We document the first record of the shipworm Teredo clappi (Bartsch, 1923) in Venezuelan coastal waters. We obtained individuals of T. clappi during 2 years at the El Morro Lagoon, Margarita Island. This record confirms the establishment of this species in the region.
Registramos por primera vez el molusco perforador de madera Teredo clappi (Bartsch, 1923) en aguas de las costas de Venezuela. Se obtuvieron individuos de T. clappi durante 2 años en la laguna El Morro, Isla de Margarita. Este registro confirma el establecimiento de esta especie en la región.
The members of the family Teredinidae (shipworms) are considered one of the most destructive marine organisms and are estimated to cause billions of dollars of damage to unprotected and treated timber structures per annum (Borges, Merckelbach, Sampaio, & Cragg, 2014; Distel et al., 2011). Despite the numerous studies concerning the diversity of marine mollusks in the Caribbean Sea, only a few of them have shown updated data regarding the distribution of teredinids for the region (Miloslavich et al., 2010). In light of this, local records may help to increase the knowledge of this group of mollusks in this geographic zone, which could clarify the distribution range of these bivalves. In the present study, the shipworm Teredo clappi is recorded for the first time in Venezuela.
Eight live specimens of T. clappi were collected in the El Morro Lagoon (10°57′15″N, 63°49′31″W) Margarita Island, Venezuela; during July 2008. The identification of the individuals was based on the pallets using the illustrations and descriptions available in Turner (1966, 1971). Subsequently, the specimens were photographed and preserved in 95% ethanol and deposited in the Malacology Collection at the Oceanology Museum Hno. Benigno Román (MOBR-M) of the Marine Station Research of Margarita (EDIMAR), Margarita Island, Venezuela. Catalog numbers MOBR-M-3979 (7 specimens) and MOBR-M-3980 (1 specimen). To verify the identification, the samples were compared to the specimens located in the Malacology Collection of the Museum of Comparative Zoology (MCZ), Harvard University, Cambridge, Massachusetts.Teredo clappiBartsch, 1923
Synonyms. Teredo trulliformis Miller, 1924, Teredo hermitensis Roch, 1929, Teredo adanensis Roch, 1935, Teredo renschi Roch, 1935.
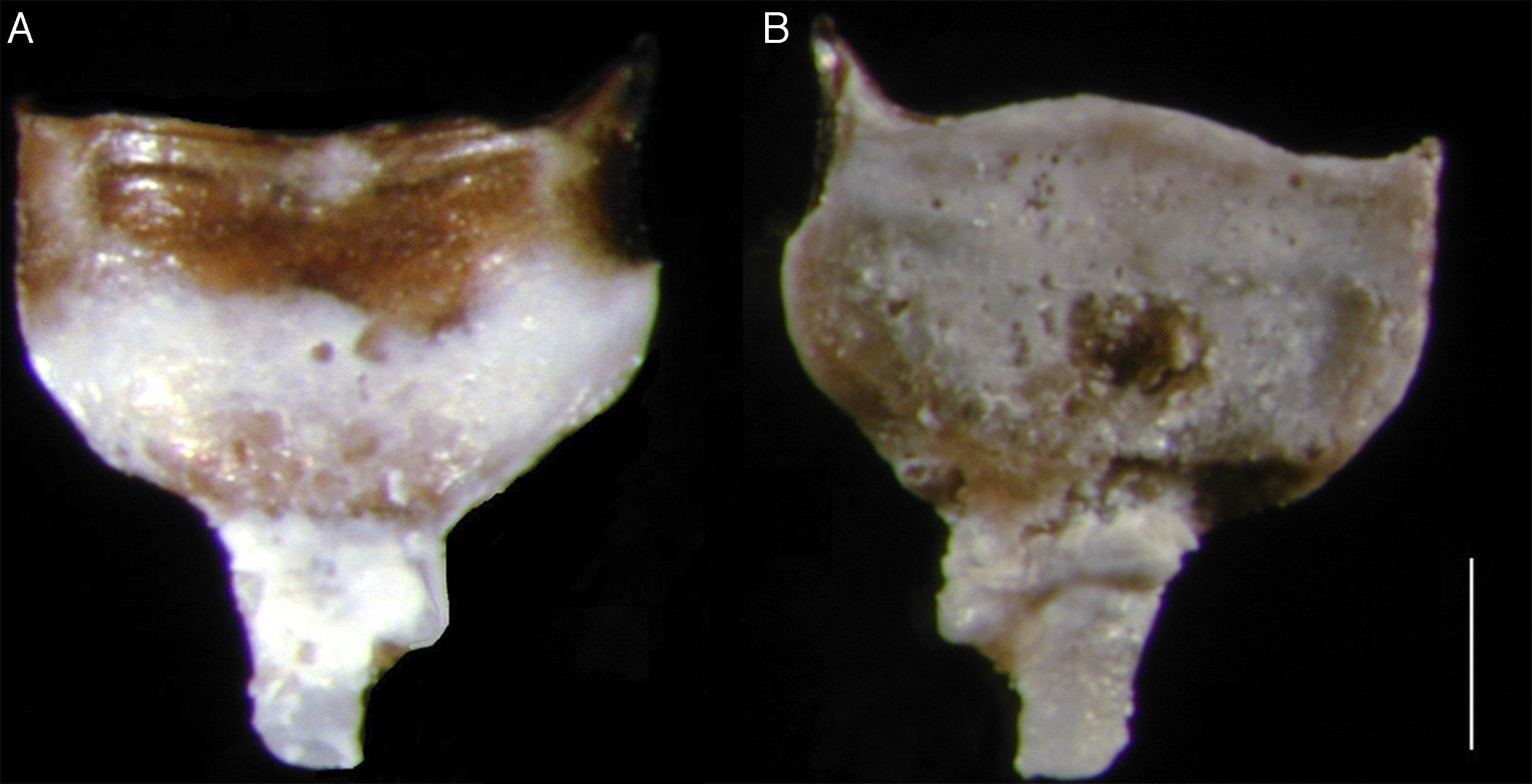
Pallets have a cup-shaped red-brown periostracum which covers the distal half of the solid calcareous blade. The internal face of the pallets may be covered by a white thin calcareous layer; stalk with smooth curvature of variable length, with a slight division near the base of the blade (Fig. 1A and B). The size of the pallets varied between 1.5 and 2.3mm. The siphons are united only at the base and length is twice the size of the pallets.
