The coyote (Canis latrans) is one of the most widely distributed and opportunistic carnivores in North America. It feeds on a variety of different species, ranging from small- (rodents) to medium-sized mammals (Lagomorpha), reptiles, and birds. Among sea turtles, the main species nesting on the coasts of Baja California is Lepidochelys olivacea. Solitary turtles arrive to beaches in low numbers. The aim of this study was to assess the effect of coyote predation on sea turtle nests on pristine beaches of Baja California Sur, Mexico. Of a total of 43 nests observed, 34 (79.1%) were considered as recent, and 9 (20.9%) as old nests; of these, 35 (81.4%) and 8 (18.6%) showed evidence of digging/not digging by predators, respectively. Eggshells were observed around and inside all preyed upon nest holes. Coyotes should be considered an important predator of turtle nests in the Baja California Peninsula.
El coyote (Canis latrans), es uno de los carnívoros más ampliamente distribuidos y oportunistas en América del Norte. En su dieta incluye diferentes especies, que van desde pequeños (roedores) a mamíferos medianos (Lagomorpha), reptiles y aves. Entre las tortugas marinas, la principal especie que anida en las costas de Baja California es la tortuga golfina. Son solitarias para anidar y llegan a las playas en un reducido número. El objetivo de este estudio fue evaluar el efecto de la depredación en nidos de tortugas marinas en las playas prístinas de Baja California Sur, México. De un total de 43 nidos localizados, 34 (79.1%) se consideraron como recientes y 9 (20.9%) como nidos antiguos; 8 (18.6%) se encontraron sin ninguna actividad de excavación por depredadores; 35 (81.4%) se encontraron con actividades de excavación por depredadores. Se encontraron cáscaras de huevo cerca de todos los nido depredados. En las playas de la península de Baja California debe considerarse al coyote como un importante depredador de nidos de tortuga.
Baja California is characterized by productive coastal marine waters coupled with unproductive inland habitats (Polis & Hurd, 1996). Inland food scarcity promotes the displacement of coyotes to coastal areas, where they have been observed in high densities (Rose & Polis, 1998).
The coyote (Canis latrans) is one of the most widely distributed and opportunistic carnivores in North America (Bekoff, 1977). This species is the most common predator in arid zones across Baja California (Álvarez-Castañeda, 2000), being considered an omnivorous opportunist (Elliot & Gueting, 1990; Grajales-Tam, Rodríguez-Estrella, & Cancino-Hernández, 2003; Ortega, 1987; Reichel, 1991; Servín & Huxley, 1993; Windberg & Mitchell, 1990). Coyotes prey on different species, ranging from small- (rodents) to medium-sized mammals (Lagomorpha), reptiles and birds; as well, they are scavengers (Álvarez-Castañeda & González-Quintero, 2005; Arnaud, 1993), feeding on dead reptiles and arthropods (Grajales-Tam et al., 2003; Hernández, Delibes, & Hiraldo, 1994).
The role of the coyote as a common predator of turtle nests has been widely documented (Drake, Hagerty, Behm, & Goldenburg, 2001; Minckley, 1966; Pritchard & Márquez, 1973). Therefore, the impact of coyotes in sea-turtle nesting areas should be assessed.
Five of the 7 sea turtle species have been recorded in the southwestern coast of the Baja California Peninsula: black turtle, Chelonia mydas; loggerhead turtle, Caretta caretta; olive ridley turtle, Lepidochelys olivacea; and, to a lesser extent, hawksbill turtle, Eretmochelys imbricate; and leatherback turtle, Dermochelys coriacea (Kampalath, Gardner, Méndez-Rodríguez, & Jay, 2006). Both E. imbricata and D. coriacea are considered as critically endangered by IUCN (Mortimer & Donnelly, 2008). Chelonia mydas and Caretta caretta are considered as endangered (Marine Turtle Specialist Group, 1996; Seminoff, 2004) and L. olivacea as vulnerable (Abreu-Grobois & Plotkin, 2008). All of them are protected by the Mexican government (Nom-059-Semarnat-2010 [Semarnat, 2011]).
