Recent molecular studies on the Burseraceae phylogeny point out that the Bursera - Commiphora complex is monophyletic. Both genera develop a brightly colored paseudaril, and it is possible to presume which tissue is a homologous character. This work analyzes the development of this tissue in 14 species of Bursera, with the aim to determine the existence of a single type of ontogenetic origin. The overall development of the pseudaril in Bursera is described from a histological point of view and it is compared with the development of this tissue in Commiphora, as reported in the literature. Results indicate that the exocarp and endocarp sensu stricto derive from the external and internal epidermis, respectively, whereas the pseudaril differentiates from mesocarp in both genera. The primary difference in fruit development between the 2 sections of Bursera consists in the pseudaril differentiation, which initiates in earlier developmental stages in species of section Bullockia compared to those of section Bursera. To conclude, ontogeny and function of the pseudaril of Bursera agree with that described for Commiphora; thus, it is strongly suggested that the aforesaid tissue is homologous in both genera.
Estudios moleculares recientes sobre la filogenia de Burseraceae indican que el complejo Bursera - Commiphora es monofilético. Debido a que ambos géneros desarrollan un pseudoarilo brillantemente coloreado, se plantea como hipótesis que este tejido es un carácter homólogo entre ambos géneros. Realizamos el análisis del desarrollo de este tejido en 14 especies de Bursera con el objetivo de determinar si tiene un mismo origen ontogenético. Se describe el desarrollo general del pseudoarilo en Bursera desde un punto de vista histológico y se compara con el desarrollo de este tejido detallado en la literatura para especies de Commiphora. En ambos géneros el exocarpo y endocarpo sensu stricto derivan de la epidermis externa e interna respectivamente, mientras que el pseudoarilo se diferencia del mesocarpo. La principal diferencia entre las 2 secciones del género Bursera es que la diferenciación del pseudoarilo comienza en etapas más tempranas del desarrollo en las especies de la sección Bullockia en comparación con las de la sección Bursera. La ontogenia y la función del pseudoarilo de Bursera concuerdan por completo con lo descrito para Commiphora, sugiriendo fuertemente que este tejido es homólogo en ambos géneros.
Historically, the tribal division of Burseraceae was based on fruit features. The first subdivision of the family included the Protieae, Boswellieae and Canarieae tribes (Engler, 1931). Afterwards, Lam (1932) renamed Boswellieae as Bursereae, establishing 2 subtribes, Burserinae and Boswelliinae, the former included Bursera and Commiphora. Bursera was divided into sections Bullockia and Bursera by McVaugh and Rzedowski (1965); the first one encompassed species with a bilocular ovary, whereas the second one grouped species with trilocular ovary, among other differences. Later, Rzedowski and Kruse (1979) posed that Bursera was a diphyletic group, due to similarities found between section Bullockia and Commiphora. Toledo (1982) divided section Bullockia into 2 groups, Copallifera and Glabrifolia, according to leaf, fruit and flower, and germination characteristics. The first one included species in which the pseudaril covers more than two-thirds of the pyrene, while in the second one coverage is less than two-thirds.
During the last decade, several molecular phylogenies have been published that pose different hypotheses and relationships in the family. Clarkson et al. (2002) proposed that Canarieae and Protieae were monophyletic, in contrast to Bursereae, which was polyphyletic, and Beiselia was found to be the earliest diverging lineage of the family. Becerra (2003) proposed that Bursera was monophyletic, and Weeks et al. (2005) supported the early divergence of Beiselia and suggested that one of the Bursera sections is more closely related to Commiphora than to the other section, but with poor statistical support. The phylogeny obtained by Thulin et al. (2008) also placed Beiselia as the earliest diverging lineage within the family and proposed that it be comprise a separate tribe; nonetheless, they reallocate some genera in Canarieae and Protieae. Furthermore, Aucoumea was included in tribe Bursereae even though it has fruits with winged pyrenes that contrast with fleshy fruits with pseudaril of the other 2 genera of the tribe. More recently, in the analysis of De-Nova et al. (2012), it was established once again that Bursera and Commiphora are monophyletic. All the aforementioned suggests the need for reviewing morphological synapomorphies in the Bursera - Commiphora complex. Since a pseudaril is present in both genera, with similar features, it is important to conduct developmental studies of this tissue to obtain detailed ontogenetic, structural and morphological characters that may help to determine the potential homology of these structures in both genera (Daly et al., 2011).
The fruit of both genera is drupaceous and presents fleshy or leathery valves and a pyrene (including 1-2 connate or connivent abortive locules, rarely developing); the pyrene is basally attached to the receptacle and partly or entirely covered by a brightly colored pseudaril, that usually covers only part of the pyrene. In Commiphora, the pseudaril develops from one of the mesocarp layers (van der Walt, 1975). In comparison, the origin of the pseudaril in Bursera has been associated with the endocarp (Daly et al., 2011), although heretofore this has not been assessed by developmental studies. To learn about the similarities and differences between the 2 genera, we conducted a research on the development of pseudaril in 14 species of Bursera, 8 of them from section Bursera and 6 of section Bullockia. The results were compared with those reported by van der Walt (1975) for Commiphora, as both genera are sister taxa.
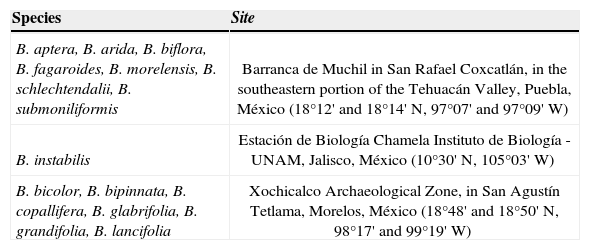
Materials and methodsThe studied species were B. aptera Ramírez, B. arida (Rose) Standl., B. fagaroides (Kunth) Engl., B. grandifolia (Schtldl.) Engl., B. instabilis McVaugh et Rzed, B. lancifolia (Schtldl.) Engl., B. morelensis Ramírez, and B. schlechtendalii Engl. of section Bursera, and B. bicolor (Willd. ex Schltdl.) Engl., B. biflora (Rose) Standl., B. bipinnata (DC.) Engl., B. copallifera (DC.) Bullock, B. glabrifolia (Kunth) Engl. and B. submoniliformis Engl. of section Bullockia. Species were collected in 3 different sites (Table 1), where the dominant vegetation type is seasonally dry tropical forest (Bullock and Solís-Magallanes, 1990; Gómez-Garzón, 2002; Ramos-Ordoñez et al., 2008; Ceccon and Hernández, 2009). Most species are dioecious and flowered annually (Guízar-Nolazco and Sánchez-Vélez, 1991; Rzedowski et al., 2004; Rzedowski et al., 2005).
