Temnocephala colombiensis n. sp., is described as ectosymbiont of Pomacea sp. from San José del Nús, Antioquia, Colombia. Temnocephalans were removed from the mantle cavity and its eggs from the umbilicus and the basal region of the operculum. The new species is characterized by: cirrus curved toward hindbody, approximately 90°; introvert's swelling with 20 to 26 longitudinal rows of fine spines and 11 to 13 spines per row; dorsolateral excretory syncytial plates rectangular with rounded corners and excretory pores eccentric, displaced to the anterior portion of the plate. Additionally we have done a comparison of cirrus morphology for Temnocephala described to date, based on literature review. This is the first Temnocephala described from Colombia.
Temnocephala colombiensis n. sp., se describe como ectosimbionte de Pomacea sp. de San José del Nús, Antioquia, Colombia. Los temnocéfalos fueron removidos de la cavidad del manto y sus huevos, del ombligo y región basal del opérculo. La nueva especie se caracteriza por un cirro curvado hacia la región posterior del cuerpo, con aproximadamente 90°; introverto ensanchado con 20–26 filas de espinas longitudinales y 11–13 espinas por fila; placas sincitiales dorsolaterales rectangulares con extremos redondeados y poros excretores excéntricos en la porción anterior. Adicionalmente se realiza una comparación de la morfología del cirro de las especies descritas a la fecha. Esta es la primera especie de Temnocephala descrita para Colombia.
The Temnocephala Blanchard, 1849 species are commensals of crustaceans, mollusks, insects and turtles in the Neotropical region, which include thirty species described to date (Moquin-Tandon, 1846; Monticelli, 1899, 1902, 1903, 1913; Haswell, 1893; Vayssiere, 1898; Pereira and Cuocolo, 1941; Dioni, 1967a, 1967b; Jennings, 1968; Lamothe-Argumedo, 1974; Moretto, 1978; Ponce de León, 1979, 1989; Cannon, 1993; Damborenea, 1994; Amato et al., 2003, 2006, 2007, 2011; Ibáñez and Jará, 2003; Amato and Amato, 2005; Damborenea and Brusa, 2008; Volonterio, 2007, 2010; Seixas et al., 2011). Of them, Temnocephala iheringiHaswell, 1893, T. rochensisPonce de León, 1979, T. haswelliPonce de León, 1989 and T. lamotheiDamborenea and Brusa, 2008 are known from ampullariid snails, showing a geographical distribution restricted to southeastern of South America (Damborenea and Brusa, 2008; Seixas et al., 2010a, 2010b, 2010c).
The Temnocephala genus is characterized by having eyes with red, fugacious pigment; a characteristic pattern of syncytial plates, and excretory pores enclosed in the excretory plates (Damborenea and Cannon, 2001). The cirrus has been the main taxonomic character (Damborenea, 1991) to differentiate the Temnocephala species. Recently, detailed descriptions of dorsolateral excretory syncytial plates (DLSPs) shape and excretory pores position has improved the identification of these (Damborenea and Cannon, 2001; Amato et al., 2003, 2005, 2006, 2010; Amato and Amato, 2005; Volonterio, 2007, 2009, 2010; Seixas et al., 2010a, 2010b, 2010c, 2011).
In the present study, we describe the first species of Temnocephala from Colombia, associated to mollusks Pomacea sp. Considering the cirrus as an important trait to differentiate the Temnocephala species, we have done a comparison of the cirrus characteristics of the species described, based on literature review.
Materials and methodsNinety three Pomacea sp. were collected manually in the Estación Piscícola de San José del Nús, San Roque, Antioquia (6°29'51” N, 74°50.028' W), from April to May of 2008, and transported live to the laboratory. A total of 77 temnocephalans (36 adults and 41 juveniles) were recovered from mantle cavity of the host snail, fixed in hot A.F.A. and after 24h, transferred to 70% ethanol, under slight cover slip pressure (Volonterio, 2007). The specimens were stained as follows: 3 in Giemsa, 4 in Meyer's paracarmine, 4 in Borax carmine, 17 in hematoxylin and 9 in silver nitrate; 6 dissected cirrus were mounted in Canada Balsam; description based on 28 adult specimens.
Morphology of DLSPs was studied in specimens stained with hot silver nitrate (SN), without pressure, between slide and cover slip. Temnocephalans were stained with hot SN and exposed to sun light for about 5 minutes, and washed in distilled water, and preserved in 70% ethanol. Measurements are in micrometers (μm) unless otherwise indicated: ranges are followed by the arithmetic mean, standard deviation values and the number of specimens measured for a given character; range (mean, standard deviation; n). Drawings were made using a drawing tube Nikon 1.25X microscope. Photographic images were taken with a Sony Cyber-shot DSC-W35. The line drawings were prepared using Corel Draw X5. Type specimens were deposited in the Colección Colombiana de Helmintos (CCH.116), and the mollusk hosts were deposited in the Colección de Moluscos Vectores (VHET-37), Universidad de Antioquia, Medellín, Colombia. A comparison of external cirrus structure in Temnocepahala was done, which includes drawings adapted from to literature found for 29 species (except T. peruensis).