Of the sea turtles mentioned above, the main species nesting along Baja California coasts is the olive ridley turtle (Lepidochelys olivacea) (WWF & UABCS, 2004). Most olive ridley turtles breed annually and display an annual migration from pelagic foraging areas to coastal breeding and nesting grounds, and then back to the open sea. This sea turtle is generally found on beaches with high humidity levels, which are suitable for nesting, mostly near river mouths or estuaries (Casas-Andreu, 1978; Márquez, Peñaflores, & Vasconcelos, 1996).
The Baja California Peninsula is the northernmost nesting area for the olive ridley turtle (Casas-Andreu, 1978); however, in contrast to its nesting behavior in other areas mentioned above, this sea turtle arrives as solitary individuals in low numbers (López-Castro, Carmona, & Nichols, 2004; Márquez et al., 1996).
On the other hand, coyotes are also found in some coastal areas of Baja California; as opportunistic carnivores, their diet includes crustaceans and fish (Grajales-Tam et al., 2003). Therefore, it is necessary to document whether coyotes are observed on the same beaches where turtles arrive to nest; and if so, the magnitude of turtle nest predation by coyotes should be assessed.
Materials and methodsWith the aim to evaluate the extent of turtle nest predation, therefore surveys along 40km on a pristine beach called Punta Marques, in the western part Baja California Peninsula (23°53′32.99″N, 110°47′57.17″O; 23°39′16.15″N, 110°30′50.18″O) were performed monthly from June to September 2013. No human settlements, crop fields, tourist developments, or commercial fishing activities were found in this area. The only access to this beach is by traveling 40km of unpaved road (Fig. 1).
Map of the location of each of the study areas in Baja California Sur, México. The narrow bold line is the survey area. The dash line areas are the sandy coastal areas in which the turtles can lay their eggs. The bold line shows the rocky coastal areas, and the dash-points are the highways. Las Barracas is one of the most important areas for turtle nesting in the peninsula.
Transects were covered using 2 ATV motorcycles in 2 parallel lines at an average speed of 20km/h. The surveys began from north to south in the morning, and the same route was covered heading north in the afternoon. All turtle tracks and dead specimens found on the beach were recorded. Each nest was geo-referenced. The distance between nests was obtained using geo-references in Google Earth (https://earth.google.com). During the study, some olive ridley turtles (Lepidochelys olivacea) were found digging their nests. Photographs of the tracks of those specimens were used for assigning species to the tracks found during the study. Four canid species inhabit the study area. The gray fox (Urocyon cinereoargenteus) and the desert fox (Vulpes macrotis) have very small tracks, whereas the coyote (Canis latrans) and the dog (C. familiaris) have large tracks such as those found around all nests. The tracks found are most likely to belong to coyotes (C.latrans), for the following reasons: (A) the area does not include human settlements from which dogs could travel to the beach; (B) the study area has a high density of coyotes; (C) the size of canid tracks was similar in all nests, and (D) the shape and size of the tracks correspond to coyote tracks previously observed in tha Baja California Peninsula. The latter 3 points are supported in more than 25 years of working with mammals across the Baja California Peninsula by the second author of this paper.
For analysis, nests were divided into 2 groups: (A) new nests, showing turtle tracks from sea-beach-sea; (B) old nests, with no evidence of turtle tracks. The third survey (September 15, 2013) was performed one week after 2 tropical storms that had hit the area (August 25, 2013, “Ivo”; September 7, 2013, “Lorena”), thus ensuring each nest with tracks around it was new. A nest was considered as preyed on when eggshells were found nearby or in a dug hole (Fig. 2). Descriptive and summary statistics were calculated including means and error standards. Due to the non-normal distribution of nests as shown by the Kolmorogov–Smirnov test, non-parametric statistical analyses were performed using the Mann–Whitney U test to make comparisons between groups; statistical significance was set at α≤0.05.
