In assessing the health of rivers, the standardization of environmental and biological information as a baseline is essential in order to determine the set of conditions that are closest to the natural state of ecosystems. This is the case especially in peri-urban rivers where anthropogenic transformations occur rapidly and constantly. The objective of this work was to determine hydromorphological, physicochemical and biological parameters in 10 mountain rivers of the Mexico Basin, in order to establish a network of potential reference conditions and to validate the regional ecological quality. The potential reference conditions in this study are defined as oligotrophic water bodies with well-oxygenated concentrations and low ion concentrations. These conditions were recorded in 4 sub-basins with high hydromorphological quality. These results were corroborated through a base assembly composed of the macroinvertebrate families Baetide, Chrironomidae, Dugesiidae, Heptageniidae, Limnephilidae, Tipulidae and the class Arachnida (Acarina). The algal community was represented by Nostoc parmelioides, Placoma regulare, Batrachospermum gelatinosum, Paralemanea mexicana, Draparnaldia mutabilis, Prasiola mexicana and Vaucheria bursata. The major disturbances were structural changes in the riverbed that affect the structure and function of rivers.
El establecimiento de líneas base de información biológica y ambiental son fundamentales para la determinación de la salud actual de los ríos y las condiciones que se acercan a su naturalidad, en especial en sistemas periurbanos donde la transformación antrópica es rápida y constante. El objetivo del estudio fue evaluar un conjunto de parámetros hidromorfológicos, fisicoquímicos y biológicos en 10 ríos de montaña de la cuenca de México, para reconocer las características que definen las condiciones de referencia potenciales y el estatus de calidad ecológica en la región. Las condiciones de referencia potenciales fueron definidas por aguas oligotróficas, bien oxigenadas y de baja concentración iónica, condiciones registradas en 4 subcuencas que mantienen características hidromorfológicas naturales. Estos resultados fueron corroborados a través del ensamble base de macroinvertebrados, integrado por las familias Baetide, Chironomidae, Dugesiidae, Heptageniidae, Limnephilidae, Tipulidae y la clase Arachnida (Acarina). La comunidad algal característica estuvo representada por las especies resilentes Nostoc parmelioides, Placoma regulare, Batrachospermum gelatinosum, Paralemanea mexicana, Draparnaldia mutabilis, Prasiola mexicana y Vaucheria bursata. Las alteraciones más importantes son modificaciones estructurales del caudal que afectan la estructura y función de los ríos.
The study of aquatic resources associated with large urban centers is of vital importance, because they provide 3 of the most important ecosystem services related to human well-being: water purification and provision, retention of biodiversity in terrestrial ecosystems, and regional climatic regulation (Niemelä et al., 2010). In this sense, aquatic ecosystems have a great influence on the cultural and economic aspects of the local human communities, mainly because they are subject to a broad array of public policies and strategies aiming to manage the aquatic resources through the construction of dams, diversion of waterways, generation of power, and extraction of in situ water (Perló & González, 2005). Some of these structural interventions are physical stressors that alter the ecosystem, including the associations within the biological communities (Caro-Borrero, Carmona-Jiménez, González-Martínez & Mazari-Hiriart, 2015). Physical alteration of ecosystems can have an even greater impact than some alterations in water chemistry; even though local regulations are based on chemical parameters assessment (Acosta, Ríos, Rieradevall, & Prat, 2009; Caroni, van de Bund, Clarke, & Johnson, 2013).
The Metropolitan Area of the Mexico Basin has grown exponentially during the past 6 decades. Mechanisms employed to supply water to this area are based mainly on extraction of deep water from the aquifer and importation of water from neighboring basins (Perló & González, 2005). Currently, the aquifer is overexploited and water importation is a complex and expensive process for the city. An alternative management program designed to preserve the aquatic ecosystems, as well as to provide an adequate supply and distribution of water in the region should be based on a comprehensive and sustainable approach toward the surface water resources (Legorreta, 2009). This will necessitate assessment of the health of the rivers to determine the set of conditions that most closely resemble the natural state; this requires a record of chemical, physical and biological parameters at several sites with similar physical features that represent the least disturbed conditions and provide an estimate of the natural variability in biological conditions and habitat quality (Acosta et al., 2009; Cortés, Hughes, Rodríguez-Pereira, & Pinto-Varandas, 2013). Furthermore, information about biological communities could be translated into indicators of hydrological ecosystem function (Caro-Borrero, Carmona-Jiménez, & Mazari-Hiriart, 2015).
The ecological reference conditions are outlined as an environment with few anthropogenic pressures and minimal ecological impacts, and are not necessarily representative of pristine environments (Wallin, Wiederholm, & Johnson, 2003). In this sense, the greatest challenge in the selection of sites to determine the reference conditions is finding an approach that allows unification of a range of criteria to be combined in its characterization. The breadth of the concept and the freedom in the selection of parameters and assessment methods limit the comparison of results and the potential setting of regional patterns (Pardo et al., 2012). This highlights the need for intercalibration based on local characteristics of ecosystems in order to facilitate determination of the evaluation criteria and thresholds for rejection or acceptance of the parameters measured (Pardo et al., 2012).
The final step is the validation of physicochemical data through the composition of the biological community, a complex task in regions where pristine ecosystems are practically nonexistent; consequently, a biological baseline has been established in places already affected by human activity (Friberg et al., 2011). Also, the methods based on community analysis that have been used to assess ecological quality have been applied mainly in developed countries, where environmental research is detailed and available, and biodiversity parameters are sufficiently precise and incorporated within ecological responses (Nijboer & Verdonschot, 2004). In Mexico, insufficient study of the criteria for evaluation of ecological quality has impeded establishment of a network of potential reference sites.
The objective of this study was to evaluate a set of parameters, including hydromorphological, physicochemical and biological information about the mountain rivers of the Mexico Basin, in order to define the ecological quality at a regional scale and to establish a baseline that will allow reference conditions to be formulated.