DescriptionTemnocephala colombiensis n. sp. (Figs. 1b-g, 2, 3, 7c, 8d-g)
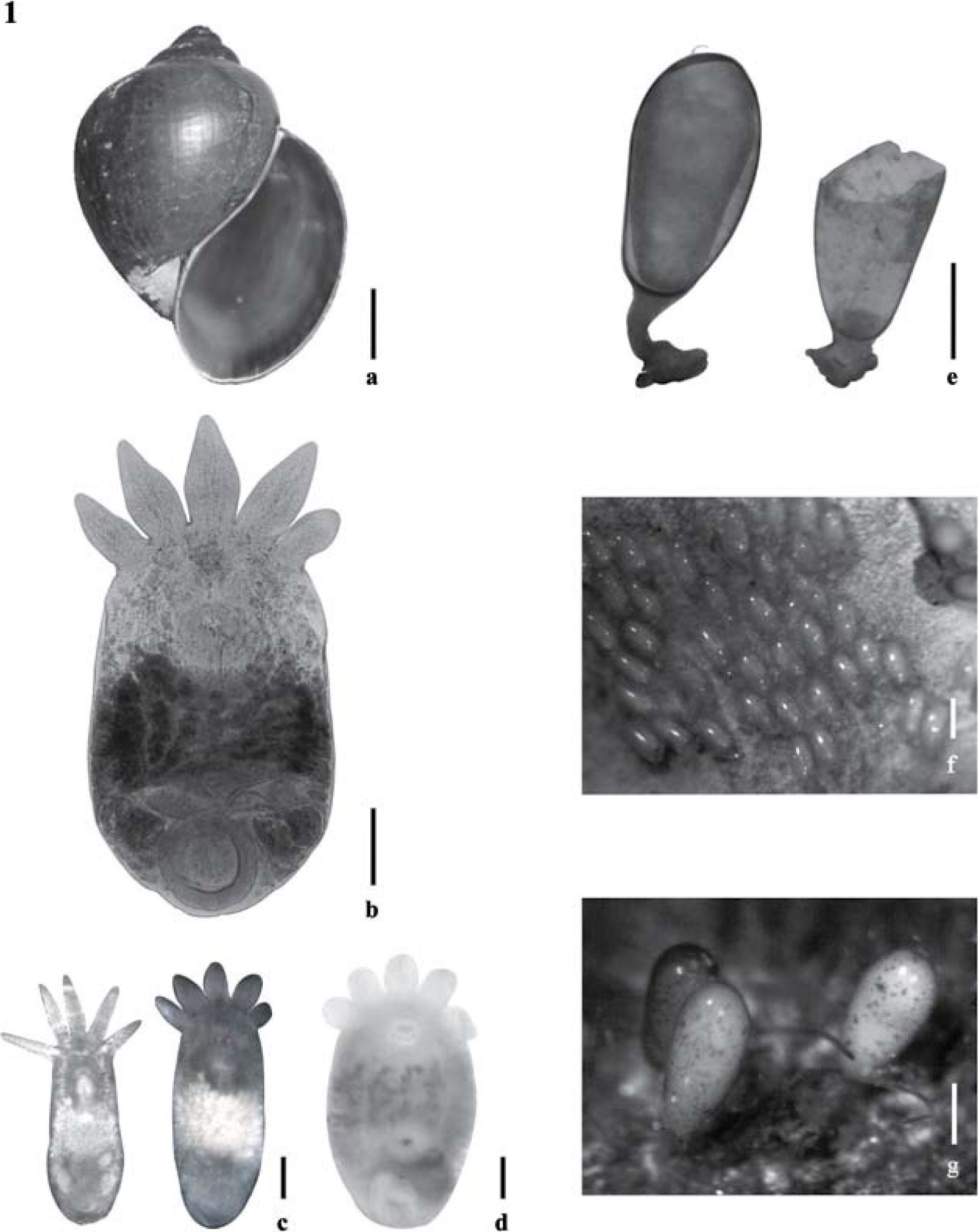
Pomacea sp. and Temnocephala colombiensis n. sp. a), Pomacea sp., ventral view bar = 10mm; b-g), Temnocephala colombiensis n. sp., b), adult specimen, stained in hematoxylin, bar = 250μm; c), specimens showing the typical body shape and redeyes, bar = 250μm; d), adult specimen with egg ready to be laid, bar = 250μm; e), egg shape and plane of fracture of the operculum, bar = 100μm; f-g), several eggs deposited on the operculum of Pomacea sp., bar = 250μm and 100μm respectively.