Photographs of 2 predated turtle nests found in the study area. (A) Turtle and coyote tracks on the sand, the open nest, and the egg shells left by the coyote. (B) An open nest with coyote tracks, eggshells, and complete eggs still in the nest. The Black vulture (Coragyps atratus) and Western gull (Larus occidentalis) were observed scavenging eggs upon our arrival.
During the surveys, a specimen of Lepidochelys olivacea digging and laying its eggs was observed. Since the tracks were similar in size and pattern to those found near preyed upon nests, we assumed that the latter also belonged to L. olivacea. Four coyote individuals were observed during the trips; the size and shape of their tracks and the distance between individual footprints found around nests matched those of the coyote (Aranda, 2000). No tracks of Urocyon cineroargenteus or Vulpes macrotis (Álvarez-Castañeda, 2000) were found near the nests. A total of 43 turtle nests were observed. Of these, 34 (79.1%) were considered as new and 9 (20.9%) as old. On the other hand, 8 (18.6%) showed no digging activity by predators; 35 (81.4%) were found with evidence of digging by predators, and all had with eggshells near the hole; from these, 28 (80.0%) were considered as new nests and 7 (20.0%) as old nests. Mainly all the nest were empty at the time of the survey.
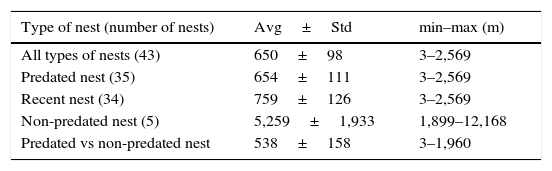
The mean distance±standard error between nests in general was 650±98m (3–2,569m), but between nests preyed upon was 654±111m (3–2,569m; Table 1). All nests which showed predation were not isolated; none matched the cluster type. No significant differences (U: 127.0(1,41); p=0.68) were found in terms of mean distances between non-preyed on and the closest preyed on nest (538±158m) versus mean distance between preyed on nests (654±111m). However, mean distance between non-preyed on nests (4,440±1,372m) was significantly different (U: 58(1,41); p=0.0089) versus mean distance between nests in general, between 2 preyed on nests, and between non-preyed on and the closest preyed on nest (Table 1). The mean distance of turtle nests from the high tide mark was 15m (range 20–10m). Nest density in the area was 0.53 per ha, and density of preyed on nests was 0.47 nests per ha. We found that nests located (Table 2) near or among the vegetation had a lower predation rate.
Distance (in meters) between turtle nests in general, predated nests, recent nests (with tracks), non-predated nests, and distance between non-predated and the closest predated nest. In each case, average (Avg), standard error (Std), minimum (min) and maximum (max) distances are shown.
| Type of nest (number of nests) | Avg±Std | min–max (m) |
|---|---|---|
| All types of nests (43) | 650±98 | 3–2,569 |
| Predated nest (35) | 654±111 | 3–2,569 |
| Recent nest (34) | 759±126 | 3–2,569 |
| Non-predated nest (5) | 5,259±1,933 | 1,899–12,168 |
| Predated vs non-predated nest | 538±158 | 3–1,960 |
Nest locations along survey transects with georeferences. NP=non predated; P=predated; E=empty hole, and notes.
