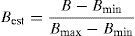The Yellow-footed Gull (Larus livens) is one of the few members of the genus Larus inhabiting the Gulf of California. Its breeding biology, nest phenology, and behavior have been long studied but little is known about seasonal changes in its diet. In this study, we tested if the diet of Yellow-footed Gull differed between reproductive (April and July) and non-reproductive (December). The frequency of occurrence of food items in gull's pellets and food niche breadth (FNB) and Levin's diversity (Best) indexes for each sampling period (April, July, and December), as well as Pianka's dietary overlap (O%) and Morisita's (MI) dietary similarity indices between periods were estimated. We identified 9 taxa classes, whose frequency of occurrence varied significantly between all comparisons (April and December, July and December, April and July), and between reproductive (April–July) and non-reproductive seasons. FNB and Best diversity indexes indicated that Yellow-footed Gull has a broader niche spectrum during the breeding season (April–July), while dietary overlap estimators (O% and MI) were higher between July vs. December and April vs. December, but lower in April vs. July. Results are contrasted with previous studies of gull's feeding ecology, and possible effects of local human activity are discussed.
La gaviota de patas amarillas (Larus livens) es uno de los pocos miembros del género Larus que habitan el Golfo de California. Su biología reproductiva, fenología de nido y comportamiento se han estudiado durante mucho tiempo, pero poco se sabe acerca de los cambios estacionales en su dieta. En este estudio se probó si la dieta de la gaviota de patas amarillas fue diferente entre temporadas reproductivas (abril y julio) y no reproductivas (diciembre). Se evaluó la frecuencia de presencia en egagrópilas, la amplitud de nicho trófico (FNB) y el índice de diversidad de Levin (Best) para cada período de muestreo (abril, julio y diciembre), así como la superposición de la dieta de Pianka (O%) y el índice de similitud de Morisita (MI) entre períodos. Se identificaron 9 clases de taxones, en los que la frecuencia varió significativamente entre todas las comparaciones (abril y diciembre, julio y diciembre, abril y julio) y entre los períodos reproductivos (abril-julio) y no reproductivos. Los índices FNB y Best indicaron que la gaviota de patas amarillas tiene un espectro de nicho más amplio durante la temporada reproductiva (abril-julio), mientras que los estimadores de solapamiento dietéticos (O% y MI) fueron más altos entre julio vs. diciembre y abril vs. diciembre, pero menor en abril vs. julio. Los resultados se contrastan con estudios previos de ecología de alimentación de gaviotas y se discuten los posibles efectos de la actividad humana local.
The Yellow-footed Gull (Larus livens) is the only bird species that is endemic to the Gulf of California (Anderson & Palacios, 2008), and is subject to special protection under Mexican law (Semarnat, 2010). Although historically considered as a subspecies of the Western Gull (L. occidentalis), it is long recognized as a distinct species (McCaskie, 1983). The breeding grounds of the Yellow-footed Gull are within the northern and western part of the Gulf of California, and the size of the colonies decreases from the midriff islands region to the southern part of the gulf, mainly due to the low productivity and smaller size of the islands in this region (Velarde & Anderson, 1994).
Seasonal changes in food availability for the Yellow-footed Gull are expected in the midriff region because primary productivity is highly dependent on seasonal changes in climate, tides, currents, and circulation (Álvarez-Borrego, 2002; Álvarez-Borrego & Lara-Lara, 1991). For example, seasonal changes in water surface temperature (between 6 and 16°C in winter to up to 31°C in summer) (Álvarez-Borrego, 2002) leads to the migration of many marine species (invertebrates, algae, and tropical vertebrates) in winter, while temperate species tend to do the same in summer (Brusca & Findley, 2005). Consequently, vertebrates inhabiting these islands that consume marine organisms, such as the Yellow-footed Gull, may be affected by variations in the type and abundance of marine resources related to seasonal changes in oceanographic conditions (García-Rodríguez & Aurioles-Gamboa, 2004; Velarde, Ezcurra, Cisneros-Mata, & Lavin, 2004). As with many other bird species in the midriff island region (Anderson & Palacios, 2008), the Yellow footed-Gull has its breeding activity between April and July (Hand, Hunt, & Warner, 1981), with this timing probably related to differences in food abundance throughout the year (Anderson & Palacios, 2008). In this study, we examined if the diet of an island population of Yellow-footed Gull differed between reproductive (April and July) and non-reproductive (December) seasons. Accordingly, we determined gull's diet composition and diversity for each sampling period, and estimated dietary similarity between periods.
