weeds, the ontogeny of the fruits and seeds of the 3 Asteraceae species was studied. This information will contribute to fruit terminology and characterization of the species and the respective tribes.
Materials and methodsFlowers, buds, developing fruits and seeds of the Asteraceae species were collected in the city of Maringá, in the state of Paraná, Brazil, at the following coordinates: C. japonica, altitude 506m, 23o24’13.3” latitude and 51º56’21.17’’ longitude; P. ruderale, altitude 519m, 23o24’13.9” latitude and 51o56’20.7” longitude; T. procumbens, altitude 523m, 23o24’16.5” latitude and 51o56’22.2” longitude. Voucher materials were deposited at UEM Herbarium with the following collection numbers: C. japonica (20833HUEM); P. ruderale (20831HUEM) and T. procumbens (20829HUEM).
Anatomical studies were performed on material which had been fixed in glutaraldehyde (1% in 0.1M phosphate buffer, pH 7.2) (Karnovsky, 1965) and then conserved in 70% ethanol. The botanical material was dehydrated through an alcohol series, embedded in hydroxymethacrylate (Gerrits, 1991), sectioned via rotary microtome (cross- and longitudinal sections), and stained with toluidine blue 0.05% in phosphate buffer pH 4.7 (O’Brien et al., 1964).
Specific microchemical tests were carried out for lipid substances (using Sudan IV dye) (Rawlins and Takahashi, 1952), starch (iodine-potassium iodide test), lignin (phloroglucinol test) (Berlyn and Miksche, 1976), and calcium crystals (sulphuric acid) (Sass, 1951).
Photomicrographs were prepared using a Leica EZ4D stereoscope and an Olympus BX50 optical microscope with a digital camera. All samples were prepared on the same micrometric scale.
Micromorphological analysis of the flowers, fruits and seeds was undertaken on material fixed in Karnovsky solution (Karnovsky, 1965). Samples were processed and then mounted on aluminum stubs, gold coated, and subsequently examined using scanning electron microscopy (Shimadzu SS-550 Superscan), and then digital images were then taken.
ResultsDeveloping fruit (pericarp)Fruits with persistent calyx (pappus) originate from an inferior ovary composed of 2 carpels and 1 loculus. The flower pappus is bristly and plumose, with each element (Figs. 1, 4, 7) consisting of a uniseriate epidermis with thin-walled elongate cells and unicellular trichomes; the mesophyll is parenchymatous (Figs. 1, 4, 7) consisting of a reduced number of cells in C. japonica. In the developing fruit, the mesophyll cells of the pappus undergo wall thickening (Figs. 2, 5, 8), but this process is less intense in C. japonica. Pappus trichomes have tapered ends; trichomes of C. japonica and P. ruderale (Figs. 3, 6) are shorter than those of T. procumbens (Fig. 9). The vascular system of the pappus is reduced in P. ruderale and T. procumbens (Figs. 5, 8) and absent in C. japonica (Fig. 2).
Pappus structure of Crepis japonica (1-3), Porophyllum ruderale (4-6) and Tridax procumbens (7-9). Flower pappus in cross section (1, 4, 7); fruit pappus in cross section (arrow indicates the vascularization) (2, 5, 8); pappus under scanning electron microscopy (SEM) (3, 6, 9). Scale bars=20µm (1, 2, 4, 5, 7); 50µm (3, 6, 8), 500µm (9).