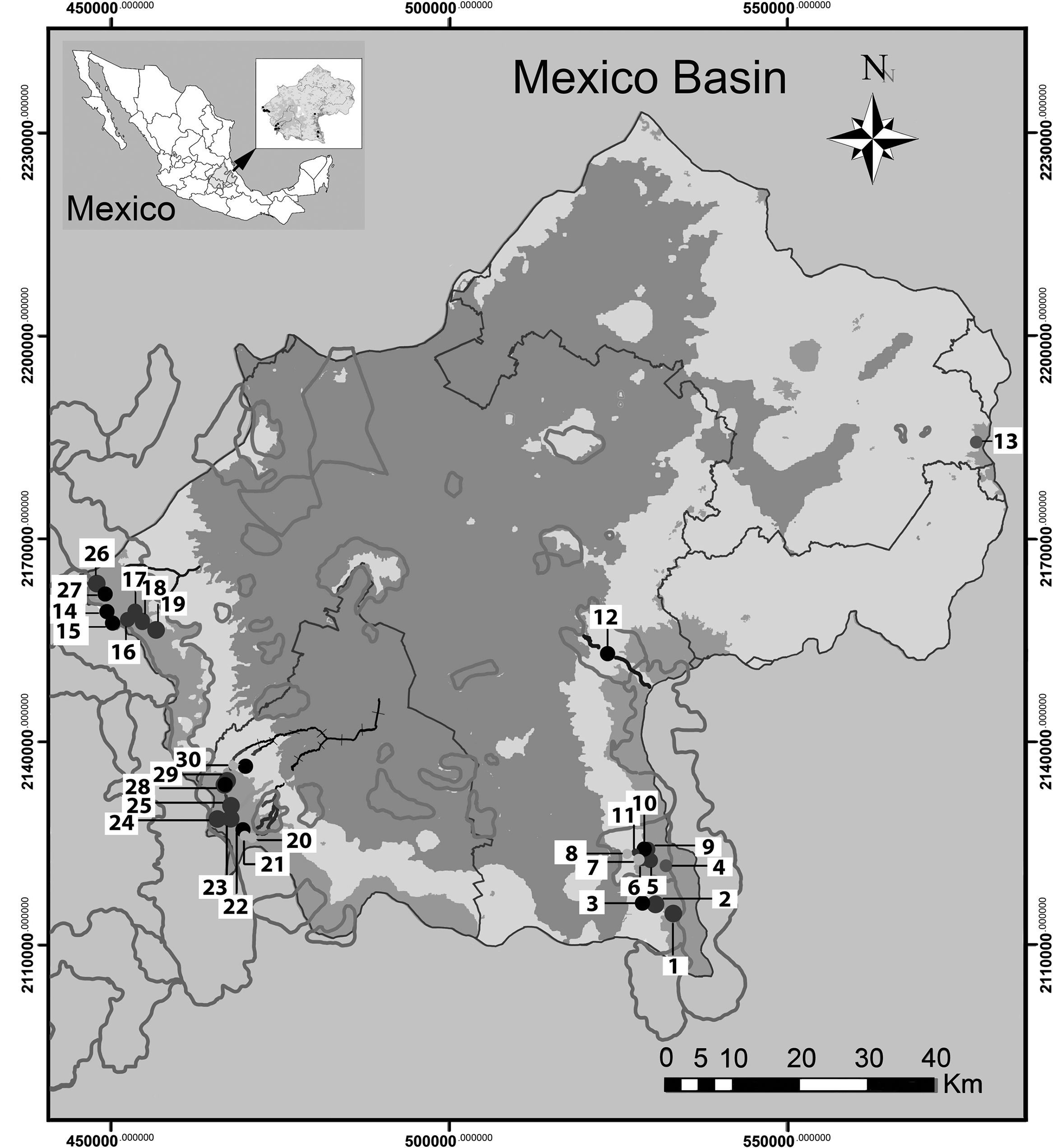
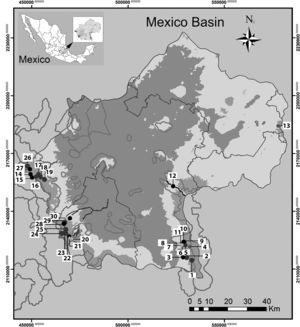
Materials and methodsThe Mexico Basin (Fig. 1) lies in the morphotectonic region of the Trans-Mexican Volcanic Belt at 19°00′–19°40′ N, 98°30′–99°30′ W and has a total surface area of 9,600km2, of which 5,518km2 are mountain ranges that rise above 2,400m asl. (Ferrusquía-Villafranca, 1998; Legorreta, 2009). The climate of the region is sub-moist and temperate (annual median temperature 13.4°C, annual median precipitation between 1,200 and 1,500mm), with abundant rains from June to October and a dry season from November to May (García, 2004). Its geological traits consist of rock pockets alternating with andesitic to basaltic lavas (Ferrusquía-Villafranca, 1998), above which forests of Abies religiosa, Pinus hartwegii and Quercus spp. grow in the upper area of the watershed, with mixed forests in the middle and lower areas (Ávila-Akerberg, 2010). Thirty sites were selected, representing 10 sub-basins with perennial rivers. The areas considered as potential reference sites were preselected on the grounds of minimal human intervention in forested areas with some legal conservation status, such as a protected area and/or soil conservation reserves at the headwaters; this employed updated cartographic information on land use and a mapped hydrological network at 1:50,000 (GDF, 2012; Inegi, 2013). Once sampled, the reference conditions were validated in a post-selection step. This entailed measurement of the physicochemical traits of the water and its hydromorphological quality, and validation of the benthic macroinvertebrates and macroscopic algae communities.
Sampling sites in the Mexico Basin; gray shading, soil conservation areas. Numbers indicate sites referred in Table 1. La Castañeda alto. 2. La Castañeda Cascada. 3. La Castañeda bajo. 4. San Rafael. 5. San Rafael Vereda. 6. San Rafael Canal. 7. Canal San Rafael. 8. Agua dulce. 9. Inicio Canal San Rafael. 10. Cascada Compañía. 11. Cosamala. 12. Santa Catarina. 13. Rancho nuevo. 14. Los Organillos. 15. Nacimiento Presa Iturbide. 16. Manantial Capoxi canal. 17. Río Capoxi. 18. Manantial San Pedro. 19. Xopachi. 20. Monte Alegre alto. 21. Monte Alegre bajo. 22. Manantial Eslava. 23. Chautitle alto. 24. Chautitle cañada. 25. Truchero alto. 26. Las Palomas. 27. Truchero Don Alvaro. 28. Santa Rosa manantial. 29. Santa Rosa alto. 30. Santa Rosa media.
Sampling was carried out between March 2012 and June 2015, during the rainy season (June-November), dry cold (December-February) and warm dry season (March-May). The following physicochemical parameters were recorded in situ with a Hanna Multiparameter probe 991300 (Dallas, USA): water temperature, specific conductivity and pH. Also, oxygen saturation (YSI-85 meter, YSI, Ohio, USA) and current velocity (Global Water FP111, Texas, USA) were recorded. Stream discharge was calculated according to Gore (1996). At each sampling station, 500ml samples of water were filtered in situ and analyzed in the laboratory according to the criteria established in the official Mexican guidelines and international standards (APHA, 2005; DOF, 2003). Nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), ammonium nitrogen (NH4-N), dissolved inorganic nitrogen (DIN) and soluble reactive phosphorus (SRP, in theory mostly in the form of orthophosphate, PO4-P) were analyzed with a DR 3900 laboratory Spectrophotometer (Hach, Loveland, CO; Hach, 2003). The criteria used to validate the water quality and its fitness for human contact were those of the Mexican norm (DOF, 2003). Hydromorphological quality (HQ) and anthropogenic activities were evaluated and adapted from the Ecological Quality of Andean Rivers Index (Acosta et al., 2009), which uses a scale of 24–120 points to classify the naturalness of the fluvial habitat in high-mountain streams; sites with values higher than 100 were considered as potential reference sites. The conservation status of riparian vegetation was evaluated according to local descriptions (Ávila-Akerberg, 2010; Espinosa & Sarukán, 1997; Rzedowski & Rzedowski, 2001).