Collection sites of the 14 species of Bursera used in this study
| Species | Site |
|---|---|
| B. aptera, B. arida, B. biflora, B. fagaroides, B. morelensis, B. schlechtendalii, B. submoniliformis | Barranca de Muchil in San Rafael Coxcatlán, in the southeastern portion of the Tehuacán Valley, Puebla, México (18°12' and 18°14' N, 97°07' and 97°09' W) |
| B. instabilis | Estación de Biología Chamela Instituto de Biología - UNAM, Jalisco, México (10°30' N, 105°03' W) |
| B. bicolor, B. bipinnata, B. copallifera, B. glabrifolia, B. grandifolia, B. lancifolia | Xochicalco Archaeological Zone, in San Agustín Tetlama, Morelos, México (18°48' and 18°50' N, 98°17' and 99°19' W) |
During the 2010-2011 fruiting season, we collected flower buds, flowers and fruits at different stages of development in 10 randomly selected trees of each species. In June and July 2010, at least 50 reproductive structures of each tree (buds, flowers and recently formed fruits) were collected. During December 2010 and May 2011, immature and mature fruits were also collected. Additional material was herborized and deposited in the Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma de México. Collection sites and dates were established by means of prior observations during the 2005–2006 and 2006-2007 fruiting seasons, literature search and consultations with local people. Fruiting has an annually pattern in all species; flowering began in June during the 2010–2011 season. Species of section Bullockia presented some mature (dehisced) fruits with an exposed pseudaril in the month of July, having its peak of ripening in December, whereas the fruits of all Bursera species ripen between December and May (Ramos-Ordoñez, unpublished data).
All material was fixed in FAA (formol, acetic acid, 96% ethanol and water 1:0.5:5:3.5) and was processed in the laboratory of Desarrollo en Plantas, Facultad de Ciencias, UNAM. Additionally, dehisced fruits or pyrenes with exposed pseudaril were collected. One part was fixed in FAA and the other one was stored under dry conditions within plastic containers in order to perform histochemical tests. Inclusion methods and contrast staining were used for describing structural characteristics of pseudaril development. Flowers and buds, as well as fruits smaller than 4mm diameter were included into RLWhite and cut between 1 and 3μm in an ultramicrotome RMC MT990 (Boeckeler Instruments) with glass blades. Subsequently, they were stained with toluidine blue and 50% safranine. At least 10 samples of each reproductive structure (bud, flower or fruit) per species were used, resulting in about 450 glass slides, with an average of 25 stained sections each. Fruits over 4mm of diameter were included into Paraplast and were cut into sections of 4 -7μm using a rotary microtome. They were stained with safranin -fast green. At least 10 fruits per species without abortion signals were used, obtaining about 1450 glass slides with an average of 20 stained sections each. For determining the presence of lipids in the pseudarils, the pulp stored in FAA was washed, while the stored dry pulp was rehydrated with distilled water. Thin cuts were made freehand, and Oil Red O and Sudan III tests were applied. Techniques were performed according to López et al. (2005). All glass slides were observed and photographed under a light microscope. Microphotographs were taken whit an optical microscope (Olympus Provis AX70), and macrophotographs were taken under a stereoscopic microscope (Zeiss).