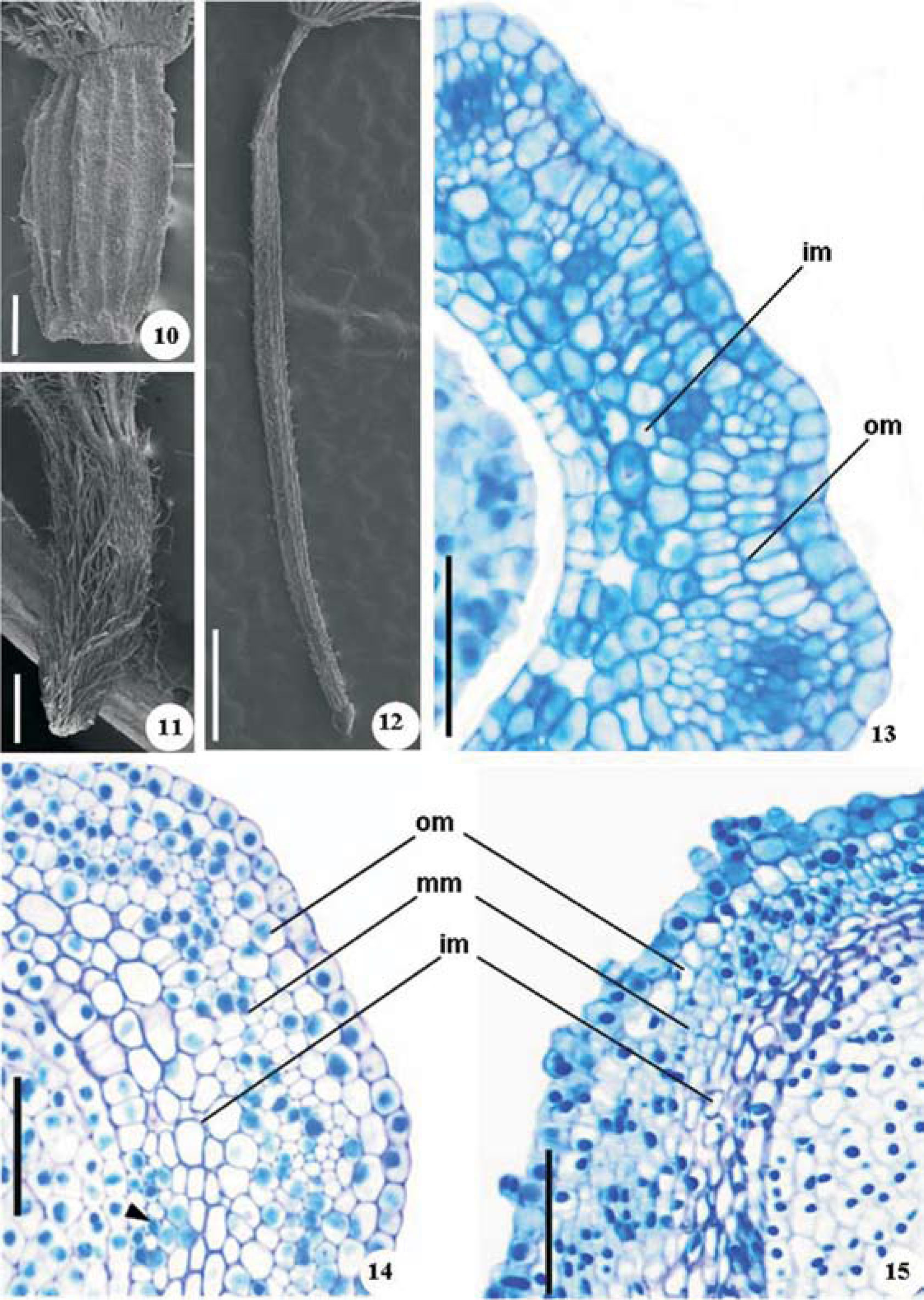
The ovary is cylindrical in C. japonica and P. ruderale (Figs. 10, 12) and is obovate and smaller in T. procumbens (Fig. 11). The outer epidermis is uniseriate (Figs. 13-15) and only the ovary of T. procumbens (Figs. 11, 15) is covered with trichomes at the time of blossoming; the other species present trichomes at the initial stages of differentiation. The inner epidermis (Figs. 13-15) is uniseriate and glabrous consisting of elongate cells. Cells of transmitting tissue occur in the inner epidermis and subepidermal mesophyll (Fig. 14), which are readily distinguished from those of the neighboring layers by reduced size and relatively dense cytoplasm. The ovary mesophyll is parenchymatous in the 3 species (Figs. 13-15), but differs in structural details. The mesophyll of C. japonica is composed of 2 regions (Fig. 13): the outermost layers (1 or 2 layers) present thin-walled cells which differ in form, and collateral vascular bundles which are surrounded by fiber primordia; the inner region is 2 to 3 cell layers thick, with intercellular spaces and elongated cells. The mesophyll of P. ruderale and T. procumbens present 3 regions (Figs. 14, 15). The outermost mesophyll of P. ruderale has 2 cell layers of parenchyma devoid of intercellular spaces, in which the subepidermal layer consists of radially elongated cells and the other layer is composed of longitudinally elongated cells; T. procumbens has just 1 column-like cell layer or elongated cells. The middle mesophyll has the same general structure in the 2 species, with tangentially elongated cells and vascular bundles. The inner mesophyll of both species has practically the same structure, in which the parenchyma is spongy.