| Latitude | Longitude | Nest condition | Notes | ||
|---|---|---|---|---|---|
| 1 | 1 | 23°49.987′N | −110°44.624′O | NP | Coyote tracks |
| 2 | 23°49.190′N | −110°43.861′O | P | ||
| 3 | 23°48.923′N | −110°43.601′O | P | ||
| 4 | 23°48.854′N | −110°43.535′O | P | ||
| 5 | 23°48.814′N | −110°43.495′O | P | Coyote tracks | |
| 6 | 23°48.769′N | −110°43.453′O | P | ||
| 7 | 23°48.523′N | −110°43.214′O | P | ||
| 8 | 23°47.830′N | −110°42.559′O | P | Coyote tracks | |
| 9 | 23°46.813′N | −110°41.603′O | P | Coyote tracks | |
| 10 | 23°46.766′N | −110°41.562′O | P | Coyote tracks | |
| 11 | 23°45.747′N | −110°40.562′O | P | Coyote tracks | |
| 2 | 12 | 23°45.123′N | −110°39.887′O | NP | Coyote tracks |
| 13 | 23°44.907′N | −110°39.650′O | P | Coyote tracks | |
| 14 | 23°44.867′N | −110°39.614′O | P | Coyote tracks | |
| 15 | 23°44.808′N | −110°39.539′O | P | Coyote tracks | |
| 16 | 23°44.655′N | −110°39.355′O | P | Coyote tracks | |
| 17 | 23°44.390′N | −110°39.024′O | P | ||
| 18 | 23°44.189′N | −110°38.772′O | P | Coyote tracks | |
| 19 | 23°43.935′N | −110°38.482′O | P | Coyote tracks | |
| 20 | 23°43.618′N | −110°38.107′O | P | Coyote tracks | |
| 21 | 23°43.595′N | −110°38.067′O | P | Coyote tracks | |
| 22 | 23°43.367′N | −110°37.801′O | P | ||
| 23 | 23°43.276′N | −110°37.679′O | P | Coyote tracks | |
| 24 | 23°43.062′N | −110°37.408′O | P | Coyote tracks | |
| 3 | 25 | 23°42.964′N | −110°37.286′O | NP | Coyote tracks |
| 26 | 23°42.961′N | −110°37.285′O | P | Coyote tracks | |
| 27 | 23°42.635′N | −110°36.871′O | P | Coyote tracks | |
| 28 | 23°41.523′N | −110°35.278′O | E | Coyote tracks | |
| 29 | 23°41.459′N | −110°35.154′O | P | Coyote tracks | |
| 30 | 23°41.298′N | −110°34.891′O | P | Coyote tracks | |
| 31 | 23°41.298′N | −110°34.890′O | P | Coyote tracks | |
| 32 | 23°41.295′N | −110°34.883′O | P | ||
| 33 | 23°41.180′N | −110°34.687′O | P | Coyote tracks | |
| 34 | 23°40.577′N | −110°33.696′O | E | ||
| 35 | 23°40.302′N | −110°33.195′O | P | Coyote tracks | |
| 36 | 23°40.272′N | −110°33.162′O | E | Coyote tracks | |
| 37 | 23°40.182′N | −110°32.980′O | P | Coyote tracks | |
| 4 | 38 | 23°39.878′N | −110°32.298′O | NP | Coyote tracks |
| 39 | 23°39.694′N | −110°31.905′O | P | Coyote tracks | |
| 40 | 23°39.703′N | −110°31.913′O | P | Coyote tracks | |
| 41 | 23°39.671′N | −110°31.842′O | P | Coyote tracks | |
| 5 | 42 | 23°39.556′N | −110°31.560′O | NP | Coyote tracks |
| 43 | 23°39.451′N | −110°31.340′O | P | Coyote tracks |
Most mammal tracks found on the beach followed the dune line between the edge of the dune vegetation and the high tide mark (Engeman, Martin, Constantin, Noel, & Woolard, 2003). This arrangement of coyote tracks is similar to observations in Costa Rica. There, on Playa Naranjo beach, up to 74% (159 of 215) of L. olivacea nests were found to be preyed on by coyotes (Drake et al., 2001); this figure is similar to the percentage found in the present study.