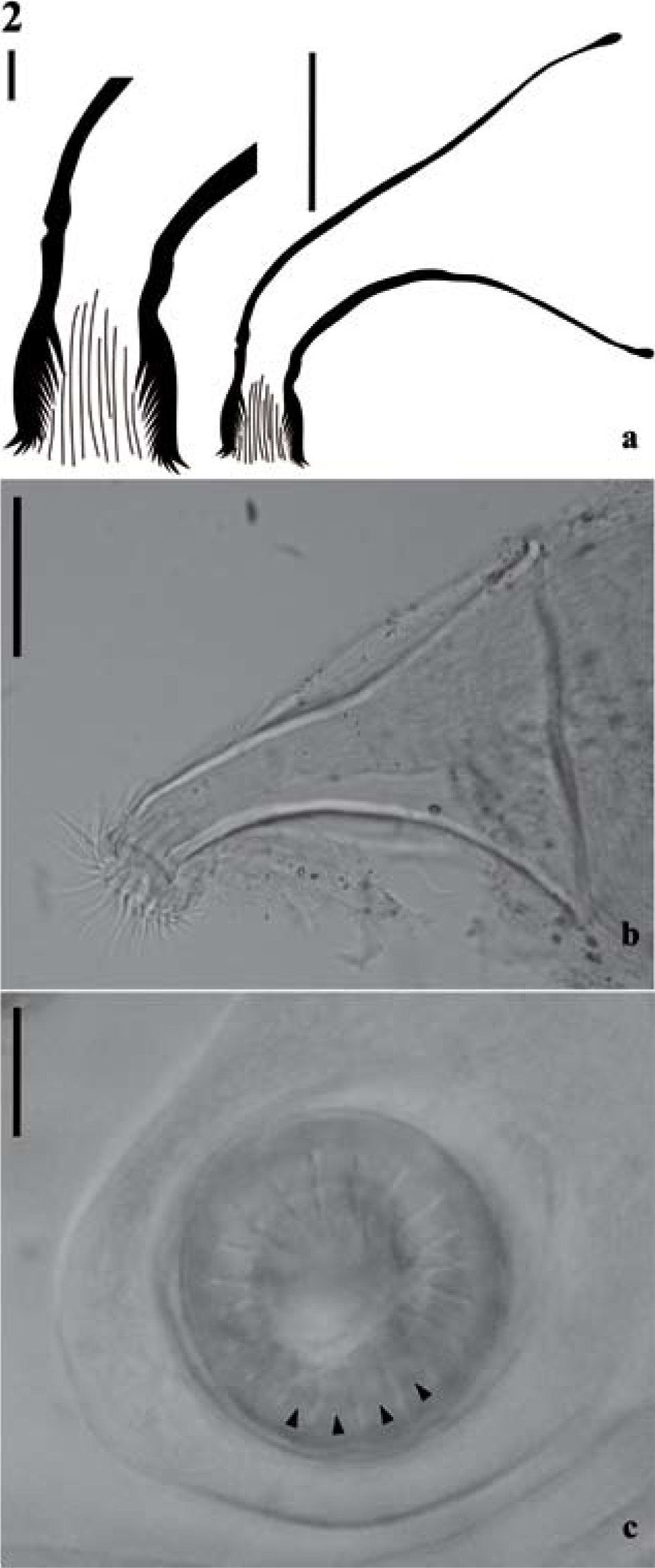
Cirrus structure of Temnocephala colombiensis n. sp. a), line drawings of introvert and entire cirrus, bars = 10μm and 50μm respectively; b), photography from a dissection, extroverted cirrus showing fine spines, bars = 50μm; c), photography of frontal view of the introvert, showing 20 longitudinal rows of spines, arrows indicating longitudinal rows, bars = 10μm.
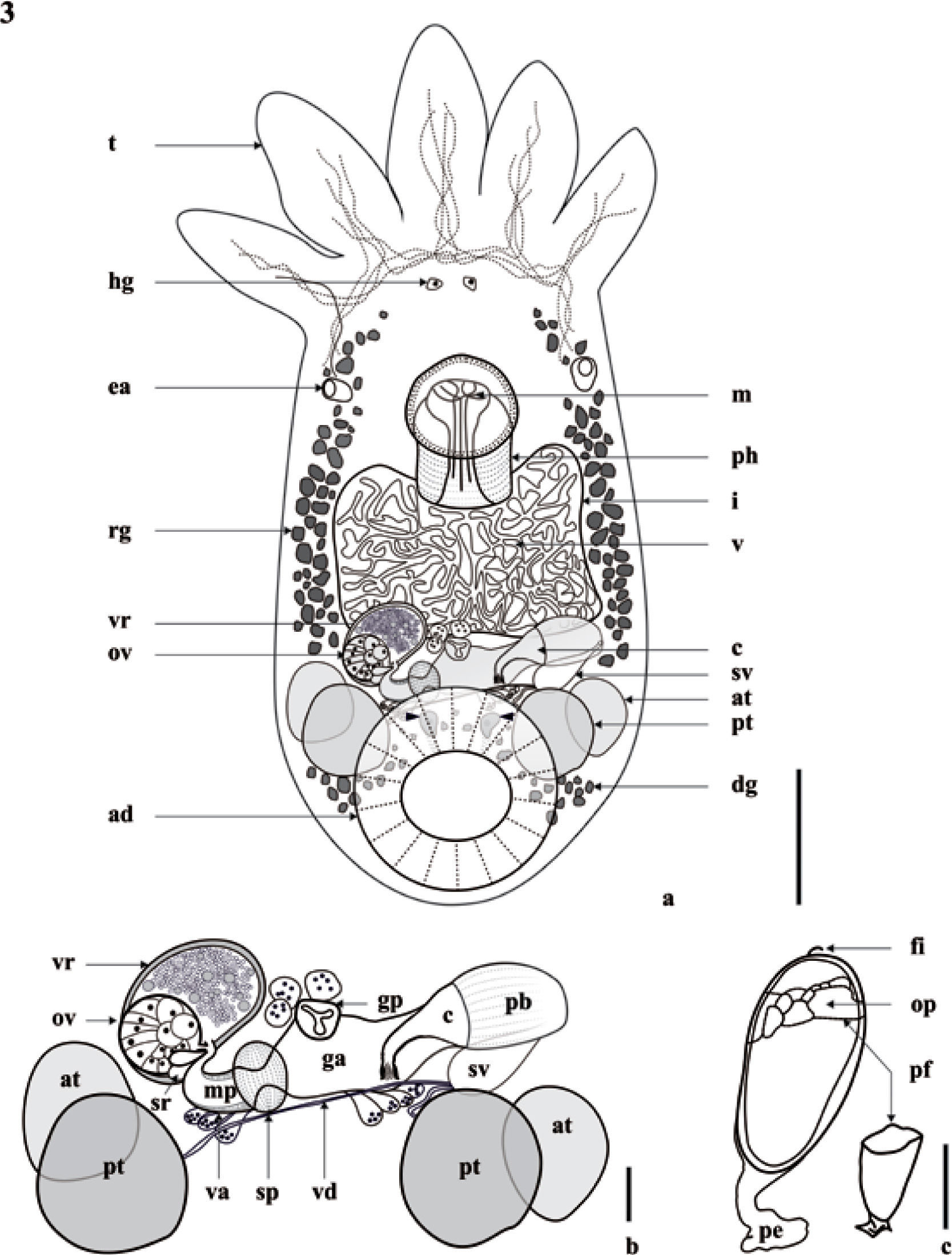
Temnocephala colombiensis n. sp., holotype. a), adult specimen in ventral view, showing: adhesive disk (ad), anterior testes (at), cirrus (c), disc glands (dg) and 2 lobed central cells (arrows), excretory ampullae (ea), Haswell glands (hg), intestinal sac (i), mouth (m), ovary (ov), pharynx (ph), posterior testis (pt), rhabditogenic glands (rg) extending along sides of intestinal sac, seminal vesicle (sv), tentacles (t), vesicula resorbens (vr), vitellarium (v), bar= 250μm; b), reproductive system: anterior testes (at), cirrus (c), genital atrium (ga), genital pore (gp), distal vagina with muscular portion (mp), ovary (ov), posterior testis (pt), prostatic bulb (pb), seminal vesicle (sv), vagina (va), vaginal sphincter (sp), seminal receptacle (sr), vasa deferentia (vd), vesicular resorbens (vr), bar = 100μm; c), whole egg and plane of fracture of the operculum, filament (fi), opercular plate (op), peduncle (pe), plane of fracture (pf), bar= 100μm.