Ovary structure of Crepis japonica (10, 13), Porophyllum ruderale (12, 14) and Tridax procumbens (11, 15). Ovary under scanning electron microscopy (SEM) (10-12); ovary in cross section showing the inner (im) and outer (om) mesophyll (13); ovary in cross section evidencing the outer, middle (mm) and inner regions (head arrow indicates transmitting tissue) (14, 15). Scale bars=50µm (13-15), 100µm (10), 500µm (12), 1mm (11).
During maturation of the pericarp, processes such as trichome differentiation, tissue sclerification, phytomelanin deposition and breakdown of tissues can be observed. Papillae (Figs. 16, 20) and trichomes differentiate in the exocarp of C. japonica fruit. The formation of twin hairs is restricted to the exocarp of P. ruderale and T. procumbens (Figs. 17, 18). They consist of a few cells, 2 of which are elongated, of different sizes, thick-walled and individualized pointed ends, while the basal cells are short and thin-walled (Fig. 19).
Immature fruits of Crepis japonica (16, 20), Porophyllum ruderale (17, 19, 21) and Tridax procumbens (18, 22). Trichomes under scanning electron microscopy (SEM) (16-18); twin hair in longitudinal section (19); pericarp in cross section showing the mesocarp and phytomelan (head arrow) (20-22). Scale bars=20µm (21), 50µm (16, 17, 19, 20, 22), 100µm (18).
Sclerization affects the outer mesocarp of C. japonica (Fig. 20), the middle mesocarp of P. ruderale and T. procumbens (Figs. 21, 22), and less intensely the inner mesocarp of T. procumbens. The fruit of C. japonica is entirely lacking in phytomelanin (Fig. 20). In the other 2 species the phytomelanin occurs in the space between the outer mesocarp and the middle mesocarp (Figs. 21, 22). The collapse of cells occurs mainly in the inner tissues of the pericarp of the 3 species.
The mature fruit (Figs. 23-25) is composed of epidermal exocarp with papillae (Fig. 27) and trichomes (Figs. 26, 29, 30) and is more or less collapsed in P. ruderale and T. procumbens (Figs. 28-30). The mesocarp of C. japonica (Figs. 26, 27) consists of 2 regions, the outer mesocarp composed of sclerified parenchyma and fibers, through which the vascular bundles run, and the inner mesocarp composed of parenchyma more or less collapsed. Three tissue regions occur in the mesocarp of the other 2 species of Asteraceae: outer mesocarp with phytomelanin, which consists of collapsed parenchyma in P. ruderale (Fig. 28) and thick-walled columnar cells in T. procumbens (Figs. 29, 30); lignified fibrous middle mesocarp in both species (Figs. 28, 29); and inner mesocarp composed of parenchyma slightly crushed in the 2 species (Figs. 28, 29). The endocarp is also crushed (Figs. 27-29).
Mature fruits of Crepis japonica (23, 26, 27), Porophyllum ruderale (24, 28) and Tridax procumbens (25, 29, 30). Fruits under scanning electron microscopy (SEM) (detail: twin hair) (23-25); pericarp in longitudinal section evidencing outer (om) and inner (im) mesocarp (head arrow indicates intercellular space with phytomelan) (26, 30); pericarp in cross-section showing middle mesocarp (mm) (head arrow and asterisk indicate exotesta and endosperm, respectively) (27-29). Scale bars=50µm (26-30), 500µm (23, 25), 1mm (24).