Materials and methodsStudy site and collecting methodsPrey remains (pellets) of the Yellow-footed Gull were collected in Isla Partida Norte (28°53′30″ N, 113°2′25″ W) in April 17, July 7, and December 19 of 2006. The locality is a small island (1.38km2) located in the midriff island region of the Gulf of California, approximately 50km off the coast of Bahia de Los Angeles, Baja California (Fig. 1). Isla Partida Norte is a volcanic island, probably originated during the Pleistocene (Carreño & Helenes, 2002), with a mid-latitude winter, subtropical summer, and less than 5 rainy days per year (Álvarez-Borrego, 2002). Vegetation on the island is mostly desert scrub (Cody, Rebman, Moran, & Thompson, 2002; Rzedowsky, 2006). The Yellow-footed Gull occupies the shores of Isla Partida Norte during the breeding and non-breeding seasons (Flores-Martínez, pers. obs.), and, unlike typical clustered colonies of other gull species, the arrangement of territories of this species in the island is commonly linear (Hand et al., 1981). Therefore, we collected pellets within a 200×10m transect located on the eastern shore of the island, covering an area of 2,000m2. We collected fresh pellets and placed them in individual plastic bags for further examination. Although diet reconstruction using pellets is non-invasive and provides relatively large sample sizes, the method might overemphasize the presence of prey with indigestible hard parts, underscoring the importance of soft prey (Brown & Ewins, 1996; Duffy & Jackson, 1986). Nonetheless, it has been shown that this method closely reflects bird diet, allowing the detection of both seasonal and geographic variations (Barrett et al., 2007; Herrera, Punta, & Yorio, 2005). To verify that pellets belonged to the Yellow-footed Gull, we used direct observation of regurgitations (Flores-Martínez, pers. obs.), collected the pellets, and used them to compare all subsequent pellets collected along the transect. Yellow-footed Gull pellets have a characteristic oval-spherical ball shape, often with prey primary feathers coming out of one side. All remains within each pellet were inspected using a stereo microscope. The minimum number of prey individuals present in the pellets was calculated based on the most common bone found, or body part that represented one single individual. For vertebrates, we used boney remains, such as skulls, humeri, ulnae, tibiotarsi, spine, and pelvic bones, considering unique or paired bone pieces. We used chelas (left or right for crabs) and thorax (for insects) to quantify arthropods. Given that to estimate the number of individual remains of fish, bivalves, anthozoans, and algae is extremely difficult, we only recorded their presence in the pellets. We identified the items within each pellet to the lowest possible taxonomic level. Only in the cases of mammals and birds were we able to achieve identification at the species level. Prey remains were identified using field guides (Norris, 2010; Reid, 2006; Sibley, 2003) and museum specimens (Colección Osteológica de Comparación and Colección Arqueozoológica, Instituto Nacional de Antropología e Historia; and Colección Nacional de Aves, Universidad Nacional Autónoma de México).