ResultsThe fruits of the studied species are fleshy drupes with septicidal (longitudinal) dehiscence. The ovary is superior and placentation is axile. The gynoecium is 3-carpelled (Bursera section) or 2-carpelled (Bullockia section). There are 2 ovules in each locule, but usually only one completes its development into seed. The sequence development of the fruit wall will be exemplified with Bursera copallifera (section Bullockia, Copallifera group), which also corresponds to the remaining species studied herein.
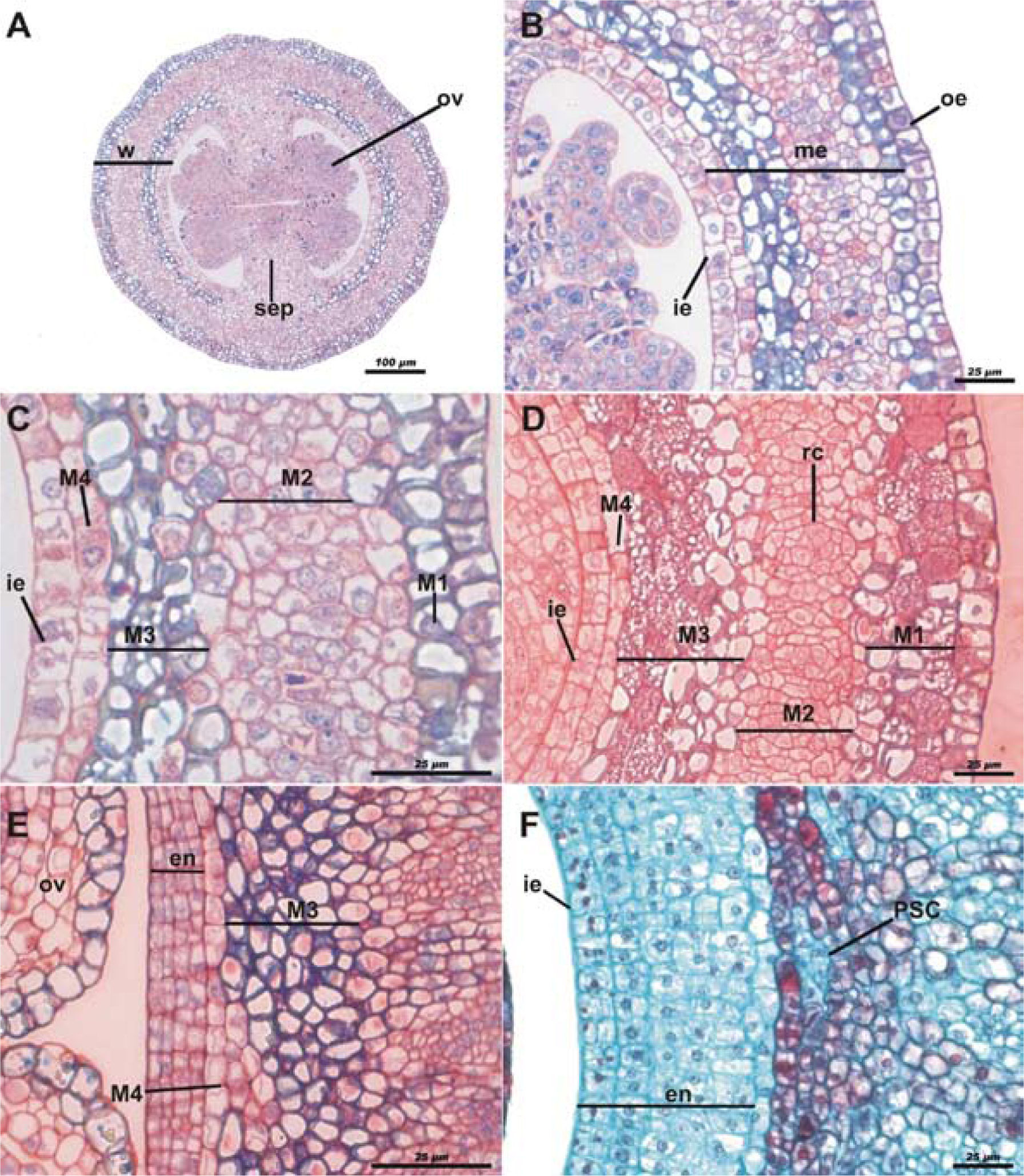
During the floral bud stage (Fig. 1A), the ovary wall is formed by a monostratified outer epidermis, a pluristratified pre-mesocarp layer whose strata have different cell characteristics and an inner epidermis that is also monostratified at first (Fig. 1B). Due to the histologic characteristics and only for descriptive purposes, the mesocarp was divided into 4 layers: M1 encompasses the stratum below to the outer epidermis with thick wall cells and a large amount of starch; M2 comprises the parenchymatic mesocarp underlying M1, with thin-walled cells and large nuclei; M3 is the third mesocarp layer, pluristratified, and its cells have the same histological features as M1; finally, M4 is a parenchymatic cell stratum adjacent to the inner epidermis (Fig. 1C). The external epidermis, known as exocarp after fecundation, has anticlinal divisions; nevertheless, it remains monostratified up to fruit maturation. In the anthetic flower, the outer layer of the mesocarp (M1) multiplies rapidly forming a pluristratified hypodermis. Cells align radially in M2 in order to form resiniferous canals. Cellular division occurs rapidly within the third layer (M3). These cells are slightly smaller than those in M1. Hence, although these layers have similar thickness, M3 has at least 2-fold cell strata with regard to M1. The fourth mesocarp layer (M4) remains without apparent changes: it can be seen monostratified with anticlinal divisions. The internal epidermis is divided and it forms 2 strata (Fig. 1D).
Pseudaril development of Bursera copallifera in early stages. Cross-sections of reproductive structures. A, floral bud showing the ovary wall (w), the ovule primordium (ov) and the placental septum (sep); scale bar= 100μm; toluidine blue and 50% safranine staining. B, ovary wall showing the 3 main layers, the outer epidermis (oe), the mesocarp (me) and inner epidermis (ie); scale bar= 25μm; toluidine blue and 50% safranine staining. C, mesocarp layers; monostratified outer layer, with cells filled of starch and thick wall (M1), parenchymatous mesocarp (M2), multistratified third layer showing cells filled with starch (M3) and stratum of cells (M4) adjacent to the inner epidermis (ie); scale bar= 25μm; toluidine blue and 50% safranine staining. D, anthetic flower M1 divides forming the hypodermis; the resiniferous canals (rc) begin their development in M2, the third layer of the mesocarp divides (M3) as well as the inner epidermis (ie); scale bar= 25μm; toluidine blue and 50% safranine staining. E, post-anthetic flower; M4 divides and the new cells are integrated to M3, the derivatives of the inner epidermis begin to form the endocarp (en); scale bar= 25μm; toluidine blue and 50% safranine staining. F, newly formed fruit showing the endocarp; in M3 the pseudaril cells (PSC) begin to differentiate; scale bar= 25μm; safranine - fast green staining.
In a post-anthesis stage and very close to fertilization, cellular division continues in the first 3 layers of mesocarp as it happens in the inner epidermis, which already forms a pluristratified endocarp (Fig. 1E). Periclinal divisions have been seen in M4. Cells from the division take the histological features of the M3 cells, thus integrating into this layer and, consequently, M3 has its origin in M4. As development proceeds (Fig. 1E), both layers have different histological characteristics.