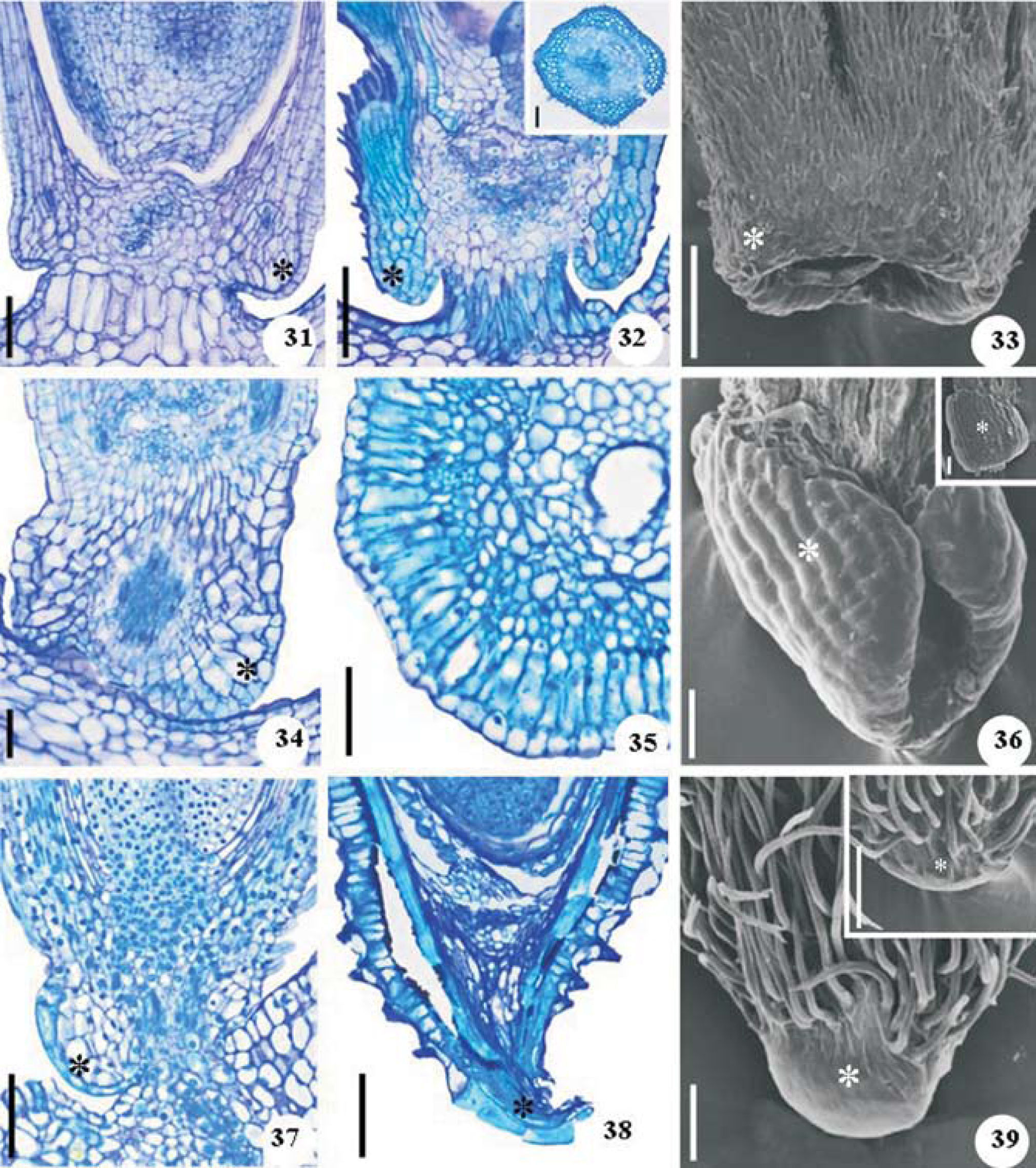
The carpopodium is symmetrical in C. japonica (Fig. 33) and asymmetrical in P. ruderale and T. procumbens (Figs. 36, 39). The ovary carpopodium consists of uniseriate epidermis and parenchyma (Figs. 31, 34, 37). The mature carpopodium of C. japonica (Fig. 32) is characterized by hairy epidermis and sclerified tissue with reticulate thickenings on the cell walls. The carpopodium of P ruderale (Fig. 35) has uniseriate epidermis, hypoderm layer of macrosclereids and parenchyma interspersed with sclereid groups. On the other hand, the carpopodium of T. procumbens (Fig. 38) consists of epidermal cells elongated in the tangential direction and collapsed tissue.