Data analysesWe followed recommendations of standardized presentation of results (Barrett et al., 2007; Duffy & Jackson, 1986) to facilitate further comparisons with other dietary studies. Therefore, all comparisons were made to Class level. We estimated the importance of each prey type as its frequency of occurrence (%) and its numerical abundance (N), as described by Duffy and Jackson (1986). We did this for April, July, December, and the 3 sampling periods combined. To assess dietary diversity, we used a food niche breadth index (FNB) following Levins (1968):
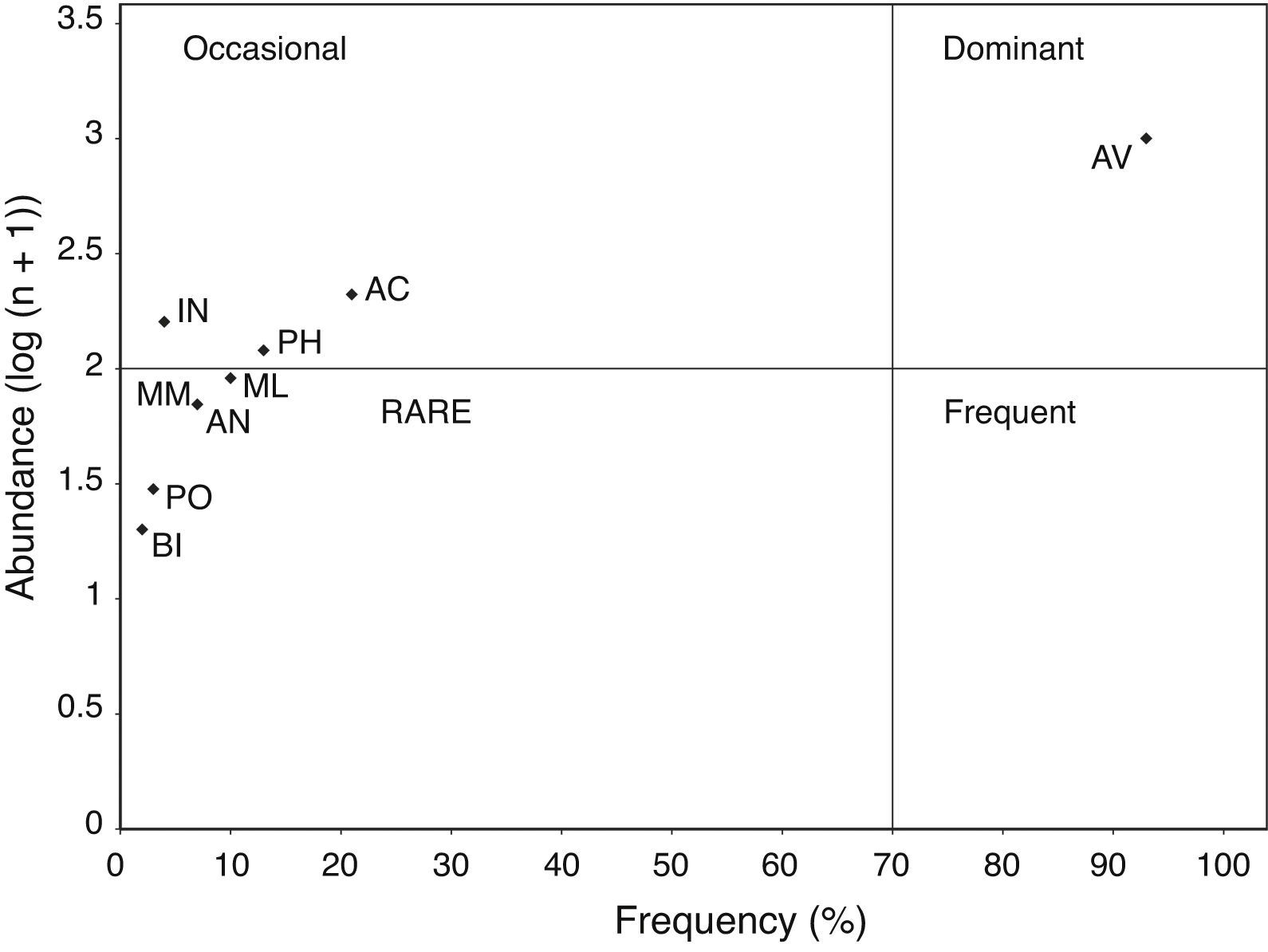
where pi is the proportion of prey category i in the Yellow-footed Gull diet. The values of this index range from 1 to N (number of prey categories in a diet sample), and large values indicate a broader niche dimension. We also estimated the Levins’ standardized index:where Bmin=1 and Bmax=total number of prey; this index is independent of the number of prey and indicates the specialization in the use of a type of prey as values approach zero (Colwell & Futuyma, 1971). To estimate dietary overlap between sampling periods, we used Pianka's (1973) dietary overlap index (O%) and Morisita's index of dietary similarity (MI) (Morisita, 1959). The dietary overlap index is defined as:where pi is the proportion of prey type i in one dietary sample and qi the proportion of the same type in the other dietary sample. This index ranges from zero (meaning no overlap) to 1 (complete overlap), being a measure of diet similarity. We multiplied values by 100 and presented as percent similarity between diet samples (Marti, 1987). The Morisita's index is similar in meaning and ranges from zero when the samples are completely distinct to 1 when they are identical. Frequency of occurrence of food items was compared between April and December, July and December, April and July, and reproductive (April–July) and non-reproductive (December) seasons, using chi-square tests. Additionally, we used references given by Velarde, Ávila-Flores, and Medellín (2007) to estimate mammal and bird prey biomass (15.6g for O. microsoma, 30.0g for O. melania, and 27.5g for Myotis vivesi). The biomass contribution of mammal and bird species to the diet was estimated as the percentage biomass. To obtain this estimator, we multiplied the number of individuals within the pellets by the estimated body mass of each prey species, and then divided it by the total sum of estimated biomass.Finally, the food items within pellets were plotted using an Olmstead–Tukey corner test for association (Olmstead & Tukey, 1947). In this diagram, the frequency of occurrence of the food items (number of different