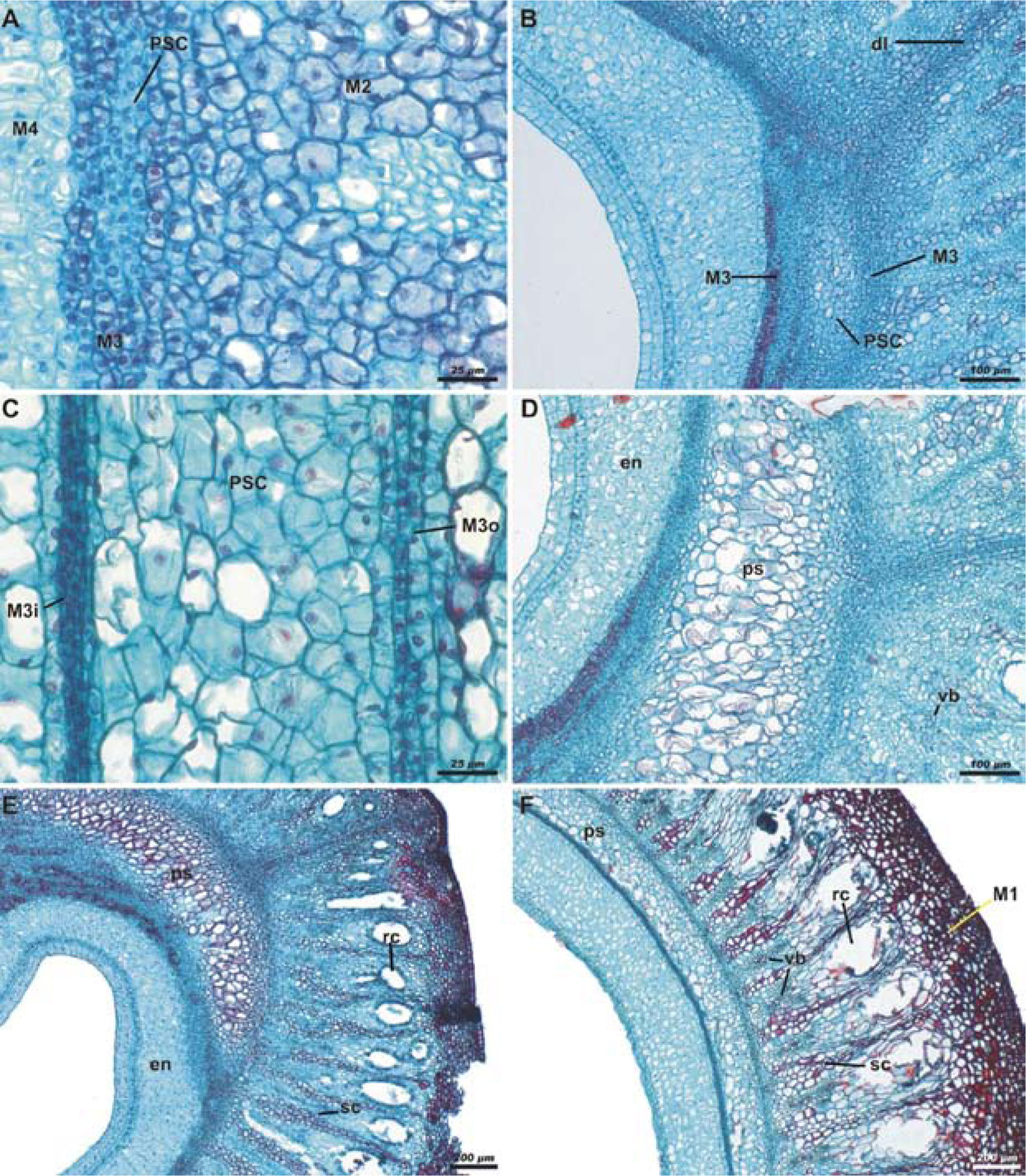
Already in early development of the fruit, when the perianth has become dry, the endocarp is pluristratified (Fig. 1F). The vascular tissue and resiniferous canals are seen in M2. The main change occurs in the third layer of the mesocarp (M3), wherein cell proliferation begins. These new cells are distinguished by their staining and are centrally located between the cellular layers of M3. They are also recognizable as a distinct tissue. These cells are differentiated in the pseudaril; hereinafter referred to as pseudaril cells (PSC) (Fig. 1F). M3 promotes its own cellular division either anticlinal or periclinal, rather than by elongation. Thus, from this stage, they appear smaller than those of the adjacent layers, M2 and M4 (Fig. 2A). The PSC proliferate and the central ones begin to elongate, separating the original strata of M3. This takes place at highest rate in the dehiscence lines or suture of the carpels. The process of cell production for constituting the PSC keeps going, and their volume is increased. They also become more transparent and exhibit thinner walls in comparison to the original layers of M3 (Fig. 2B). The inner strata of M3 present a more compact array than the outer strata (Fig. 2C). The pseudaril is totally differentiated before the fruit reaches its final size, which happens in the first 2 to 5 days after fertilization. During elongation of the PSC, calcium oxalate crystals are produced in M4. As the fruit ripens, the endocarp cells consist of sclereids; subsequently, this tissue lignifies (Fig. 2D). Tannins are produced in the exocarp of the mature fruit. In addition to tannins, calcium oxalate crystals (druses) and other compounds occur in the hypodermis (M1). The parenchymatous mesocarp (M2) presents large resiniferous canals, sclereids and vascular bundles (Figs. 2E, F).
Pseudaril development of immature fruits in Bursera copallifera. A, multiplication of the cells of the third layer of the mesocarp (M3) and the pseudaril cells (PSC); scale bar= 25μm. B, elongation of the pseudaril cells (PSC) causing the separation of the third layer of mesocarp (M3); scale bar= 100μm. C, pseudaril in differentiation, showing the compact arrangement of the inner strata of the third layer of the mesocarp (M3i) contrasting with the loose arrangement of the outer strata (M3o); scale bar= 25μm. D, pseudaril (ps) almost differentiated close to the dehiscence line, the sclerification begins in the endocarp (en), and in the parenchymatous mesocarp the vascular bundles (vb) are visible; scale bar= 100μm. E and F, differentiated pseudaril (ps) close to the dehiscence line (E) and on the front face of the fruit (F), showing the sclerified endocarp (en), the resiniferous canals (rc) associated with vascular bundles (vb) and sclereids (sc) in the parenchymatous mesocarp, and hypodermis (M1) whose cells have different compounds; scale bar= 200μm. Safranine - fast green staining; cross-sections.