Since coyotes are opportunistic species, we believe they come to the beach to scavenge on marine species that probably died at sea and were washed ashore. Our hypothesis is that coyotes are not specifically looking for sea turtle nests; but when they come across turtle tracks they follow the tracks, dig and consume the eggs. On the Baja California coastline, coyote frequency is 13:3 in relation to inland, and more than 69% of their scats contain marine items (Rose & Polis, 1998). This finding suggests that marine inputs to continental land are highly important for scavengers in desert areas with limited resources (Polis & Hurd, 1996).
Individual L. olivacea nests usually contain around 110 eggs (Semarnap-Conanp, 2009), i.e. about 3.3kg of eggs per nest. L. olivacea eggs are a good source of nutrients, with one egg supplying ∼5.73g protein, ∼0.26g carbohydrates and ∼0.31g lipids (Mora-Castro, Chávez, & Herrera, 1997). In all recently preyed on nests found in this study, some intact eggs were found inside holes. The black vulture (Coragyps atratus) and the western gull (Larus occidentalis) were seen scavenging turtle eggs, which opens up the possibility that coyotes frequently do not eat all eggs. This issue gives other scavenging species the opportunity to feed on the remaining eggs. Ultimately, the entire nest is lost for the reproduction process, either by predation or exposure, hence affecting eggs by dehydration.
Potential conservation implications of turtle nest predationMolecular data have demonstrated that female and male turtles are philopatric to specific geographical areas in which they perform courtship and lay eggs (Fitzsimmons, Moritz, Limpus, Pope, & Prince, 1997), returning precisely to their natal beach, with geographical differences of 5–15km (Lee, Luschi, & Hays, 2007). On the other hand, this study site is located within the northermost limit of the reproductive distribution range of L. olivacea (López-Castro et al., 2004).
The data in our study reveal that nest predation is significantly high (81.4%), and the number of non-preyed on nests is low (18.6%). This leads us to assume that recruitment of new turtles to the population in this area is low therefore, given the philopatric behavior, the number of adult turtles that may return to the same area to lay their eggs would be reduced accordingly.
This situation leaves a question in the air. Could predation by coyotes on turtle eggs on this beach limit the range of the breeding area for this turtle species? If such predation is causing a decrease in the number of nests and fewer individuals with philopatric behavior are recruited by the population, eventually a reduced number of turtles will be arriving to nest in the area studied and nests will become increasingly more isolated. However, an alternative explanation could be that this sea turtle species is broadening its reproductive range as response to the rise in sea water temperature and perhaps the turtles currently arriving to the study area could be just beginning to use the beach as a nesting ground, and nests would be expected to be arranged in clusters as the number of turtles visiting the beach increases. However, our data shows that nests with a cluster arrangement suffer more predation than isolated nests. This finding could be related to the physical characteristics of the beach. In a previous study, Fritts and Stinson (1982) compared the density of nests between Punta Marqués and Cabo Falso, a beach located on the southern end of the Baja California Peninsula. These authors reported a higher nest density in Cabo Falso, where the beach is relatively flat and wide (30–100m of open sand between surf and vegetation areas), with a slight ridge ∼1m high. Meanwhile, beaches at Punta Marqués are steeper and more variable in width but consistently display vegetation areas closer to the surf zone. Alongside these characteristics, sand humidity is also an important factor for the construction and success of the nest (Fritts & Stinson, 1982). Therefore, beach areas with characteristics that are best suited as nest sites will also have a higher presence of coyotes, hence posing a higher risk of nest predation. Areas less favorable for use as nesting grounds (e.g., vegetation areas) are also less frequented by coyotes, hence offering a higher nest survival rate; this in turn would improve turtle survival beyond the predation effect. These 2 hypotheses need to be evaluated through a long-term study to assess the predation effect by coyotes and the plausible expansion in reproductive range as a response to warmer oceans resulting from the global climate change.
We express gratitude to T. Papenfuss and D. Dorantes for their comments. Financial support (grant 151189) was provided by the Consejo Nacional de Ciencia y Tecnología (Conacyt) and sabbatical grants (203952 LMR and 207093 STAC). Maria Elena Sánchez-Salazar edited the English manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.