The macroinvertebrates sampling points were selected at each sampling location, following a multihabitat criterion and using a Surber-type D-net with 250μm mesh and a 30cm width. Sampling was performed along a 10m transect. Sediment was removed by kicking during a 5-minute period and organisms were moved to a tray for extraction. Organisms were also caught by manual examination and extraction from the submerged faces of large rocks, pieces of dead wood and leaves. At least 100 individuals were collected from each sampled site as a representative and preserved in 70% alcohol. Individuals were separated out under an Olympus SZX7 stereoscopic microscope (Olympus Corporation, Tokyo, Japan) and identified to family level with reference to Bueno-Soria (2010), DeWalt, Resh, and Hilsenhoff (2010), Merritt, Cummins, and Berg (2008) and Voshell (2010).
The macroscopic algae sampling consisted of 5 quadrats, each separated by 2m. Quadrats were positioned within each site on areas with >1% of algal cover. Their direction and localization was chosen randomly in an interval between 0° and 180°. This procedure was repeated along the sampling quadrats (in an upstream direction). The abundance of macroscopic algae (percentage cover) was evaluated with a circular sampling unit of 10cm radius (area 314cm2) (Bojorge, Carmona-Jiménez, Cartajena, & Beltrán, 2010; Necchi, Branco, & Branco, 1995. Algae were identified to species level by reference to taxonomic keys and bibliographic resources (Anagnostidis & Komárek, 2005; Carmona-Jiménez & Necchi, 2002; Carmona-Jiménez & Vilaclara, 2007; Ettl & Gartner, 1988; Komárek, 2013; Rieth, 1980; Wher & Sheath, 2003). For taxonomic analyses, an Olympus BX51 microscope with an SC35 microphotography system was used.
Hydromorphological and physicochemical parameters were used to establish ecological quality and the possible source of degradation of the aquatic communities. Relationships between physicochemical parameters, hydromorphological quality and biological diversity were evaluated with agglomerative hierarchical clustering (AHC, Euclidean distance and UPGMA arithmetic mean). Mean values for each season were transformed to natural logarithm (x+1). In order to be considered, percentage algal cover and relative abundance in macroinvertebrates had to be greater than 1% in all the sampling sites. Species diversity was assessed by the Shannon-Wiener diversity test (Hlog10). Numerical analyses were performed with XLSTAT software (Addinsoft, 2003).
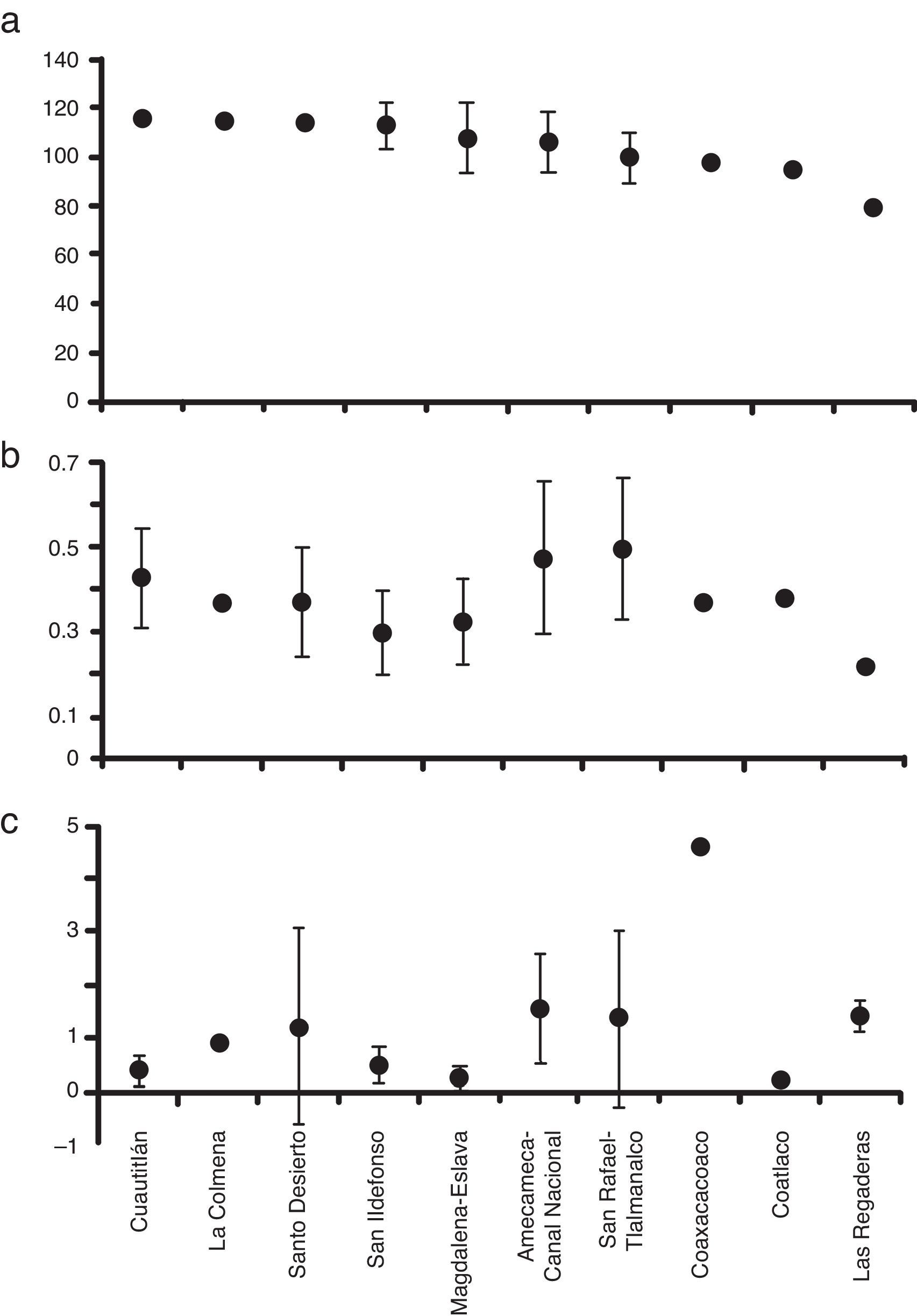
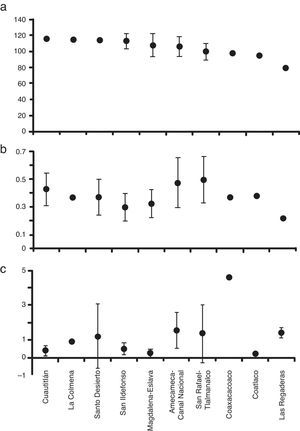
ResultsEnvironmental characterizationThe streams within the Mexico Basin showed relatively stable physicochemical characteristics (Table 1). The water temperature was temperate (5–17.6°C), with variable dissolved oxygen values (41–129%), low specific conductivity (35–255μScm−1), and slightly acidic pH (4–7.5) due to the low-mineralized basaltic substratum. According to HQ assessment, 7 sub-basins had potential reference conditions (up to 100 points), and 3 sub-basins presented poor HQ conditions (Fig. 2a). According to the Mexican norms (DOF, 2003), the nutrient concentrations were within the category “permissible for direct human contact” although point sources of pollution were detected through their high values of SRP (Fig. 2b) and NID (Fig. 2c).