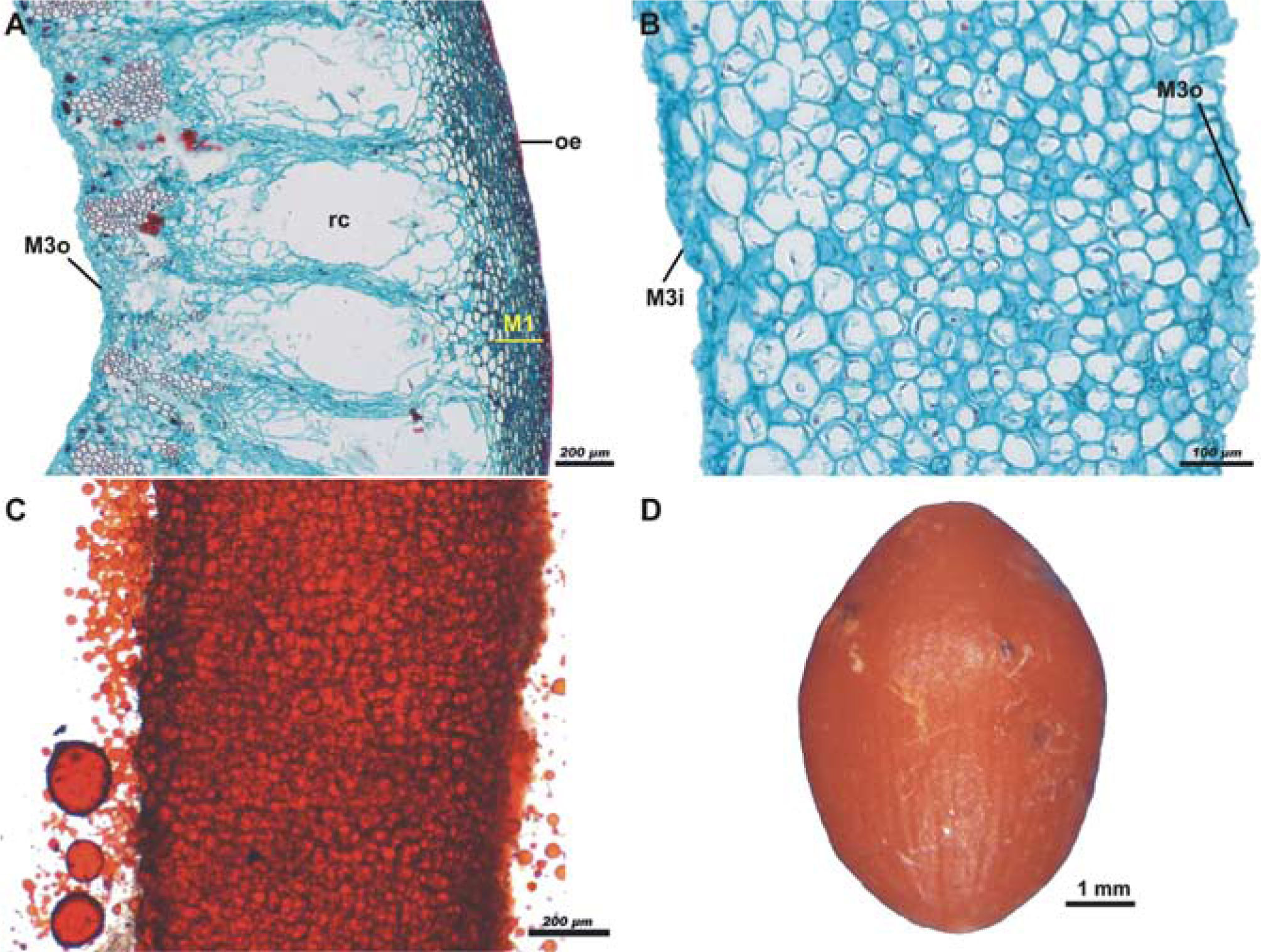
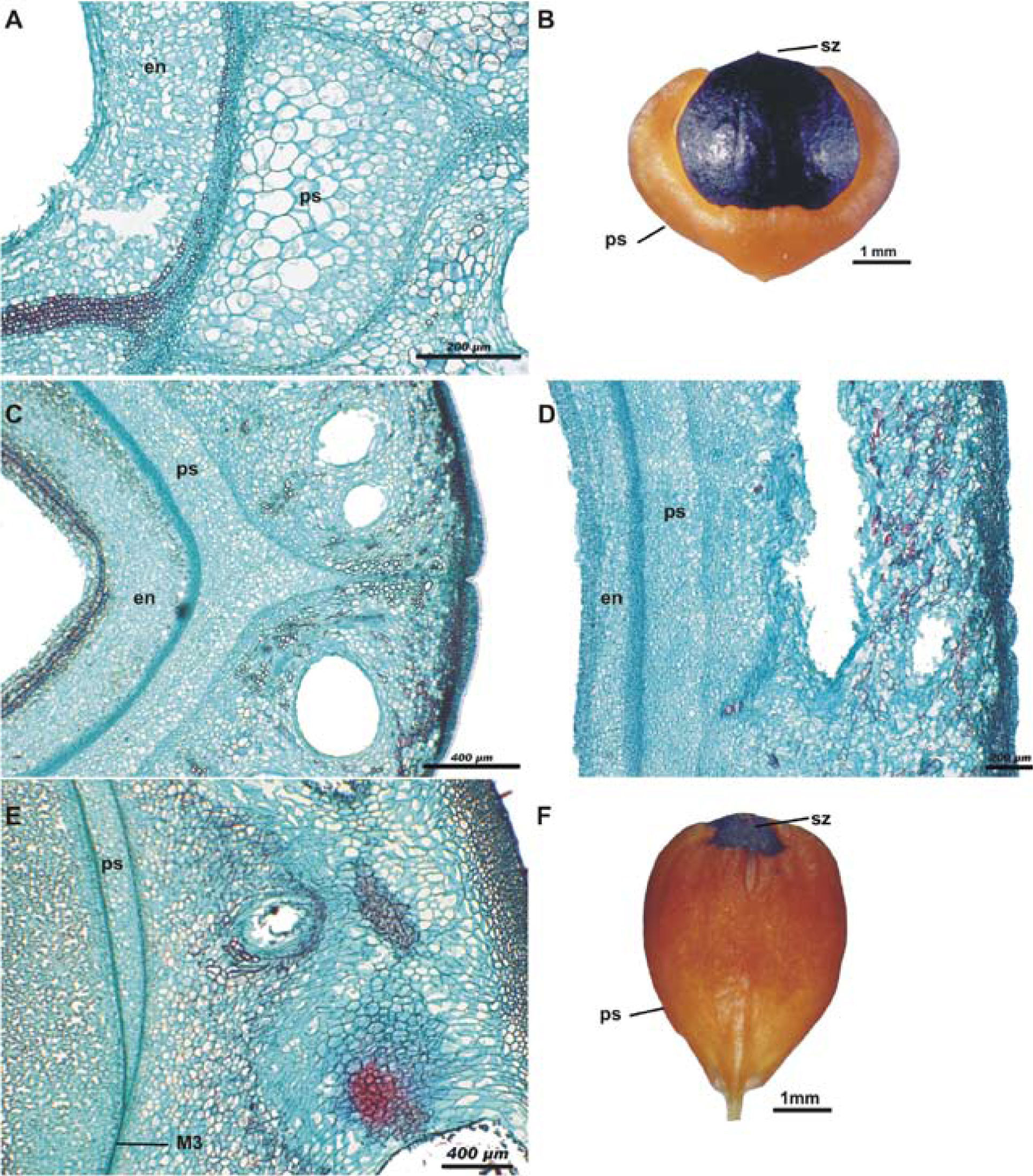
During dehiscence of the fruit, the outer strata of M3 becomes fragmented. One part stays adhered to M2, so that M2, the hypodermis and the exocarp, constitute the valves of the fruit (Fig. 3A), and, when these detach, the pseudaril becomes exposed (Fig. 3B). The pseudoaril in turn covers the inner strata of M3, the M4, and the pluristratified endocarp enclosing the seed. Detachment of the pseudaril and pyrene is accomplished by means of fragmentation of the inner strata of M3, along with, probably, that of M4. When the fruit ripens, the pseudaril is brightly colored with tones from orange to red, depending on the species, and it has a high lipid content (Fig. 3C). Pseudaril and pyrene (endocarp + seed) constitute the unit of dispersal (Fig. 3D).