Description based on 28 adult specimens. External characteristics. Body shape elliptical (Figs. 1b-d, 3a) with 5 anterior tentacles, concave ventrally. Body without tentacles 1 090.26-2 838.79 (1 621.44±3 61.02; 28) long by 596.56–1 686.82 (938.91±252.08; 28) wide; posterior circular adhesive disc 350.77–661.68 (479.21±89.110; 27) in diameter; eyespots round to irregular shaped, with red pigment in live specimens.
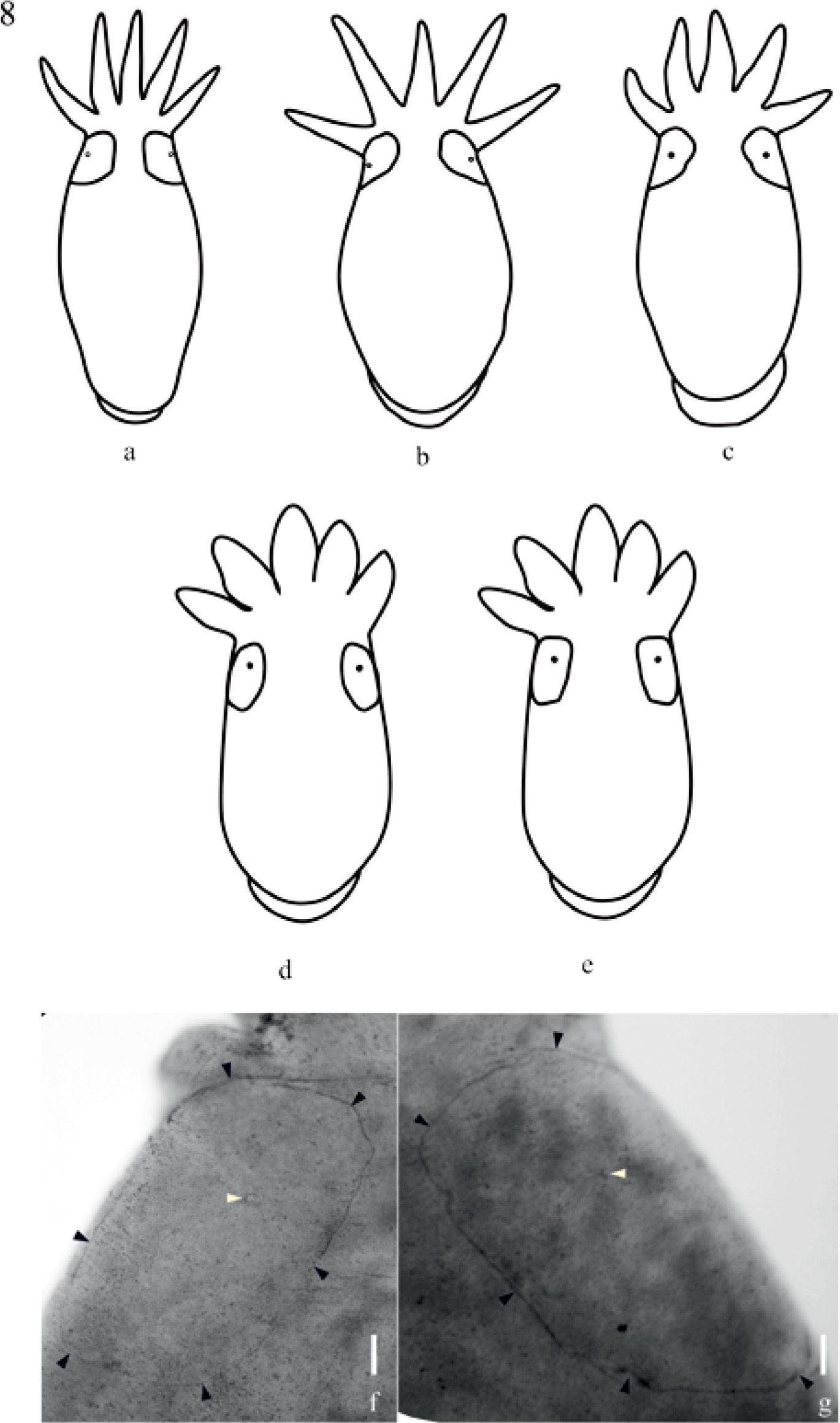
Epidermal mosaic (demonstrated through staining with SN) with 2 dorsolateral excretory syncytial plates (DLSPs) rectangular with rounded corners (Figs. 8d-g), extending from just below the base of first and fifth tentacles, respectively; left plate 221.82–514.93 (389.94±78.37; 9) long by 150.52–324.80 (196.29±106.41; 9) wide; right plate 261.43–522.852 (392.58±95.06; 9) long by 95.06–348.57 (198.93±102.45; 9) wide. Excretory pores eccentric displaced to the anterior portion of plate (Figs. 8f-g). Alimentary system: mouth between first and second thirds of body, surrounded by a large muscular sphincter; pharynx longer than wide, 111.61–286.99 (204.14±46.70; 28) long by 103.63–454.40 (201.86±78.11; 28) wide; intestine saccular, without septa. Excretory system: 2 excretory ampullae at level of mouth. Glands: rhabdite producing glands, numerous, forming bunches, in lateral fields of the body; extending from pharynx to the anterior part of adhesive disc. Haswell cells in front of the eyespots and the brain. Disc glands between adhesive disc and genital complex, integrated for numerous lateral cells, forming bunches around the disc and 2 lobed central cells (Fig. 3a).