Carpopodium (*) of Crepis japonica (31-33), Porophyllum ruderale (34-36) and Tridax procumbens (37-39). Flower carpopodium in longitudinal section (31, 34, 37); fruit carpopodium in longitudinal section and (35 and detail) in cross section (32, 38); carpopodium under scanning electron microscopy (SEM) (details: opposite face of the carpopodium) (33, 36, 39). Scale bars=50µm (31, 34-36), 100µm (32, 33, 37-39); details 50µm (32, 36), 100µm (39).
In the apical plate of the flower, the region that supports the pappus, is composed of uniseriate epidermis and parenchyma (Figs. 40-42). The subepidermal tissue is strikingly different in the mature fruit, consisting of sclerified and lignified cells in C. japonica (Fig. 43), subepidermal macrosclereids and thin-walled cells in P. ruderale (Fig. 44) and tight thick-walled cells in T. procumbens (Fig. 45).
Developing seedSeeds originated from anatropous, unitegmic and tenuinucellate ovules (Figs. 46-50). The ovules have short funicle in C. japonica (Figs. 46, 49) and they are sessile in the other 2 species (Fig. 50). The integument (Figs. 46-48) consists of uniseriate glabrous outer epidermis with cells that vary in shape (cuboid to tabular), multiseriate parenchymatous mesophyll, and inner epidermis, which is uniseriate or biseriate with radially elongated cells. Hypostase is well developed in the 3 species consisting of thick-walled cells (Figs. 46-48). The calazal region of P. ruderale and T. procumbens (Figs. 47, 48) is longer than in C. japonica (Fig. 46). The vascular supply (Fig. 46) consists of a single vascular strand extending around the seed from the funicle more or less to the micropyle.
Ovules of Crepis japonica (46, 49), Porophyllum ruderale (47) and Tridax procumbens (48, 50). Longitudinal section showing outer epidermis (oe), mesophyll (me), inner epidermis (ie) and vascular bundle (vb) (46-48); ovules under scanning electron microscopy (SEM) (49, 50). Scale bars=50µm (46), 100µm (47, 49), 150µm (48), 200µm (50).
The differentiation of the seed (Figs. 51-53) shows notable longitudinal growth in the species P. ruderale and T. procumbens. The seed coat arises by sclerization of the exotesta in C. japonica, development of spiraled wall thickenings in P. ruderale, cellular collapse in the exotesta of P. ruderale and T. procumbens, cellular dissolution of the mesotesta and endotesta cells in the 3 species, and collapse of the middle tissue of the chalaza in P. ruderale and T. procumbens.
The mature seed of C. japonica is exotestal with exotesta cells presenting U-shaped thickening (Figs. 54, 55). The seed coat of the other 2 species (Figs. 56-59) is unspecialized; the exotesta is composed of thin-walled cells, which eventually collapse. Exotesta cells of P. ruderale with spiral thickening are limited to the hilum region (Fig. 57). Mesotesta have thin-walled cells, crushed cells in P. ruderale and T. procumbens (Figs. 56-59). Inner epidermis develops as an endothelium in the 3 species, collapsing later.
The endosperm consists of a thin-walled cell layer in C. japonica and thick-walled cells in the other species (Figs. 56, 58). The embryo is straight with short hypocotylradicle axis in C. japonica (Fig. 60) and elongated in the other 2 species (Fig. 65). The cotyledons differ in size only in T. procumbens (Figs. 65, 66) and present homogeneous mesophyll in C. japonica (Figs. 60, 61) and dorsiventral mesophyll in P. ruderale (Fig. 63) and T. procumbens (Figs. 66, 67). The plumule consists of a conical protuberance in the 3 species (Figs. 62, 64, 67).
Embryo of Crepis japonica (60-62), Porophyllum ruderale (63, 64) and Tridax procumbens (65-67). Embryo in longitudinal section (60, 61, 66); embryo in cross section (63); detail of the plumule in longitudinal section (62, 64, 67); embryo under scanning electron microscopy (SEM) (head arrow indicates endosperm) (65). Scale bars=50µm (61-64, 67), 100µm (60,66), 500µm (65).