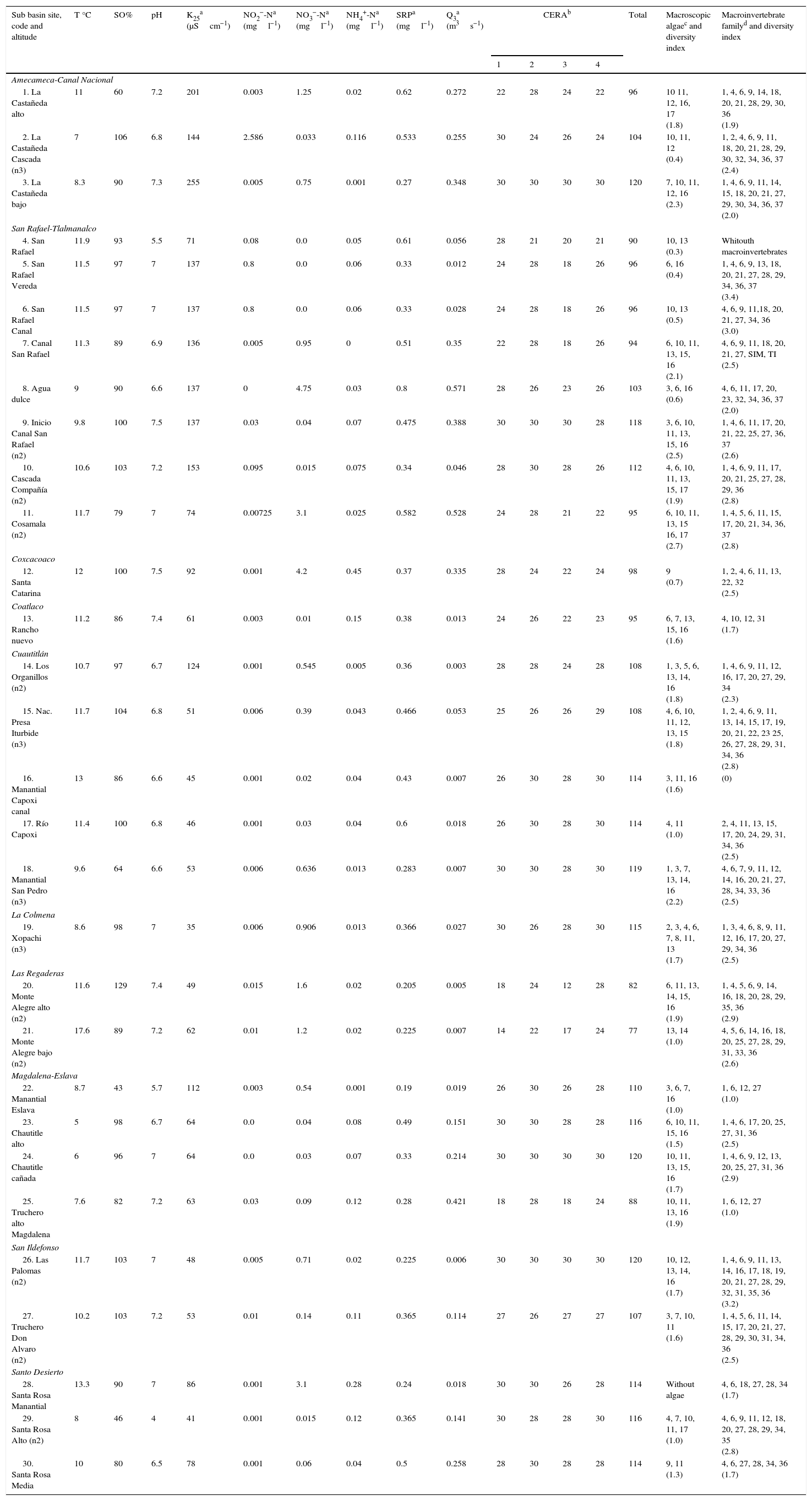
Physicochemical and biological characteristics of sites selected as potential reference sites in the Mexico Basin.
| Sub basin site, code and altitude | T °C | SO% | pH | K25a (μScm−1) | NO2−-Na (mgl−1) | NO3−-Na (mgl−1) | NH4+-Na (mgl−1) | SRPa (mgl−1) | Q3a (m3s−1) | CERAb | Total | Macroscopic algaec and diversity index | Macroinvertebrate familyd and diversity index | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||||||||
| Amecameca-Canal Nacional | ||||||||||||||||
| 1. La Castañeda alto | 11 | 60 | 7.2 | 201 | 0.003 | 1.25 | 0.02 | 0.62 | 0.272 | 22 | 28 | 24 | 22 | 96 | 10 11, 12, 16, 17 (1.8) | 1, 4, 6, 9, 14, 18, 20, 21, 28, 29, 30, 36 (1.9) |
| 2. La Castañeda Cascada (n3) | 7 | 106 | 6.8 | 144 | 2.586 | 0.033 | 0.116 | 0.533 | 0.255 | 30 | 24 | 26 | 24 | 104 | 10, 11, 12 (0.4) | 1, 2, 4, 6, 9, 11, 18, 20, 21, 28, 29, 30, 32, 34, 36, 37 (2.4) |
| 3. La Castañeda bajo | 8.3 | 90 | 7.3 | 255 | 0.005 | 0.75 | 0.001 | 0.27 | 0.348 | 30 | 30 | 30 | 30 | 120 | 7, 10, 11, 12, 16 (2.3) | 1, 4, 6, 9, 11, 14, 15, 18, 20, 21, 27, 29, 30, 34, 36, 37 (2.0) |
| San Rafael-Tlalmanalco | ||||||||||||||||
| 4. San Rafael | 11.9 | 93 | 5.5 | 71 | 0.08 | 0.0 | 0.05 | 0.61 | 0.056 | 28 | 21 | 20 | 21 | 90 | 10, 13 (0.3) | Whitouth macroinvertebrates |
| 5. San Rafael Vereda | 11.5 | 97 | 7 | 137 | 0.8 | 0.0 | 0.06 | 0.33 | 0.012 | 24 | 28 | 18 | 26 | 96 | 6, 16 (0.4) | 1, 4, 6, 9, 13, 18, 20, 21, 27, 28, 29, 34, 36, 37 (3.4) |
| 6. San Rafael Canal | 11.5 | 97 | 7 | 137 | 0.8 | 0.0 | 0.06 | 0.33 | 0.028 | 24 | 28 | 18 | 26 | 96 | 10, 13 (0.5) | 4, 6, 9, 11,18, 20, 21, 27, 34, 36 (3.0) |
| 7. Canal San Rafael | 11.3 | 89 | 6.9 | 136 | 0.005 | 0.95 | 0 | 0.51 | 0.35 | 22 | 28 | 18 | 26 | 94 | 6, 10, 11, 13, 15, 16 (2.1) | 4, 6, 9, 11, 18, 20, 21, 27, SIM, TI (2.5) |
| 8. Agua dulce | 9 | 90 | 6.6 | 137 | 0 | 4.75 | 0.03 | 0.8 | 0.571 | 28 | 26 | 23 | 26 | 103 | 3, 6, 16 (0.6) | 4, 6, 11, 17, 20, 23, 32, 34, 36, 37 (2.0) |
| 9. Inicio Canal San Rafael (n2) | 9.8 | 100 | 7.5 | 137 | 0.03 | 0.04 | 0.07 | 0.475 | 0.388 | 30 | 30 | 30 | 28 | 118 | 3, 6, 10, 11, 13, 15, 16 (2.5) | 1, 4, 6, 11, 17, 20, 21, 22, 25, 27, 36, 37 (2.6) |
| 10. Cascada Compañía (n2) | 10.6 | 103 | 7.2 | 153 | 0.095 | 0.015 | 0.075 | 0.34 | 0.046 | 28 | 30 | 28 | 26 | 112 | 4, 6, 10, 11, 13, 15, 17 (1.9) | 1, 4, 6, 9, 11, 17, 20, 21, 25, 27, 28, 29, 36 (2.8) |
| 11. Cosamala (n2) | 11.7 | 79 | 7 | 74 | 0.00725 | 3.1 | 0.025 | 0.582 | 0.528 | 24 | 28 | 21 | 22 | 95 | 6, 10, 11, 13, 15 16, 17 (2.7) | 1, 4, 5, 6, 11, 15, 17, 20, 21, 34, 36, 37 (2.8) |
| Coxcacoaco | ||||||||||||||||
| 12. Santa Catarina | 12 | 100 | 7.5 | 92 | 0.001 | 4.2 | 0.45 | 0.37 | 0.335 | 28 | 24 | 22 | 24 | 98 | 9 (0.7) | 1, 2, 4, 6, 11, 13, 22, 32 (2.5) |
| Coatlaco | ||||||||||||||||
| 13. Rancho nuevo | 11.2 | 86 | 7.4 | 61 | 0.003 | 0.01 | 0.15 | 0.38 | 0.013 | 24 | 26 | 22 | 23 | 95 | 6, 7, 13, 15, 16 (1.6) | 4, 10, 12, 31 (1.7) |
| Cuautitlán | ||||||||||||||||
| 14. Los Organillos (n2) | 10.7 | 97 | 6.7 | 124 | 0.001 | 0.545 | 0.005 | 0.36 | 0.003 | 28 | 28 | 24 | 28 | 108 | 1, 3, 5, 6, 13, 14, 16 (1.8) | 1, 4, 6, 9, 11, 12, 16, 17, 20, 27, 29, 34 (2.3) |