taxa found within each pellet) was plotted versus their abundance (total number of individuals in all pellets, log (n+1) transformed). The vertical line dividing the diagram shows the food item present in less than 50% of the pellets on the left side, and the food item present in more than 50% of the pellets on the right side. The horizontal line dividing the diagram shows the most abundant preys in the top part and the least abundant preys in the bottom part. Thus, dominant food items in the gull's diet are those with the highest frequency and abundance values. Occasional food items occur infrequently at the different sampling sites, but show a high abundance. Frequent food items are those most commonly used, but with a lower than mean abundance. Rare food items show low abundance and frequency values.
ResultsFrequency and biomass spectrumA total of 99 pellets were analyzed in this study: 41 in July, 34 in April and 24 in December. We identified a total of 178 prey items and a mean of 1.7 (S.E.=0.09) prey items per pellet. In July, the total number of items of all taxonomic classes found and the overall numerical abundance (N) of taxa identified were slightly higher than in April (Table 1). In December the number of items detected was about half of the number found in April or July, and many taxa were not present in the pellets at all (Table 1). Pellets collected in April, July, and December contained remains of birds, fish, insects and crabs, whereas mollusks, corals, sponges and brown algae were present only in April and July. The frequency of occurrence of all prey classes varied significantly between April and December (χ2=14.6, df=7, p<0.05), between July and December (χ2=16.3, df=8, p<0.05), between April and July (χ2=32.7, df=8, p<0.05), and between reproductive (April–July) and non-reproductive seasons (χ2=14.2, df=7, p<0.05). When samples from all periods were pooled, small birds were the most frequent prey, followed by fish, insects, crustaceans, and mammals, in that order (Table 2). Other prey items found in the pellets (coral, sponges and bivalves) represented less than 4% of the frequency (Table 2). At the species level and in terms of frequency of occurrence, the dominant prey was the Least Storm-petrel (Oceanodroma microsoma) (48.3%). The estimated biomass of tetrapods indicated that Yellow-footed Gulls consumed 3 times more biomass from O. microsoma than from O. melania (1,341.6 and 420g, respectively), and 7 times more O. microsoma than Myotis vivesi (192.5g).
Taxonomic identification and overall numerical abundance (N) of prey items of the Yellow-footed Gull diet in Isla Partida Norte, collected in April, July and December of 2006.