The term cypsela adopted here for the fruits of C. japonica, P. ruderale and T. procumbens, was based on the terminology used by Marzinek et al. (2008), who define cypsela as a complex fruit, dry, indehiscent, unilocular, with a single seed not adnate to the pericarp (linked only by the funicle) and originating from an inferior ovary; the achene is similar to the cypsela but originating from a superior ovary, as in Plumbaginaceae. It is important to emphasize that many botanists have ignored this distinction and continue to use the term achene (Spjut, 1994).
Concerning their inner structure, the pappus members may be considered reduced, less differentiated forms of foliage leaves, with undifferentiated or sclerenchymatous mesophyll and reduced vascular system (Roth, 1977). A pappus study of 6 species of Asteraceae showed that each bristle is composed of a multiseriate group of cells with vascular system, except the pappus of Mikania micrantha H. B. K., in which the vascularization is entirely lacking (Marzinek and Oliveira, 2010). Regarding the 3 studied species, C. japonica is the most reduced with pappus devoid of vascularization and mesophyll usually with 3 cells; P. ruderale occupies an intermediate position with vascularized pappus, and T. procumbens is formed by more developed bristles, whose elements consist of numerous sclerified cells and reduced vascular system.
The trichomes may have taxonomic value, although the so-called twin hairs, which are very characteristic of the pericarp of many Compositae (Asteraceae) (Roth, 1977), are of no value, as very distinct types occur within the same subfamily and even within the same genus (Hess, 1938). On the other hand, Marzinek and Oliveira (2010) reveal that the trichomes found in species of Eupatorieae have taxonomic significance. In the studied Asteraceae, the cypsela trichomes are different among the species, being unicellular pointed in C. japonica; in the other 2 species the trichomes are twin hairs, although they are quite elongated in T. procumbens.
The general microscopic structure of the Asteraceae ovary consists of strikingly different tissues, while the inner ovary tissue, formed by spongy parenchyma, is relatively similar among the species. The ovary of other Asteraceae species may be 3 (Julio and Oliveira, 2009) or 2 tissue regions (Galastri and Oliveira, 2010; Marzinek and Oliveira, 2010). The mesophyll structure of the investigated species is the same as in the other species, with 2 tissues in C. japonica and 3 in P. ruderale and T. procumbens. In these 3 species the transmitting tissue runs near the surface of the ovary wall and pericarp, but there is no mention in the other species except Vernonia anthelmintica (L.) Willd. (Misra, 1972).
A special subepidermal layer of cells (hypodermis), which are distinguished from the underlying cells by their shape and function, occur in Compositae (Asteraceae) fruits; the capacity of the hypoderm to absorb water and to spread it homogeneously over the entire pericarp periphery is of great importance for the softening and soaking of the fruit and the water supply of the embryo (Roth, 1977). Only the species C. japonica and T. procumbens have a subepidermal layer (outer mesocarp), more sclerified in the first species, which may be considered as a hypodermis, though its function in water absorption has yet to be proven.
A dark brown or black pigment layer (phytomelan layer) is characteristic of many achenes (cypselas), which may serve as a protective screen against excessive insolation or as a protection of the pericarp (Roth, 1977). According to Roth, the phytomelan layer varies within the outer pericarp: it may be in the outer epidermis, in the subepidermal layer beneath the outer epidermis, or on the outer or inner surface of the sclerenchymatous tissue. Julio and Oliveira (2009) and Marzinek and Oliveira (2010) report the phytomelanin deposition in the spaces between the outer and the middle mesocarp or outer and the inner mesocarp, respectively. Although the phytomelanin layer is entirely lacking in C. japonica, the phytomelanin deposition in P. ruderale and T. procumbens is similar to that in fruits found by Julio and Oliveira (2009), occurring between the outer and the middle mesocarp; however, in both species the phytomelanin is in the inner surface of the sclerenchymatous tissue. The occurrence of phytomelanin may be considered as a synapomorphy of Asteraceae, including more than 5 000 species in the Phytomelanin Cypsela Clade (Panero, 2007).