Sections of the mature fruit of Bursera copallifera. A, valve formed by the outer epidermis (oe), the hypodermis (M1), the parenchymatous mesocarp with large resiniferous canals (rc), and the outer strata of the third layer of the mesocarp (M3o); scale bar= 200μm; safranine - fast green staining. B, pseudaril detached from the rest of the layers of fruit, showing the remnants of the outer (M3o) and inner (M3i) strata of the third layer of the mesocarp; scale bar= 100μm; safranine - fast green staining. C, lipidic pseudoaril demonstrated by Sudan III test; scale bar= 200μm. D, dispersal unit formed by the pseudaril which completely covers the pyrene; scale bar= 1mm.
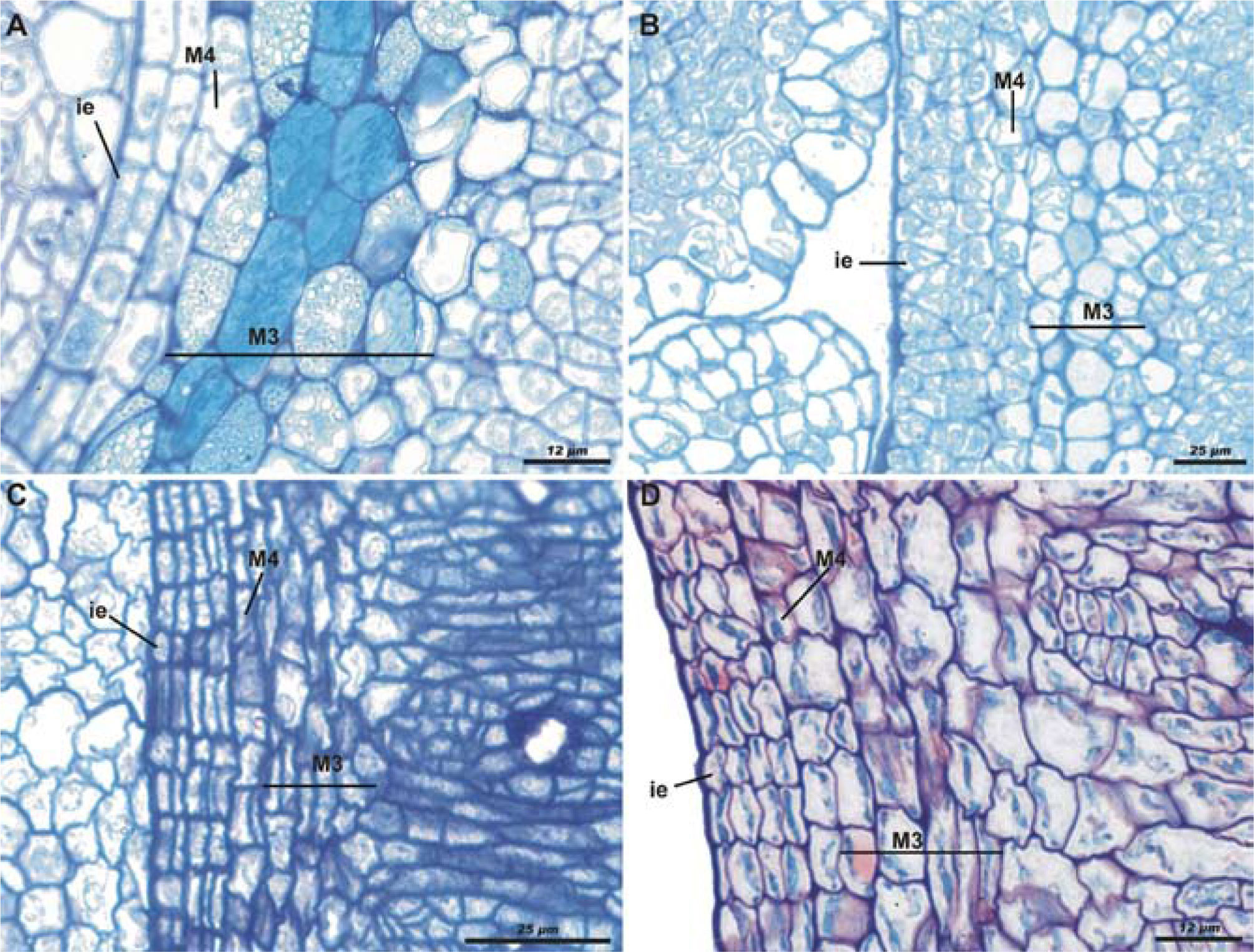
Differences were found in the other species analyzed with regard to the timing in which the layers are developed, but the ontogenic pattern was the same for all species. The development stages of Bursera submoniliformis (section Bullockia, Copallifera group; Fig. 4A) show the most similar patterns to those in B. copallifera. Development is earlier in B. biflora (section Bullockia, Glabrifolia group), when compared to B. copallifera. At the floral bud stage, the internal epidermis has already divided, giving rise to at least 4 strata. M3 also has a major development at this stage: it is pluristratified, with cells of varying size in division, but with less starch (Fig. 4B). In all species of the Bursera section, endocarp differentiation starts during the bud stage; when the ovules reach their final size, the internal epidermis has already divided into 3 more strata. In these species, layers M3 and M4 have the same histological characteristics. The division of M4 is observed, confirming that it gives rise to M3; nevertheless, the starch in the M3 cells begins to be visible in far earlier stages, shortly post-anthesis (Figs. 4C, D).