| Phylum/Division | Class | Order | Family | Genus | Species | N | |||
|---|---|---|---|---|---|---|---|---|---|
| April | July | December | All | ||||||
| Chordata | Aves | Procellariiformes | Hydrobatidae | Oceanodroma | microsoma | 31 | 31 | 24 | 86 |
| Chordata | Aves | Procellariiformes | Hydrobatidae | Oceanodroma | melania | 5 | 4 | 5 | 14 |
| Chordata | Mammalia | Chiroptera | Vespertilionidae | Myotis | vivesi | 0 | 5 | 2 | 7 |
| Chordata | Actinopterygii | Beloniformes | Belonidae | – | – | 11 | 7 | 3 | 21 |
| Arthropoda | Insecta | Coleoptera | – | – | – | 14 | 1 | 1 | 16 |
| Arthropoda | Malacostraca | Decapoda | – | – | – | 3 | 6 | 1 | 10 |
| Mollusca | Bivalvia | – | – | – | – | 1 | 1 | 0 | 2 |
| Cnidaria | Anthozoa | – | – | – | – | 1 | 6 | 0 | 7 |
| Porifera | – | – | – | – | – | 0 | 3 | 0 | 3 |
| Phaeophyta | Phaeophyceae | Fucales | Sargassaceae | Sargassum | – | 1 | 11 | 0 | 12 |
| Total | 67 | 75 | 36 | 178 | |||||
Numerical abundance (N), frequency of occurrence (F), and biomass (B, estimated) of prey classes collected in pellets of Yellow-footed Gull in April, July and December of 2006. Taxonomic class keys are as given in Figure 2. Biomass was estimated only for birds and mammals. Explanations: AV, Aves; AC, Actinopterygii; PH, Phaeophyceae; ML, Malacostraca; MM, Mammalia; AN, Anthozoa; IN, Insecta; PO, Porifera; BI, Bivalvia.
| Month | AV | AC | PH | ML | MM | AN | IN | PO | BI | All |
|---|---|---|---|---|---|---|---|---|---|---|
| April | ||||||||||
| N | 36 | 11 | 1 | 3 | 0 | 1 | 14 | 0 | 1 | 67 |
| F (%) | 53.7 | 16.4 | 1.5 | 4.5 | 0 | 1.5 | 20.9 | 0 | 1.5 | 100 |
| B (%) | 100 | – | – | – | 0 | – | – | – | – | 100 |
| July | ||||||||||
| N | 35 | 7 | 11 | 6 | 5 | 6 | 1 | 3 | 1 | 75 |
| F (%) | 46.7 | 9.3 | 14.7 | 8 | 6.7 | 8 | 1.3 | 4 | 1.3 | 100 |
| B (%) | 96.8 | – | – | – | 3.2 | – | – | – | – | 100 |
| December | ||||||||||
| N | 29 | 3 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 36 |
| F (%) | 80.6 | 8.3 | 0 | 2.8 | 5.6 | 0 | 2.8 | 0 | 0 | 100 |
| B (%) | 99.9 | – | – | – | 0.1 | – | – | – | – | 100 |
| Total | ||||||||||
| N | 100 | 21 | 12 | 10 | 7 | 7 | 16 | 3 | 2 | 178 |
| F (%) | 56.2 | 11.8 | 6.7 | 5.6 | 3.9 | 3.9 | 8.9 | 1.7 | 1.1 | 100 |
| B (%) | 98.6 | – | – | – | 1.4 | – | – | – | – | 100 |
The highest FNB and Best values were found in July, followed by April and December (Table 3), indicating that the Yellow-footed Gull has a broader niche spectrum during the April and July periods (Table 3). Levin's index shows that gull's diet was more specialized in December, followed by April, and July (Table 3). Moreover, dietary overlap (estimated both by dietary overlap and Morisita's index of dietary similarity) was higher between July vs. December and April vs. December, but lower in April vs. July (Table 3). The scattergram of the Olmstead-Tukey corner test of association showed 4 prey classes (AN, BI, ML, MM, and PO) as rare, 3 (AC, IN, and PH) as occasional, and only 1 (AV) as dominant (Fig. 2).
Diversity and similarity of Yellow-footed Gull diet in 3 sampling periods. Diet diversity was measured with food niche breadth index (FNB) and Levins’ standardized niche breadth index (Best) for each sampling period, and diet similarity between sampling periods was estimated with dietary overlap index (O%) and Morisita's index of diet similarity (MI).
| Sampling period | FNB | Best | Periods compared | O% | MI |
|---|---|---|---|---|---|
| April | 2.76 | 0.22 | April vs. July | 88.75 | 0.84 |
| July | 3.74 | 0.34 | April vs. December | 92.75 | 0.87 |
| December | 1.51 | 0.06 | July vs. December | 92.83 | 0.88 |
| All | 2.87 | 0.23 | – | – | – |
Scattergram showing the results of the Olmstead-Tukey corner test of association, with the frequency and abundance of the different prey classes used by the Yellow-footed Gull Larus livens, in Isla Partida Norte, Gulf of California, Mexico. Taxonomic class keys are as given in Table 2.