The basal callus or carpopodium is often taxonomically useful in Asteraceae (Robinson, 1981). There are structural differences in the carpopodium of the studied species: it is symmetrical in C. japonica and asymmetrical in the other 2 species, it is more elaborated structurally in P. ruderale, and the carpopodium of P. procumbens consists mostly of obliterated cells.
Endothelium is present in the developing seeds of the 3 species of Asteraceae and is completely absent in the mature seeds. The endothelium is apparently involved with several processes (Werker, 1997): Misra (1964) found that the endothelium of Flaveria repanda Lag. (Asteraceae) loses its identity in advanced stages of seed development and exists in the mature seed as a cuticle closely adhering to the persistent layer of endosperm.
The seed coat of P. ruderale and T. procumbens has not completely deteriorated in the cypsela, but it consists mostly of obliterated cells in the mature seed, with spiral thickening in the developing seed of P. ruderale. On the other hand, the C. japonica seed is exotestal composed of thick-walled cells (U-shaped thickening). It is probable that in this last species, without phytomelan layer, the seed coat furnishes additional protection to the embryo. The vascular supply of the 3 species has practically the same structure of Corner (1976), with a single bundle extending to the micropyle.
The single layer of cellular endosperm persists in the mature seed of the 3 studied species but consists of thin-walled cells in C. japonica and thick-walled cells in the other 2 species. It is likely that the persistent endosperm protects the embryo, mainly in the seeds of P. ruderale and T. procumbens, which do not have a specialized seed coat. Seeds of Bignoniaceae originating from the unitegmic and tenuinucellate ovules also have an endosperm layer with a protective function (Souza and Paoli, 2009).
The embryos of 3 species of Asteraceae may be recognized as spatulate type (as described by Martin, 1946), which is characterized as erect embryo, with variable cotyledons, thin to thick and slightly expanded to broad.
Morphological features that are taxonomically important at the tribal level (Judd et al., 2002) include the pappus and cypsela structure. Comparative analysis of the pappus and cypsela characters of C. japonica, P. ruderale and T. procumbens demonstrated that they are efficient in separating species and tribes. Other characters, such as those regarding seed coat and embryo, may also be useful in the characterization of tribes, once extended to other species of Asteraceae. Fruit and seed features that are potentially significant in species characterization are summarized in Table 1.
Significant features in the characterization of the fruit and seed in development of Crepis japonica, Porophyllum ruderale and Tridax procumbens
| Characters | C. japonica | P. ruderale | T. procumbens |
| Pappus | Bristly with short trichomes | Bristly with short trichomes | Bristly and plumose with long trichomes |
| Pappus structure | Vascularization wanting and little sclerified | Reduced vascularization and sclerified | Reduced vascularization and very sclerified |
| Ovary shape | Cylindrical | Cylindrical | Obovate |
| Ovary mesophyll | Parenchymatous with 2 regions | Parenchymatous with 3 regions | Parenchymatous with 3 regions |
| Cypsela indumentum | Papillae and unicellular short non-glandular trichomes with pointed ends | Short twin hairs | Long twin hairs |
| Sclerization process in the pericarp | Outer mesocarp | Middle mesocarp | Inner and middle mesocarp |
| Phytomelan deposit | Wanting | Among the outer and middle mesocarps | Among the outer and middle mesocarps |
| Collapse of tissues in the mature pericarp | Endocarp | Exocarp, outer and inner mesocarp, endocarp | Exocarp and inner mesocarp |
| Carpopodium | Symmetrical with hairy epidermis | Asymmetrical and glabrous | Asymmetrical and glabrous |
| Carpopodium tissue | Sclerified tissue with reticulate thickenings | Hypodermis of macrosclereids and parenchyma | Collapsed tissue |
| Apical plate/subepidermical tissue | Sclerefied | Macrosclereids and parenchyma | Tight with thick-walled cells |
| Funicle | Short | Wanting | Wanting |
| Seed-coat | Exotesta with U-shaped thickening | Unspecialized, eventually collapsed | Unspecialized, eventually collapsed |
| Hypocotyl-radicle axis | Short | Long | Long |
| Embryo cotyedons | Same size and homogeneous mesophyll | Same size and dorsiventral mesophyll | Different size and dorsiventral mesophyll |