Comparative material. Florida, USA (MCZ-121641); Port au Prince, Haiti (MCZ-121199); Puerto Cortes, Honduras (MCZ-357197); Cuba, Banes (MCZ-122033); Almirante, Panamá (MCZ-357189).
T. clappi has wide distribution in tropical and subtropical waters. Gulf of Mexico (Turgeon, Lyons, Mikkelsen, Rosenberg, & Moretzsohn, 2009); Caribbean Sea, Puerto Rico (Turner & Johnson, 1971); Brazil (Rocha et al., 2013); North Atlantic (West coast), Florida, USA (Bartsch, 1923; Turner, 1966; Turner & Johnson, 1971); Red Sea, Persian Gulf (Turner, 1966); Indo-Pacific: Madagascar; Indonesia (Turner, 1966); Philippines (Betcher, 2011); Australia (Brearley, Chalermwat, & Kakhai, 2003); New Guinea (Turner, 1966; Rayner, 1983); Hawaiian Islands (Coles, DeFelice, Eldredge, & Carlton, 1997; Edmondson, 1942); India (West coast) (Santhakumaran, 1994).
The pallets of individuals collected in El Morro Lagoon showed some differences with the pallets of the specimens from Florida (comparative material), as the latter have a small cleft in the distal margin of the pallet that was not observed in the individuals from El Morro Lagoon. Turner (1971) stated that this characteristic seems to be present in younger specimens. On the other hand, the specimens from El Morro Lagoon showed a thin calcareous layer that covers almost the half of the pallet (Fig. 1A and B), a feature that was also observed in some specimens from Florida, but not in the remaining comparative material. Velásquez & López (2015) also found significant differences between the pallets of individuals of Spathoteredo spatha collected in Margarita Island, when compared to specimens of the same species collected in different localities of the Caribbean Sea.
For the Teredinidae, the pallets are an essential feature for the taxonomic identification (Turner, 1966; Voight, 2015), however, given the intraspecific variations of the pallets in some species, and the poor preservation of specimens commonly observed in collections, the taxonomic classification usually implies a task of significant difficulty (Leonel, de Moraes, & Lopes, 2006), which would probably increase depending on the genus (e.g. dry pallets of Bankia and Nausitora species). The variation of the pallets could be a response to the life style and environmental conditions (Borges et al., 2014; Cragg, Jumel, Al-Horani, & Hendi, 2009).
The thermal and haline limits of this species are poorly known. However, an experiment carried out under laboratory conditions showed that T. clappi could have successful growth and settlement rates in waters with temperatures between 28 and 30°C (Turner & Johnson, 1971). This is an aspect that may help to understand their distribution, given that abiotic factors such as temperature and salinity have an important effect on the establishment and growth for this group of mollusks (Barreto, Junqueira, & Silva, 2000; Borges et al., 2014; Cragg et al., 2009). All individuals examined here were collected from sunken wood of black mangrove trees Avicennia germinans, between 1 and 4m of depth. The long-term variations of superficial temperature and salinity conditions at El Morro Lagoon ranged from 28.8 to 32.3°C and 26.1 to 37.3°C, respectively (López, 2013). T. clappi is a larviparous species that broods the young within the mantle cavity and the larvae are released at the stage ready for immediate metamorphosis. However, it has been documented that in the absence of wood the larvae can survive 22 days in the water column (Scheltema, 1971), and then in presence of wood the metamorphosis continues normally (Turner & Johnson, 1971). The time of permanence of the larvae in the water column before settlement may affect the distribution of individuals by ocean currents to different regions (Scheltema, 1971), either via rafting in floating timber (Thiel & Gutow, 2005) or in ship ballast water (Carlton, 1999).
The collections of the samples of T. clappi were carried out in July 2008 (present study) and February 2014 (Velásquez, unpublished data) in Laguna El Morro, therefore, it can be concluded that this species is established at this locality.
The authors want to thank Adam Baldinger, Curatorial Associate of Malacological Collection of the Comparative Zoology Museum of Harvard University (MCZ), for allowing the revision of the Teredinidae samples from the collection in order to make comparisons with the specimens of this study.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.