Reproductive system (Fig. 3b). Female. Genital atrium spacious; gonopore in the center of the posterior third of the body; ovary ovoid, 47.83–175.38 (96.42±32.18; 21) long by 39.86–143.49 (91.87±28.70; 21) wide, 2–3 seminal receptacles observed; vitellaria branched, completely covering intestine sac dorsally without exceeding it; vagina short, with distal portion muscular, sphincter large and symmetrical well developed, vesicula resorbens ovoid, 71.75–334.82 (138.04±58.34; 19) long by 71.75318.88 (151.47±64.93; 19) wide, with spermatozoids inside. Eggs oval, 510.2–693.87 (617.34±6.43; 8) long by 183.67–285.71 (257.65±3.30; 8) wide, with mediansized subpolar filament; peduncles 122.45–224.49 (170.91±3.62; 8); the plane of fracture is perpendicular (Figs. 1e, 3c). Eggs deposited in umbilicus and operculum (Figs. 1f-g). Male. Testes 4, usually rounded, posterior to intestinal sac, anterior and posterior testes of different sizes, posterior testes more voluminous; right anterior testis 79.72–279.02 (157.45±63.91; 28) long by 63.78–231.19 (134.39±54.32; 28) wide; left anterior testis 63.78–318.88 (156.19±60.08; 27) long by 71.75–247.13 (140.23±48.35; 27) wide; right posterior testis 111.61–350.77 (193.04±64.42; 28) long by 79.72–294.96 (176.52±64.43; 28) wide; left posterior testis 111.61–318.88 (176.52±55.98; 28) long by 103.64–358.74 (192.75±66.31; 28) wide. Vesicle seminal pyriform, dorsal to prostatic bulb, 12–46 (23.68±8.72; 19) long by 16–50 (30.32±9.87; 19) wide. Prostatic bulb slightly ovoid with thick muscular wall, 72–216 (146.44±36.81; 27) long by 78–210 (136.89±24.74; 27) wide. Cirrus curved toward the terminal region of the body, approximately in 90°, 150–268 (213.19±31.11; 27) long; shaft base 68–146 (119.41±17.97; 27) wide; introvert 20–40 (30.60±6.04; 27) long, 24–26 (25.08±1.01; 24) wide at base; maximum introvert width at level of swelling 22–48 (30.00±5.46 27), with fine spines; proximal limit of introvert marked with small protuberances. Introvert's swelling shows 20 to 26 longitudinal rows of spines and 11–13 spines per row (Figs. 2, 7c). Ratio between total body length, without tentacles/total length of cirrus (TBL/ TLC) 7.6: 1; ratio between total length of cirrus/maximum width of shaft's base (TLC/WSB) 1.8: 1; ratio between total length of cirrus/total length of introvert (TLC/TLI) 7: 1.
Taxonomic summaryType host: Pomacea sp.
Host specimens deposited: VHET-37 (647, 648, 650–660).
Type locality: estación piscícola San José del Nús, San Roque, Antioquia, Colombia (6°29'51” N, 74°50.028' W).
Prevalence: 60 of 93 (64.5%) Pomacea sp. were infested with temnocephalans.
Site of infestation: Adults and juveniles in mantle cavity, eggs in umbilicus and operculum.
Type specimens: Colección Colombiana de Helmintos (CCH.116). Holotype: stained in Hematoxylin, CCH.116. (142). Paratypes: 27 whole mounted specimens; 4 stained in Meyer's paracarmine; 4 stained in Borax carmine; 16 stained in Hematoxylin; 3 stained in Giemsa, CCH.116. (143); 2 dissected cirrus, CCH.116. (144). Nine specimens stained in silver nitrate, and preserved in 70% ethanol, CCH.116. (145). Unhatched eggs preserved in 70% ethanol, CCH.116. (146).
Etymology: the specific name refers to the first Temnocephala described for Colombia.
Remarks. Temnocephala colombiensis n. sp. is compared with temnocephalans previously described from ampullariids, which can be distinguished by the combination of traits such as cirrus morphology, DLSPs shape and excretory pores localization.