| 15. Nac. Presa Iturbide (n3) | 11.7 | 104 | 6.8 | 51 | 0.006 | 0.39 | 0.043 | 0.466 | 0.053 | 25 | 26 | 26 | 29 | 108 | 4, 6, 10, 11, 12, 13, 15 (1.8) | 1, 2, 4, 6, 9, 11, 13, 14, 15, 17, 19, 20, 21, 22, 23 25, 26, 27, 28, 29, 31, 34, 36 (2.8) |
| 16. Manantial Capoxi canal | 13 | 86 | 6.6 | 45 | 0.001 | 0.02 | 0.04 | 0.43 | 0.007 | 26 | 30 | 28 | 30 | 114 | 3, 11, 16 (1.6) | (0) |
| 17. Río Capoxi | 11.4 | 100 | 6.8 | 46 | 0.001 | 0.03 | 0.04 | 0.6 | 0.018 | 26 | 30 | 28 | 30 | 114 | 4, 11 (1.0) | 2, 4, 11, 13, 15, 17, 20, 24, 29, 31, 34, 36 (2.5) |
| 18. Manantial San Pedro (n3) | 9.6 | 64 | 6.6 | 53 | 0.006 | 0.636 | 0.013 | 0.283 | 0.007 | 30 | 30 | 28 | 30 | 119 | 1, 3, 7, 13, 14, 16 (2.2) | 4, 6, 7, 9, 11, 12, 14, 16, 20, 21, 27, 28, 34, 33, 36 (2.5) |
| La Colmena | ||||||||||||||||
| 19. Xopachi (n3) | 8.6 | 98 | 7 | 35 | 0.006 | 0.906 | 0.013 | 0.366 | 0.027 | 30 | 26 | 28 | 30 | 115 | 2, 3, 4, 6, 7, 8, 11, 13 (1.7) | 1, 3, 4, 6, 8, 9, 11, 12, 16, 17, 20, 27, 29, 34, 36 (2.5) |
| Las Regaderas | ||||||||||||||||
| 20. Monte Alegre alto (n2) | 11.6 | 129 | 7.4 | 49 | 0.015 | 1.6 | 0.02 | 0.205 | 0.005 | 18 | 24 | 12 | 28 | 82 | 6, 11, 13, 14, 15, 16 (1.9) | 1, 4, 5, 6, 9, 14, 16, 18, 20, 28, 29, 35, 36 (2.9) |
| 21. Monte Alegre bajo (n2) | 17.6 | 89 | 7.2 | 62 | 0.01 | 1.2 | 0.02 | 0.225 | 0.007 | 14 | 22 | 17 | 24 | 77 | 13, 14 (1.0) | 4, 5, 6, 14, 16, 18, 20, 25, 27, 28, 29, 31, 33, 36 (2.6) |
| Magdalena-Eslava | ||||||||||||||||
| 22. Manantial Eslava | 8.7 | 43 | 5.7 | 112 | 0.003 | 0.54 | 0.001 | 0.19 | 0.019 | 26 | 30 | 26 | 28 | 110 | 3, 6, 7, 16 (1.0) | 1, 6, 12, 27 (1.0) |
| 23. Chautitle alto | 5 | 98 | 6.7 | 64 | 0.0 | 0.04 | 0.08 | 0.49 | 0.151 | 30 | 30 | 28 | 28 | 116 | 6, 10, 11, 15, 16 (1.5) | 1, 4, 6, 17, 20, 25, 27, 31, 36 (2.5) |
| 24. Chautitle cañada | 6 | 96 | 7 | 64 | 0.0 | 0.03 | 0.07 | 0.33 | 0.214 | 30 | 30 | 30 | 30 | 120 | 10, 11, 13, 15, 16 (1.7) | 1, 4, 6, 9, 12, 13, 20, 25, 27, 31, 36 (2.9) |
| 25. Truchero alto Magdalena | 7.6 | 82 | 7.2 | 63 | 0.03 | 0.09 | 0.12 | 0.28 | 0.421 | 18 | 28 | 18 | 24 | 88 | 10, 11, 13, 16 (1.9) | 1, 6, 12, 27 (1.0) |
| San Ildefonso | ||||||||||||||||
| 26. Las Palomas (n2) | 11.7 | 103 | 7 | 48 | 0.005 | 0.71 | 0.02 | 0.225 | 0.006 | 30 | 30 | 30 | 30 | 120 | 10, 12, 13, 14, 16 (1.7) | 1, 4, 6, 9, 11, 13, 14, 16, 17, 18, 19, 20, 21, 27, 28, 29, 32, 31, 35, 36 (3.2) |
| 27. Truchero Don Alvaro (n2) | 10.2 | 103 | 7.2 | 53 | 0.01 | 0.14 | 0.11 | 0.365 | 0.114 | 27 | 26 | 27 | 27 | 107 | 3, 7, 10, 11 (1.6) | 1, 4, 5, 6, 11, 14, 15, 17, 20, 21, 27, 28, 29, 30, 31, 34, 36 (2.5) |
| Santo Desierto | ||||||||||||||||
| 28. Santa Rosa Manantial | 13.3 | 90 | 7 | 86 | 0.001 | 3.1 | 0.28 | 0.24 | 0.018 | 30 | 30 | 26 | 28 | 114 | Without algae | 4, 6, 18, 27, 28, 34 (1.7) |
| 29. Santa Rosa Alto (n2) | 8 | 46 | 4 | 41 | 0.001 | 0.015 | 0.12 | 0.365 | 0.141 | 30 | 28 | 28 | 30 | 116 | 4, 7, 10, 11, 17 (1.0) | 4, 6, 9, 11, 12, 18, 20, 27, 28, 29, 34, 35 (2.8) |
| 30. Santa Rosa Media | 10 | 80 | 6.5 | 78 | 0.001 | 0.06 | 0.04 | 0.5 | 0.258 | 28 | 30 | 28 | 28 | 114 | 9, 11 (1.3) | 4, 6, 27, 28, 34, 36 (1.7) |
K25, Specific conductivity; Q3, Discharge; DIN, dissolved inorganic nitrogen; SRP, soluble reactive phosphorus.