Cross-sections of buds and flowers of some species of Bursera in early stages of development. Development of inner epidermis (ie) and the third layer of the mesocarp (M3), both separated by the fourth layer of the mesocarp (M4). A, anthetic flower of B. submoniliformis with similar development to B. copallifera; scale bar= 12μm; toluidine blue staining. B, floral bud of B. biflora with faster development in M3 and inner epidermis; scale bar= 25μm; toluidine blue staining. C, anthetic flower of B. morelensis showing cell division of the inner epidermis and M4; scale bar= 25μm; toluidine blue and 50% safranine staining. D, mature flower or post-anthesis of B. instabilis, similar to B. morelensis; scale bar= 12μm; toluidine blue and safranine 50% staining.
Differentiation and elongation of the PSC was faster in the first two-thirds of the fruit (from the insertion of the pedicel), in the lines of dehiscence or sutures, forming the pseudaril lobes (thick deposits of this tissue); by contrast, development was slower on the faces of the stone (in outlying areas of the carpel unions). Despite cell elongation, it did not form lumps. Towards the stigmatic zone, PSC were not differentiated and the compact arrangement of cells remained as in M3 (Figs. 5A, B). In species of section Bursera, PSC begin their equal differentiation in all zones of M3; therefore, the pseudaril shows the same thickness in any particular zone of the fruit (Figs. 5C, D) and it completely covers the pyrene (Fig. 3D). An intermediate development pattern between the 2 previously described was observed in species of the Copallifera group and that of B. glabrifolia; PSC begin their differentiation in all M3 as in species of section Bursera, i.e., with the pseudaril completely covered by the pyrene. Nevertheless, in some fruits a small portion toward the apex did not show differentiation of the PSC, resulting in an incomplete coverage by the pseudaril as it happens in B. submoniliformis (Figs. 5E, F). Fully-developed seeds were found in all mature fruits with an exposed pseudaril of B. bipinnata, B. copallifera and B. glabrifolia. However, those collected during the last days of July showed incomplete pseudoaril coverage of the stone, while those collected in December showed complete or incomplete coverage, suggesting that pseudaril coverage may be related to the time it takes the embryo to mature.
Sections of immature fruits and dispersal units of some species of Bursera. A, cross-section of the pseudaril lobe (ps) of B. biflora; en, endocarp scale bar= 200μm; safranine - fast green staining. B, dispersal unit of B. biflora, pseudaril (ps) covers the black pyrene, stigmatic zone (sz) of this species always is naked; scale bar= 1mm. C: Cross-section of B. lancifolia without pseudaril lobe (ps) in the dehiscence line; scale bar= 400μm; safranine - fast green staining. D, cross-section of B. lancifolia showing the pseudaril (ps) on the front face of the fruit, away from the dehiscence lines; scale bar= 200μm; safranine - fast green staining. E, longitudinal section of B. submoniliformis showing the pseudaril (ps) and the M3 area where the pseudaril cells have not been developed; scale bar= 400μm; safranine - fast green staining. F, dispersal unit of B. submoniliformis showing the pseudaril (ps) covering the pyrene, except for the stigmatic zone (sz); scale bar= 1mm.
The early developmental anatomy of the Bursera fruit studied here shares many ontogenic similarities with that of Commiphora as described by Van der Walt (1975), who performed the development study of pseudaril based on information of almost 40% of the Commiphora species. It is important to note that this latter work studied only young fruits. In contrast, the present study also used floral buds and flowers in different stages of development in order to describe the pseudaril ontogeny. According to van der Walt, the pseudaril of Commiphora develops from the mesocarp, and pseudaril cells differentiate after most of the inner epidermis derivatives (viz., endocarp) differentiated. This is the same development pattern observed in species of Bursera section during the anthesis and post-anthesis stages, when the endocarp is clearly visible and the third layer of the mesocarp (M3) presents the same histological characteristics as the stratum that gave rise to it (M4). As interpreted by van der Walt in Commiphora, the pseudoaril cells are formed by the division and enlargement of hypodermal cells adjacent to the endocarp, corresponding in the present study to the third layer of the mesocarp (M3) of Bursera, wherein cells divide and enlarge giving rise to PSC. The histological description of pseudaril cells is very similar between the 2 genera: they are bigger and more transparent than surrounding cells. When the fruit ripens, these relatively large, thin walled cells contain lipids.
A “separation layer” is also described in the case of Commiphora, consisting of 2 to 3 strata of small and elongated cells, outside the pseudaril cells and separating them from the rest of mesocarp. In cross-section, there are 2 expansions of this layer toward the opposite poles, through the mesocarp and exocarp, which disintegrates when the fruit ripens to allow the valves to fall away. In Bursera, the separation layer corresponds to the original strata of M3, which are externally disposed to the pseudaril. The expansions mentioned in Commiphora coincide with the lines of dehiscence or sutures of any of the species with a bilocular ovary (like, Commiphora), thereof extending across the junction of the carpels, permitting dehiscence at maturity. There is no clear separation between the exocarp and mesocarp in the mature fruit of Commiphora, except for the cellular content and structures found in the 2 layers. However, the similarities that were observed between Bursera and Commiphora are indisputable: a hypodermis composed of collenchymatic cells, some containing calcium oxalate druses, other secretions or plastids and a parenchymatous mesocarp with wide intercellular spaces, with large secretory canals in the outer side and vascular bundles in the inner side. The endocarp of the mature fruit is very similar as well, which is formed in both genera by strongly thickened isodiametric sclereids with lignified walls.
In general, the homologies of the layers constituting the pericarp of drupes is based mainly on the origin of the exocarp and endocarp, which is considered sensu stricto when these layers differentiate only from the outer and inner epidermis, respectively, or upon their direct derivatives; and sensu lato, when they develop from the epidermal strata and from adjacent subepidermal layers or other regions of the ovary wall. The tissue between the exocarp and endocarp, which is usually parenchymatic, corresponds to the mesocarp (von Teichman, 1989). In this context, the exocarp and endocarp of Bursera sensu stricto are formed from the monostratified outer and pluristratified inner epidermis, respectively; thus being clear that pseudaril arises from M3 cells in the mesocarp. The exocarp and endocarp of drupes are regarded as functional units, namely, the shell (valves) and the wall of the stone (pyrene), respectively (von Teichman, 1989). The valves of the fruit of Bursera, which consist of the exocarp, hypodermis (M1) and the parenchymatous mesocarp (M2), together account for the external functional unit or shell. From the functional point of view, the pseudaril of Bursera can be considered as part of the internal functional unit.