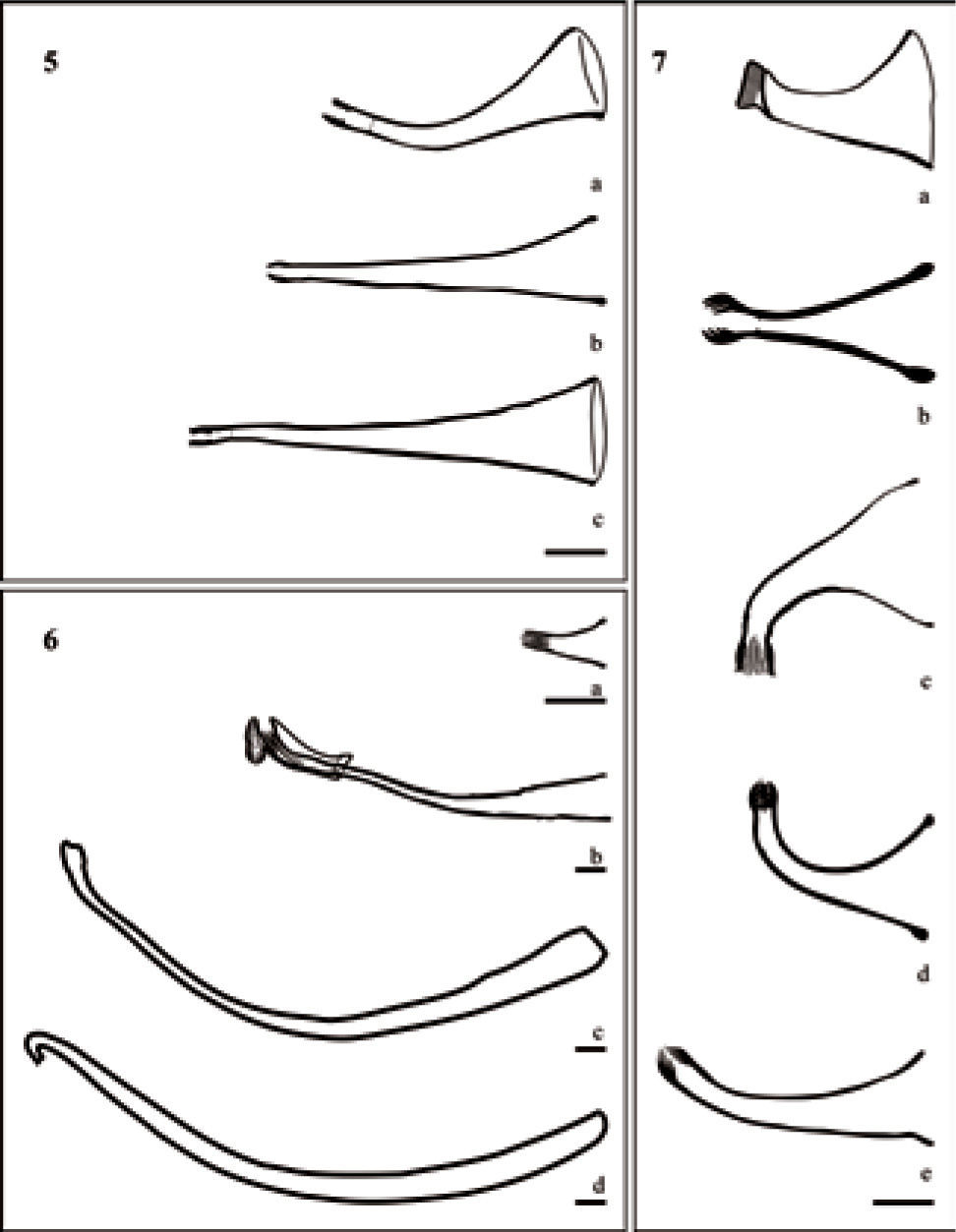
The new species shows a distinctive cirrus shape, unlike the other species, it is curved towards the hindbody, approximately in 90°. In comparison, T. haswelli, T. iheringi, T. lamothei and T. rochensis show cirrus curved towards the forebody (Figs. 7a-b, d-e). The cirrus of T. iheringi and T. lamothei are similar in length and shaped (Figs. 7a-b), with 1 flat and 1 concave side (Damborenea and Brusa, 2008). The cirrus of T. rochensis, T. haswelli and T. colombiensis n. sp. are similar in length and shape too (Figs. 7c-e), all of them with both sides curved. Temnocephala haswelli resembles the new species in the cirrus shape (curvature approximately 90°), total length (215μm and 213μm respectively) and shaft base width of cirrus (122μm and 119μm respectively), however both differs in curvature direction in relation to the body. The ratios TBL/TLC (7.6:1) and TLC/WSB (1.8: 1) from T. colombiensis n. sp. indicate that it cirrus is larger with relation to the body size, and that the width of its shaft base respect to cirrus length is wider.
Temnocephala colombiensis n. sp. shows introvert's swelling with 20 to 26 longitudinal rows of fine spines and 11–13 spines per row. Swelling is a trait described in temnocephalans ampullariids, excepting T. lamothei, and the number of longitudinal rows and spines per row is different in all of them: T. haswelli 18 and 4; T. iheringi 28 and 7; T. rochensis 22 and 6; T. lamothei 45–50 and 2, respectively.
The new species shows DLSPs rectangular with rounded corners, with a slight intraspecific variation (Figs. 8d-g), and excretory pores eccentric, displaced to the anterior portion of the plate. DLSP of molluskan temnocephalans has been described in T. haswelli, T. iheringi and T. rochensis, and these differ from the new species in shape and pore location (Figs. 8a-e).