Acosta et al. (2009): (I) riparian vegetation and naturality, (II) stream conservation state, (III) physiographic heterogeneity channel, (IV) pollution.
Macroscopic algae: 1. Batrachospermum gelatinosum. 2. Calothrix sp. 3. Cladophora glomerata. 4. Coleodesmium wrangelii. 5. Draparnaldia mutabilis. 6. Nostoc parmelioides. 7. Oedogonium sp. 8. Paralemanea mexicana. 9. Phormidium autumnale. 10. Placoma regulare. 11. Prasiola mexicana. 12. Rhizoclonium sp. 13, Spirogyra sp. 14. Tetraspora gelatinosa. 15. Ulothrix sp. 16. Vaucheria bursata. 17. ‘Chantransia’ stage of unidentified rhodophyte.
Macroinvertebrate families: 1. Arachnida (Acarina). 2. Ameletidae. 3. Athericidae. 4. Baetidae. 5. Ceratopogonidae. 6. Chironomidae. 7. Chysomelidae. 8. Cordulegastridae. 9. Dixidae. 10. Dryopidae. 11. Dugesiidae. 12. Dytiscidae. 13. Elmidae. 14. Empididae. 15. Ephydridae. 16. Gerridae. 17. Glacidorbidae. 18. Glossosomatidae. 19. Helicopsychidae. 20. Heptageniidae. 21. Hydrobiosidae. 22. Hydrophilidae. 23. Hydroptilidae. 24. Lepidostomatidae. 25. Leptoceridae. 26. Leptophlebiidae. 27. Limnephilidae. 28. Nemouridae. 29. Oligochaeta. 30. Perlolidae. 31. Polycentropodidae. 32. Psychodidae. 33. Saldidae. 34. Simuliidae. 35. Siphlonuridae. 36. Tipulidae. 37. Xiphocentronidae.
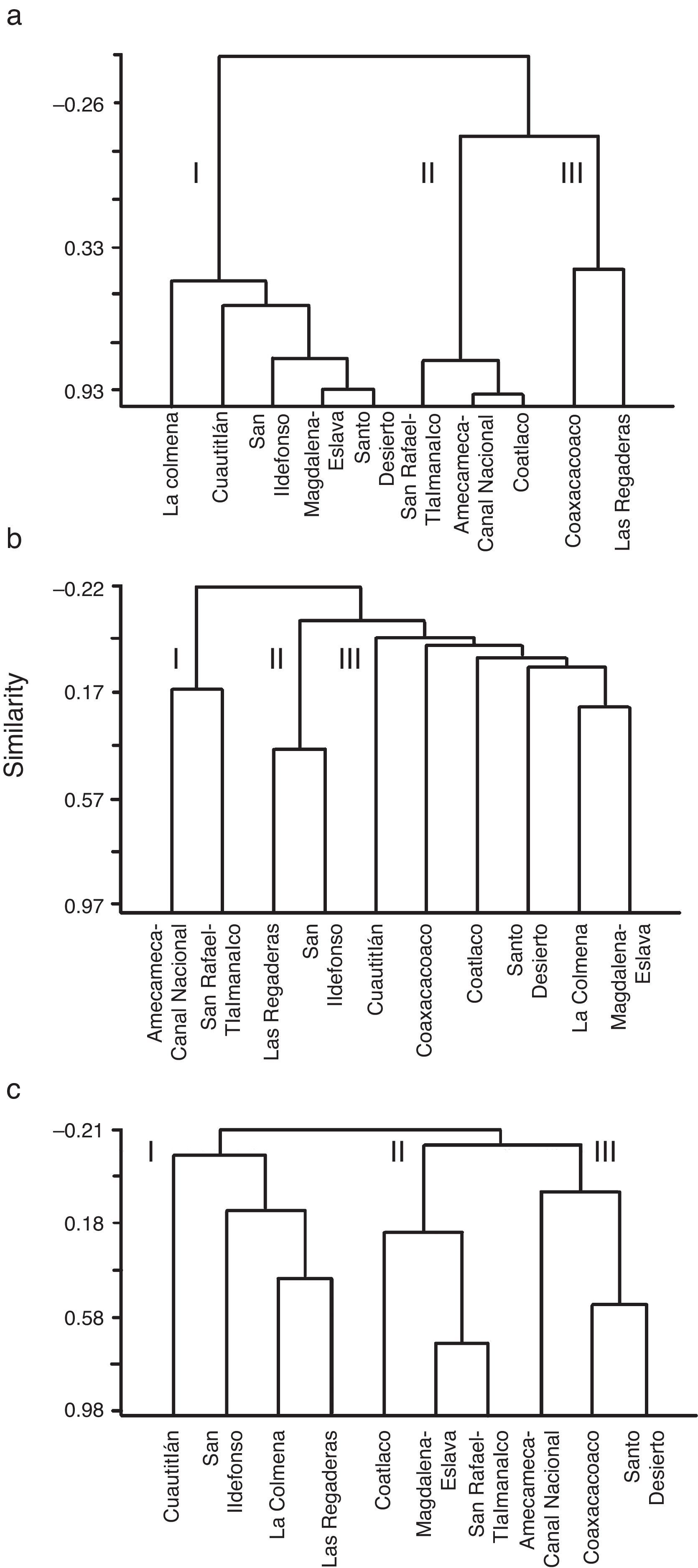
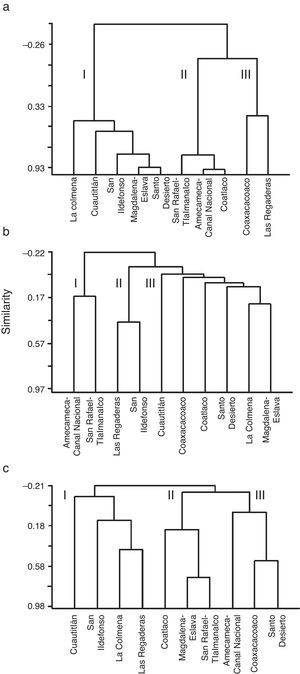
Taking into account the HQ assessment, nutrient concentration and AHC procedure, we established that 63% of sites exhibited potential reference conditions and we identified 3 main groups (Fig. 3a): 5 sub-basins in the western region; 3 sub-basins in the eastern region; and 2 sub-basins belonging to both regions, with the lowest HQ values and the highest concentrations of NO3-N. The sub-basins that had conserved a natural state were those that included at least 2 sites with high values of HQ (>100 points) at the headwater, namely Cuautitlán, La Colmena, Magdalena-Eslava and Santo Desierto. The other sub-basins had acceptable to poor HQ values (near or below 100 points) related to changes in the naturalness of riparian vegetation, modification of the structure of the channel with gabion dams, presence of human activities and the extraction and/or channeling of water.