Previous observations of feeding ecology of the Yellow-footed Gull are scarce but they can be used as a basis for comparison purposes with the present study. Hand et al. (1981), in Isla Partida Norte (mistakenly referred as “Isla Cardinosa”), observed Yellow-footed Gulls swallowing or regurgitating fish, petrels, crustaceans, squids, and pelican eggs. They also suggested that Yellow-footed Gulls may prey on themselves, by cannibalizing eggs and young of other nearby breeding conspecifics. Anderson and Keith (1980) documented that in different islands and areas within the Gulf of California, Yellow-footed Gulls prey on Brown Pelican (Pelecanus occidentalis) eggs and chicks. On the other hand, Velarde (1992) reported that during the breeding season at Isla Rasa, Yellow-footed Gulls (presumably the ones nesting at nearby Isla Partida Norte) prey on Heermann's Gull (Larus heermanni) chicks, but not on their eggs. In the present study, we observed the occurrence of fish, crabs, and petrels in the majority of the pellets throughout all the sampling periods in Isla Partida Norte. However, we failed to identify other items such as squid, pelican eggs, pelican chicks, Heermann's Gull chicks, or conspecific chicks or eggs. Although these food items might not be part of the diet of the Yellow-footed Gulls at the study site, methodological constraints might have limited their identification. For example, eggs might be consumed without the ingestion of eggshell, which in turn can only be documented by direct observation. On the other hand, we found a number of items in pellets (bats, insects, bivalves, cnidarians, sponges and brown algae) that had not been reported previously.
Contrasts of our diet reconstruction with previous studies may also be related to the low occurrence of some prey taxa in gull foraging areas, historical changes in marine communities, or the use of different collecting methods. One example of low probability of occurrence concerns the presence of Brown Pelican chicks. Although this species has been reported throughout the Gulf of California, it may be available as food source for limited periods of time (10–12 weeks before fledging), when Brown Pelican chicks are available (Anderson & Palacios, 2008). Therefore, its contribution as a prey item for the Yellow-footed Gull may be negligible or hard to identify. Changes in the marine communities in the Gulf of California in the last 60 years may have also affected the presence of nesting pelicans. For example, declining numbers of large vertebrates, echinoderms and large gastropods (Sagarin, Gilly, Baxter, Burnett, & Christensen, 2008) could be responsible of the lack of nesting Brown Pelicans, as previously reported in Isla Partida Norte (Anderson et al., 2007). On the other hand, contrary to previous observations (Velarde, 1992), we did not find evidence of Heermann's Gull chicks or egg remains in pellets. Given the nesting behavior and territoriality of the Yellow-footed Gull (Hand et al., 1981), the lack of Heermann's Gull nests in the selected study site, and the fact that there are several nesting points for Yellow-footed Gull in Isla Partida Norte and nearby islands (e.g., Cardonosa, Rasa), spatial partitioning of prey resources is a plausible explanation for this phenomena. If so, only Yellow-footed Gulls nesting close to Heermann's Gull nests will tend to prey on them. Further tests of this idea should include an assessment of the Yellow-footed Gulls diet in areas where other bird preys are also established.
In our study, the Least Storm-petrel and the Black Storm-petrel were the main preys of the Yellow-footed Gull (87–99% of total tetrapod biomass, respectively), while all the remaining items identified were of low importance (Fig. 2). One possible reason for this is that the high abundance of these species in Isla Partida Norte (500,000 Least Storm-petrels and 50,000 Black Storm-petrels; Anderson, 1983) encourages their consumption by Yellow-footed Gull. Preponderance of birds has been reported in the diet of other gull species. For example, the Atlantic Yellow-legged Gull (L. michahellis atlantis) has a tendency to focus on bird species as their main prey (>60% by biomass), obtaining as much as 82.5% of all their consumed energy from them (Matias & Catry, 2010). Likewise, the Yellow-legged Gull (L. michahellis) preys mainly on European Storm-petrels (Hydrobates pelagicus; Oro, de León, Minguez, & Furness, 2005). High specificity in prey selection has been reported in other gulls, such as the Kelp Gull (L. dominicus) that preys mostly on bivalves (∼75% of total prey; Bertellotti, Pagnoni, & Yorio, 2003), and the Heermann's Gull (L. heermanni) that feeds mostly on Pacific Sardines (Sardinops caeruleus) (60–97% of its diet; Velarde & Anderson, 1994). In contrast, Yellow-legged Gulls are omnivorous, consuming natural prey items, rubbish dump material or commercial fisheries discards (Martínez-Abrain, Maestre, & Oro, 2002; Oro, Bosch, & Ruiz, 1995), whereas the Caspian Gull (L. cachinnans) tends to have a diversified diet, with similar proportions of crustaceans, fish, echinoderms, and mollusks (Álvarez-Lao & Méndez-Iglesias, 1995; Munilla, 1997).