Like the majority of temnocephalans, T. colombiensis n. sp. has eyespots with red pigment, which are present in T. haswelli (Seixas et al., 2010c) and T. rochensis (Seixas et al., 2010b), absent in T. iheringi (Seixas et al., 2010a) and not described in T. lamothei. The new species attaches its eggs onto the host's umbilicus and the basal region of the operculum, as was described in T. lamothei, however this one differs because some eggs were fixed within spire (Damborenea and Brusa, 2008). Temnocephala iheringi, T. haswelli and T. rochensis attach their eggs onto the umbilicus, suture and spire, never on the operculum (Seixas et al., 2010a; 2010b; 2010c), sometimes T. haswelli and T. rochensis attach their eggs in the body whorl of the host shell (Seixas et al., 2010b; 2010c). In the present study all temnocephalans associated with Pomacea sp. correspond to T. colombiensis n. sp. In contrast, T. iheringi reported in Rio Grande do Sul, has always been found in concurrent cohabitation with either T. haswelli or T. rochensis (Seixas et al., 2010a).
DiscussionSpecies of Temnocephala are commensals of crustaceans, mollusks, insects and turtles (Damborenea and Cannon, 2001). The present study describes for the first time a Temnocephala from Colombia, corresponding to the fifth described from ampullariids host, which are restricted to South America, in particular to Uruguay, Brazil and Argentina. These species show similar traits in cirrus length and a no-septated intestine. The absence of paranephrocytes is a common trait on commensals of ampullariids (Damborenea and Brusa, 2008). However, T. colombiensis n. sp. show paired disc glands, T. iheringi show 2 pairs to large disc glands (Seixas et al., 2010a) and T. lamothei show adhesive disc glands that are scarce and scattered, under to the posterior testis (Damborenea and Brusa, 2008). These discs glands are referred to as central cells or paranephrocytes in temnocephlans of crustaceans (Amato et al., 2010; Seixas et al., 2011). It is unclear if the disc glands described as paranephrocytes in crustaceans are homologous to discs glands described in temnocephalans of mollusks.
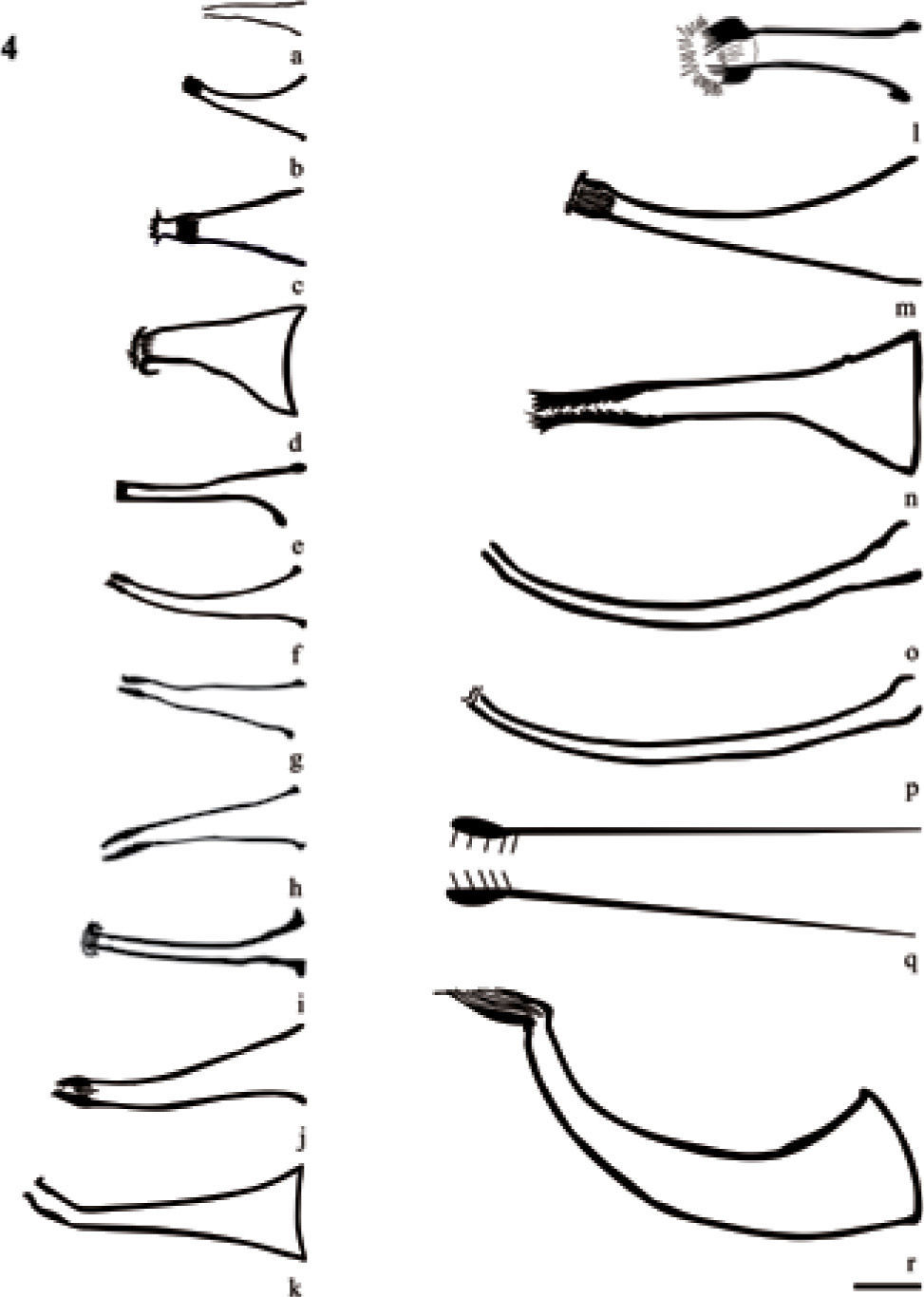
The cirrus remains the main taxonomic character for the identification of species of Temnocephala, this is a hard structure and relatively non-deformable (Damborenea, 1991), that sometimes can show slight intraspecific variation. In the present study the cirrus shape and direction with respect to the body of 30 temnocephalans species was compared (except T. peruensis, which has no detailed drawing of the cirrus). In general the cirrus are variable in size and shape, the majority are described as conical, straight or curved and with direction toward the hindbody, except for T. talicei (Fig. 4h) and T. colombiensis n. sp. (Fig. 7c), with cirrus curved toward the forebody. Temnocephalans of crustaceans show morphological variations in shape, cirrus length and width of shaft base; these variations are more evident due to the number of species described (Fig. 4). Temnocephalans of hemipterans show extra-long and curved cirrus, except T. minutocirrus which have the smallest cirri described to date within the genus (Fig. 6). The remaining groups (mollusks and turtles) are homogeneous in the cirrus total length and the width of shaft base, but differ in shape. In this regard, T. colombiensis n. sp. has a cirrus highly curved towards hindbody when it is observed in ventral and dorsal view.