The macroinvertebrates represented by 37 families, of which 30 had a high total abundance and wide distribution (Table 1). However, the diversity identified in Cuautitlán and San Ildefonso rivers represents 90% of all the families registered. In 18 sites, the family diversity index was high (H′=2.5–3.4); of these, 9 are classified as potential reference sites according to the HQ assessment and the low nutrient concentrations. The diversity of macroinvertebrates was fairly consistent with the grouping of the sub-basins according to HQ and nutrients, and again resulted in 3 groups (Fig. 3b): 4 sub-basins in the eastern region; 2 sub-basins in the west; and 2 sub-basins in the east.
The families Hydroptiliidae, Psychodidae, Lepidostomatidae, Cordulegastridae, Athericidae, Chysomedidae, Dityscidae, and Helicopsychidae were exclusive to potential reference sites. The first 2 families were found at only 1 site. A second group of representative organisms was recorded at 17 sites that had varying values of HQ and nutrients: Arachnida (Acarina) and families Dugesiidae, Baetidae, Chironomidae, Glossosomatidae, Heptageniidae, Limnephilidae, Tipulidae and Simuliidae. Finally, the Siphlonuridae and Dryopidae families were only present at sites without potential reference conditions and poor HQ values.
Records of the frequency and abundance of macroinvertebrates identified a representative assemblage of organisms in potential reference sites: Baetidae, Chironomidae, Dugesiidae, Heptageniidae, Limnephilidae, Tipulidae and Arachnida (Acarina). However, when Oligochaeta, Dryopidae, or Siphlonuridae were present in this assemblage the site was not considered to be a potential reference site.
Validation of macroscopic algaeThe 17 species-level taxa identified had a heterogeneous distribution and diversity, with 0–7 species per site (Table 1). The species diversity index was high (H′=1.5–2.7) at 18 sites. Of the 30 sites, 13 corresponded to sub-basins with potentially reference conditions according to the HQ assessment. The sub-basins of Cuautitlán and San Ildefonso (eastern region) presented the entire diversity of macroscopic algal species thus far described from the Mexico Basin. The diversity of macroscopic algae was consistent with the sub grouping obtained through the HQ and nutrient assessment, again resulting in 3 groups of sub-basins (Fig. 3c): 4 sub-basins in the eastern region with high HQ and low nutrient concentrations; 3 sub-basins (1 in the east and 2 in the west) with various HQ and nutrient concentrations; and 2 sub-basins in the east and 1 in the west, each with various HQ and nutrient concentrations.
The following species were representative of sites with high HQ values and low nutrient concentrations: Coleodesmium wrangelii, Calothrix sp., Nostoc parmelioides, Batrachospermum gelatinosum, Oedogonium sp., Spirogyra sp. and Tetraspora gelatinosa. In sites with wide variations in HQ and nutrient concentrations, the following species were identified: Placoma regulare, the ‘Chantransia’ stage of unidentified rhodophyte, Ulothrix sp., Prasiola mexicana, Rhizoclonium sp., Cladophora glomerata, and Vaucheria bursata. Two species were site-specific, Paralemanea mexicana and Draparnaldia mutabilis. A recurring assembly composed of N. parmelioides, P. regulare, Paralemaea mexicana and V. bursata was representative of sites with potential reference conditions.
DiscussionPotential reference conditionsThe rivers of the Mexico Basin share the same geological origin, as well as physicochemical characteristics that classify them as siliceous mountain rivers, and they are defined under the same fluvial typology. All the analyzed streams had a flow that was permanent but considerably variable, which can generally be attributed to seasonality. This condition is maintained up as far as 2,300m when the slope starts to flatten and floodplains begin to appear. Most of these floodplains have lost their natural state, even those within conservation areas (Legorreta, 2009). The potential reference conditions were defined by oligotrophic water with good oxygen concentrations and low ion concentrations but variation in HQ status. These patterns were recorded as follows: 4 sub-basins with HQ values higher than 100 points (Cuautitlán, La Colmena, Santo Desierto and San Idelfonso), and 3 sub-basins with sites with HQ values>100 points together with a few sites with HQ values<100 points (Magdalena-Esalava, Amecameca Canal Nacional and San Rafael Tlalmanalco).
One aspect of the HQ evaluation with important modifications was the riparian vegetation; in general, at the headwaters there was arborescent vegetation with mixed forest, pine forest, fir forest, and oak-pine forest. The natural state of riparian vegetation has a structural and functional effect on aquatic communities by providing shaded areas, substratum diversity (habitat availability and heterogeneity) and allochthonous organic matter as a food source (Acosta et al., 2009; Januschke, Jähnig, Lorenz, & Hering, 2014).
The most important causes of deterioration in the natural states of the rivers were the alteration of the riverbed structure and the presence of hydraulic infrastructure (dams, diversion channels and/or pipelining of springs). These alterations have been historically overlooked because environmental regulation in Mexico has mainly focused on detection and control of chemical and bacteriological contaminants (DOF, 2003). However, at the basin scale, physical alterations such as loss of heterogeneity or habitat fragmentation, and pressure on water resources due to extraction, constitute the main threats leading to environmental degradation (Ramussen et al., 2013). This occurred at the headwaters in the present study, where the nutrient concentrations in general did not exceed the values established by the local regulations for the protection of aquatic life. The highest nutrient concentrations were observed in the headwaters of the rivers Las Regaderas and Coaxacoaco, and might be associated with activities such as fish farming and livestock husbandry in the area (Caro-Borrero, Carmona-Jiménez, González-Martínez, et al., 2015; Legorreta, 2009).
Variable HQ values were recorded in the Amecameca-Canal Nacional, Magdalena-Eslava and San Rafael-Tlalmanalco sub-basins, in which riparian vegetation was replaced by trails and infrastructure to channel the course of rivers upstream. However, in the lower section in areas with a steep slope, the habitat had recovered with an increase in HQ values. This topographical feature can be linked to the difficulty of access to the rivers and therefore to minimal alterations to the riverbed and bank conditions.
In the remaining 3 sub-basins (Coaxacoaco, Coatlaco and Las Regaderas), with the lowest HQ values recorded in this study, the riparian vegetation is fragmented by a surface reduction intended for crops and isolated from the river channel by physical barriers and also by riverbed alterations.