Although Yellow-footed Gulls main prey items were constant throughout the seasons we sampled, a statistically significant difference in the frequency of occurrence in the diet in all comparisons was found, including between breeding (April and July) and non-breeding (December) seasons. Such differences are given mostly by taxa whose contribution by frequency of occurrence is negligible when compared to that of birds (Table 2). FNB and Best indexes consistently suggests that in the breeding season (April and July) the food resources are more diversified than in the non-breeding season (December). On the other hand, dietary overlap estimated with O% and MI indexes showed higher overlap between April and December and between July and December, than between April and July. The almost exclusive bird diet in December (80% frequency) makes diets in non-reproductive and reproductive periods more similar than the diets during the 2 reproductive periods, which in turn differ by the variations in the contribution of non-bird preys. It is not clear whether the differences in taxa present in the diet, as well as differences in diversity and similarity between sampled periods, are solely due to changes in the population sizes of less abundant prey taxa, or a changing pressure in Yellow-footed Gulls to find food resources if young are to be fed. However, we must acknowledge that differences in feeding patterns may only be specific to Isla Partida Norte and may not be consistent through time, as pellet collection was performed during limited periods of time (approximately 2 weeks per collecting period) in a single year. In other gull species it has been reported that, after collecting in many different sites, seasons and years, such differences may not be conclusive at all (Ewins, Weseloh, Groom, Dobos, & Mineau, 1994).
Additionally, we observed that the fish-eating Myotis bat was by far not as abundant in the pellets as the petrels, indicating a low importance in the diet of the Yellow-footed Gull. This may account for resource partitioning within the island with other predators, such as the Barn Owl. On both Rasa and Partida Norte islands, Velarde and Medellín (1981) found that Barn Owl pellets contained mostly the fish-eating Myotis bat (37% frequency) and black rats (Rattus rattus; 53% frequency), with a minor contribution of Least Storm-petrels (<2% frequency). A more recent study revealed that the fish-eating Myotis bat was the main prey item of Barn Owl in Isla Partida Norte (81% frequency), with minor contributions of the Least Storm-petrel (14% frequency) and the Black Storm-petrel (<2% frequency; Velarde et al., 2007).
Finally, although human activity in the area surrounding Bahia de los Angeles and the midriff islands of the Gulf of California has steadily increased since the second half of the nineteenth century (Bahre & Bourillon, 2002), little is known of its effects on feeding habits of local birds. Human activity has not only been increased by fisheries (both recreational and commercial), but also it has created disturbances by other groups (e.g., egg collectors, tourists, educational groups, scientists, etc.), which actively approach nesting areas (Anderson & Keith, 1980). In the case of Yellow-footed Gull, human intrusion to nesting sites provokes that several nests with eggs and chicks are abandoned by parents, leading to predation by other gull adults (Hand, 1980). Our results reveal that, at least during the year of study, this had not been the case for Yellow-footed Gulls nesting on Isla Partida Norte, as we were unable to identify egg or chick remains within the pellets. Nevertheless, one important shortcoming of this study is lack of data from different years and sites, making it impossible to test for differences among years and regions. This paucity of samples is a result of the limitations imposed when working in a remote area with a rare and vulnerable species, which preclude any invasive studies. However, this study is a first step toward a better understanding of the feeding ecology of the Yellow-footed Gull, and encourages further collection of data from this species.
Transportation to Isla Partida Norte was generously provided by Secretaría de Marina-Armada de México. The Prescott College Kino Bay Center provided invaluable logistic support during fieldwork. Aida Otalora, and two anonymous reviewers, kindly reviewed early versions of the manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.