Cirrus structure of temnocephalans of crustaceans. a), Temnocephala pignalberiae (Damborenea 1992); b), T. lutzi (Amato et al., 2005); c), T. longivaginata (Seixas et al., 2011); d), T. costaricensis (Lamothe-Argumedo, 1974); e), T. lanei (Pereira and Cuocolo, 1941); f), T. axenos (Volonterio, 2007); g), T. mertoni (Volonterio, 2007); h), T. talicei (Volonterio, 2009); i), T. trapeziformis (Amato et al., 2006); j), T. kingsleyae (Damborenea, 1994); k), T. mexicana (Lamothe-Argumedo, 1968); l), T. chilensis (Damborenea, 1992); m), T. cyanoglandula (Amato et al., 2003); n), T. digitata (Damborenea, 1992); o), T. microdactyla (Damborenea, 1992); p), T. santafesina (Damborenea, 1992); q), T. brenesi (Jennings, 1968); r), T. travassofilhoi (Pereira and Cuocolo, 1941); bar = 50μm.
Figure 5. Cirrus structure of temnocephalans of turtles. a), Temnocephala cuocoloi (Volonterio, 2010); b), T. brevicornis (Ferreira Yuki et al., 1993); c), T. pereirai (Volonterio 2010); bar = 50μm.
Cirrus structure of temnocephalans of turtles. a), Temnocephala cuocoloi (Volonterio, 2010); b), T. brevicornis (Ferreira Yuki et al., 1993); c), T. pereirai (Volonterio 2010); bar = 50μm.
Figure 6. Cirrus structure of temnocephalans of hemipterans. a), Temnocephala minutocirrus (Amato et al., 2007), bar 50=μm; b), T. caddisflyi (Amato et al., 2011); c), T. curvicirri (Amato and Amato, 2005); d), T. decarloi (Moreto, 1978); bar = 50μm.
Figure 7. Cirrus structure of temnocephalans of mollusks. a), Temnocephala lamothei (Damborenea and Brusa, 2008); b), T. iheringi (Damborenea, 1992); c), T. colombiensis n. sp.; d), T. haswelli (Seixas et al., 2010c); e), T. rochensis (Ponce de León, 1979); bar = 50μm.
The cirrus features and the combination with other characters of taxonomic importance observed in recent papers (such as excretory syncytial plates DLSPs, and position of excretory pores) facilitate the identification of species within this genus. The DLSPs are considered a diagnostic character, however they have only been described in 22 species of Temnocephala (Damborenea and Cannon, 2001; Amato et al., 2003, 2006, 2007, 2010, 2011; Amato and Amato, 2005; Volonterio, 2007, 2009, 2010; Seixas et al., 2010a, 2010b, 2010c, 2011) including T. colombiensis n. sp., which shows rectangular DLSPs with rounded corners and excretory pores eccentric and displaced to the anterior portion of the plate (Fig. 8f).
Representation of dorsolateral excretory syncytial plates shape of neotropical temnocephalans from mollusks. a), Temnocephala rochensis (Seixas et al., 2010b); b), T. haswelli (Seixas et al., 2010c); c), T. iheringi (Seixas et al., 2010a); d-e), T. colombiensis n. sp.; f), T. colombiensis n. sp. photography of typical DLSP shape; g), T. colombiensis n. sp. showing slight intraspecific variation in DLSP shape; bar = 50μm. Black arrows indicate the limits of the plate, white arrows indicate the eccentric excretory pores.
The ampullariidae are freshwater snails predominantly distributed in aquatic tropical and subtropical habitats in Asia, Africa, Central and South America (Cowie and Thiengo, 2003). Their wide distribution, diversity of habitatsand special association with temnocephalans, suggests that the number of Temnocephala species may increase in the next years. To date the temnocephalans of mollusks were restricted to southeastern the part of South America, and this present study increases their geographical distribution towards the northern area of South America.
To Rafael Lamothe-Argumedo, Rodrigo Ponce de León, Odile Volonterio for their advice and providing bibliography. To José Felipe Amato for providing bibliography. To Silvana A. Thiengo for her advice in the Pomacea sp. identification. This study was founded by the Comité para el Desarrollo de Investigación (CODI E-01246) and PECET, under the project entitled “Evaluación del valor nutritivo y de variables biológicas en dos poblaciones de Pomacea (Mollusca: Pilidae) de Antioquia”, Universidad de Antioquia.