Diversity of benthic macroinvertebrates and macroscopic algaeThe ecological features of the macroinvertebrate families and algal species (food and habitat preferences) defined these organisms as frequent inhabitants of oligotrophic mountain rivers. In general, the lowest values for macroinvertebrate family richness were related with sites in which modifications of the river channel structure had occurred, probably as a result of changes in the heterogeneity of the substratum and a decrease in water flow and regime velocity. These results are consistent with predictions of the “river continuum concept” (Vannote, Minshall, Cummins, Sedell, & Cushing, 1980), which states that in natural stream systems, biological communities of the headwaters form a temporal continuum of synchronized species replacements. Downstream communities are adapted to capitalize on upstream processing inefficiencies, and both the upstream inefficiency (represented by hydraulic intervention and organic pollution) and downstream adjustments can be predicted from the structure of macroinvertebrate assemblages and algal communities.
The representative assemblage of benthic macroinvertebrates associated with sites with good HQ status and permanent water flow, composed of Baetidae (gathering collectors), Dugesiidae (carnivores), Tipulidae (shredders and predators) and the Arachnida (Acarina) was consistent with other studies in this area that considered them to be good indicators of potential reference conditions (Caro-Borrero, Carmona-Jiménez, & Mazari-Hiriart, 2015). For example, the Baetidae can colonize diverse substrata and are usually associated with fast water currents, and with the highest HQ values since they feed on microalgae and particulate organic matter (Ozcos, Galicia, & Miranda, 2011). The Dugesiidae can tolerate changes related to weather seasonality, a trait linked to mountain river ecosystems (Hawking, Smith, LeBusque, & Davey, 2013). Organisms belonging to Heptageniidae (scrapers), Limnephilidae (shredders, in part) and Arachnida (Acarina) have been recorded in the headwaters of Mexico Basin of and are associated with oligotrophic conditions; the first 2 with high flow rates and the last with low rates (Caro-Borrero, Carmona-Jiménez, & Mazari-Hiriart, 2015). These families are frequently found to be sensitive to low DO concentrations and hence require clean and well-oxygenated waters (Bueno-Soria, 2010; Guilpart et al., 2012).
Regarding the Chironomidae (gathering collectors), in part because of the great species diversity and therefore their ability to colonize many habitats, it is not surprising to find them associated with sites in a decent state of conservation (Merritt et al., 2008). A good example is the subfamily Podonominae, in previous studies found associated with conditions with insignificant anthropogenic intervention (Caro-Borrero, Carmona-Jiménez, & Mazari-Hiriart, 2015).
The families that were associated with good HQ values but not with oligotrophic conditions did not form part of a representative assemblage, and their records were consistent with the conditions described in the literature. For example, Dytiscidae (swimmers and predators-carnivores) species are inhabitants of riverbanks and are facultatively stress-tolerant, since both adults and larvae breathe atmospheric air (Merritt et al., 2008); Ozcos et al., 2011). The Gerridae (predators-carnivores) can tolerate high nutrient concentrations and are associated with low-intensity water flows, such as those sampled here (Hawking et al., 2013). The Helicopsychidae (scrapers, herbivores) feed primarily on diatoms and fine organic matter, so it is highly probable to find these associated with high organic matter loading (Bueno-Soria, 2010).
The 2 families exclusively belonging to sites with poor or regular HQ values were the Dryopidae and Siphlonuridae. The Dryopidae are frequently associated with water bodies with low to moderate current flow, with or without riparian vegetation (Rico & García-Avilés, 1998). Siphlonuridae larvae are usually found in areas with little or no current, as in this study, and they most commonly forage on decaying plant material lying on soft sediment (Voshell, 2010).
Finally, the organisms belonging to the Simuliidae were widely distributed and associated with every gradient of physicochemical alteration registered in this study; this is consistent with other studies that considered them to be tolerant organisms (Caro-Borrero, Carmona-Jiménez, & Mazari-Hiriart, 2015).
The algal diversity is represented by a resilient community typically associated with mountain rivers from the central region of Mexico. Some of the constituent species, such as Prasiola mexicana and Paralemanea mexicana, were described for the first time from the Mexico Basin in the mid-19th century and are still present (Ortega, 1984). The macroscopic algae Nostoc parmelioides and Coleodesmium wrangelii are able to fix atmospheric nitrogen in environments with low nutrient concentrations and minimal alterations of the riverbed (Komárek, 2013). In the same way, Draparnaldia mutabilis, Batrachospermum gelatinosum and Paralemanea mexicana have been described in mountain rivers with low to moderate nutrient concentrations and high flow rates (Carmona-Jiménez & Vilaclara, 2007; Carmona-Jiménez, Montejano, & Cantoral, 2004; John, 2003). The specific micro-environmental conditions required by these species and their limited dispersal strategies (Branco, Bispo, Peres, Tonetto, & Branco, 2014) associate them with the reference conditions described in this study, and they therefore make good potential indicators of sites with limited hydromorphological and physicochemical alterations. The most frequent and abundant species were Placoma regulare, Prasiola mexicana and Vaucheria bursata, which were found in sites with good HQ conditions and moderate nutrient concentrations. According to the ecological indicator value of the algae in the Magdalena-Eslava river, this association is composed of detecting species (Carmona-Jiménez, Ramírez, Bojorge, González, & Cantoral, 2016; Caro-Borrero, Carmona-Jiménez, González-Martínez, et al., 2015) that can respond in a better way to environmental changes and provide information for more than one habitat configuration. The widespread distribution of the detecting species could be related to reproductive strategies that favor their propagation and multiplication despite potential human environmental stressors (León-Tejera, Montejano, & Cantoral-Uriza, 2003; Ramírez & Carmona-Jiménez, 2005). On the other hand, the tolerant and intolerant species commonly found could be considered as native species and as indicators of a resilient algal community regulated by seasonal factors, particularly by variations in water temperature and the flow rates.
This research establishes a preliminary baseline characteristic of the potential reference conditions in mountain rivers, particularly for tropical latitudes within the Mexico Basin, and their relationship with biological indicators and anthropogenic environmental change. The macroinvertebrate assemblages and algal communities associated with the reference conditions are perhaps exposed to an intermediate disturbance, which would explain their co-existence in the mountain rivers of the Mexico Basin and potentially in rivers with similar characteristics of the Trans-Mexican Volcanic Belt. The continuous presence of the river flow was a determining factor in maintaining biological diversity. Nevertheless, unregulated water extraction in situ is the main threat to the ecological quality of aquatic ecosystems.
Assessment of the impact of land use change on ecological quality, and in particular on aquatic communities, will require studies based on the ecological threshold concept. The present work attempts to define the potential fluvial reference conditions that may be used as a guideline in evaluating any water body under similar conditions. The set of conditions presented here should be considered in seeking regional agreements to establish public policies aimed at avoidance of further degradation of the last peri-urban rivers of the Mexico Basin.
The authors express their sincere thanks to Pablo Brauer and Ann Grant who made valuable comments on a previous version of the manuscript; to Raquel Ortiz (FC-UNAM) for her help with the maps; to Rogelio Rodríguez, Mauricio Ramírez, Victor Salinas and Mariana Cartajena for their help during fieldwork; to Beatriz González for taxonomic evaluation of the riparian vegetation; and to Edgar Caro-Borrero for finalizing the figures. JCJ received financial support through Research Grant PAPIIT-UNAM (IN220115) and PINCC 2012-2014.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.