As observed in Bursera morelensis, the removal of the pseudaril is necessary for germination, suggesting that this tissue somehow inhibits germination (Ramos-Ordoñez, 2009), which are removed when passing through the digestive tract of dispersers (Cipollini and Levey, 1997; Yagihashi et al., 1999; Figueroa and Castro, 2000; Ramos-Ordoñez, 2009). In addition to preventing germination before seed dispersal and attracting the dispersers (Ramos-Ordoñez and Arizmendi, 2011). It has been proposed that the fleshy pulp of endozoochorous fruits evolved to reduce the harmful effects of granivory and frugivory by containing substances which repel seeds predators and/or accelerate the passage through the digestive tract (Bolmgren and Eriksson, 2010).
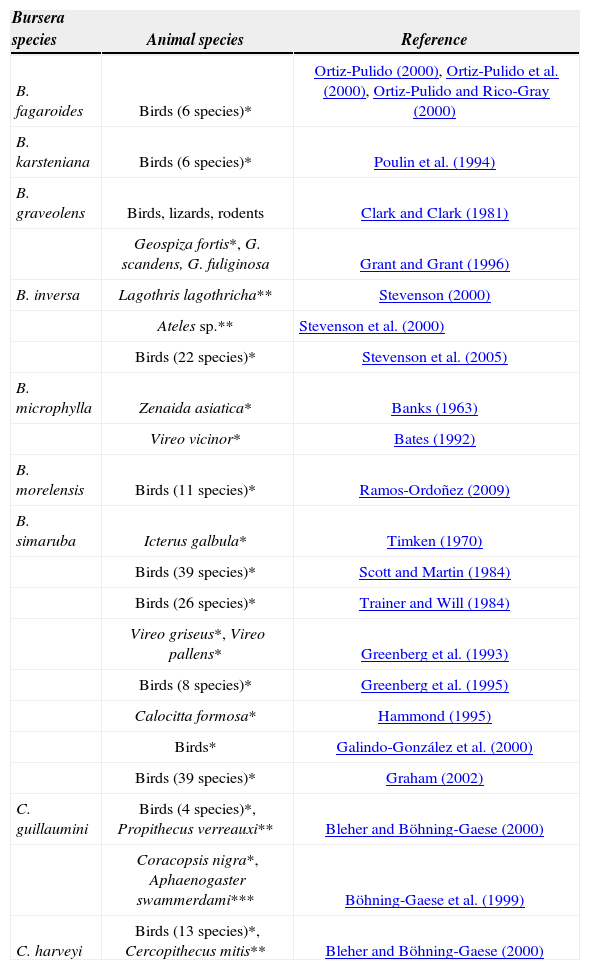
Seed dispersal of Bursera and Commiphora is clearly related to their high lipid content of pseudaril. Although no reports have been found on the amount of lipids present in the pseudaril in Commiphora, it is known that 67.74% of the fruit compounds in B. morelensis are lipids, 3.11% proteins and 2.36% carbohydrates (Ramos-Ordoñez, 2009). In B. simaruba and B. hindsiana, the pseudaril caloric content is 32.8±1.3 and 27.4±0.8kJ/g, respectively (Bates, 1992). Animal species that remove fruits of both genera are mainly birds (Table 2). Analysis of these studies indicates that, particularly in Bursera, most of them are migratory birds that travel over the areas where these plants are distributed. By consuming the pseudaril among other foods, they obtain the energy demands for their migration (Scott and Martin, 1984; Bates, 1992) and at the same time defecating some pyrenes in suitable conditions for germination and in favorable sites for establishment of the species (Scott and Martin, 1984; Hammond, 1995; Ramos-Ordoñez, 2009). Also, dispersal by primates is favoured by the long distances they travel, as well as by the size of the seeds (Böhning-Gaese et al., 1999; Bleher and Böhning-Gaese, 2000; Stevenson et al., 2000). The seeds are deposited at sites far away from the parental trees, thus increasing the survival and colonization of new sites. In addition, secondary seed dispersal is performed by ants, which remove the pyrenes with pseudaril and avoid naked pyrenes; nevertheless, this has been best recorded in Commiphora (Böhning-Gaese et al., 1999) than in Bursera (Ramos-Ordoñez et al. 2012).
Animal species that remove the fruits of Bursera and Commiphora. Only references where seed dispersers are evaluated using standardized monitoring techniques according to Schupp (1993) have been cited. *Bird species; ** primate species; *** ant species. Some studies include several plant species, only the number of animal species reported in Bursera are listed
| Bursera species | Animal species | Reference |
|---|---|---|
| B. fagaroides | Birds (6 species)* | Ortiz-Pulido (2000), Ortiz-Pulido et al. (2000), Ortiz-Pulido and Rico-Gray (2000) |
| B. karsteniana | Birds (6 species)* | Poulin et al. (1994) |
| B. graveolens | Birds, lizards, rodents | Clark and Clark (1981) |
| Geospiza fortis*, G. scandens, G. fuliginosa | Grant and Grant (1996) | |
| B. inversa | Lagothris lagothricha** | Stevenson (2000) |
| Ateles sp.** | Stevenson et al. (2000) | |
| Birds (22 species)* | Stevenson et al. (2005) | |
| B. microphylla | Zenaida asiatica* | Banks (1963) |
| Vireo vicinor* | Bates (1992) | |
| B. morelensis | Birds (11 species)* | Ramos-Ordoñez (2009) |
| B. simaruba | Icterus galbula* | Timken (1970) |
| Birds (39 species)* | Scott and Martin (1984) | |
| Birds (26 species)* | Trainer and Will (1984) | |
| Vireo griseus*, Vireo pallens* | Greenberg et al. (1993) | |
| Birds (8 species)* | Greenberg et al. (1995) | |
| Calocitta formosa* | Hammond (1995) | |
| Birds* | Galindo-González et al. (2000) | |
| Birds (39 species)* | Graham (2002) | |
| C. guillaumini | Birds (4 species)*, Propithecus verreauxi** | Bleher and Böhning-Gaese (2000) |
| Coracopsis nigra*, Aphaenogaster swammerdami*** | Böhning-Gaese et al. (1999) | |
| C. harveyi | Birds (13 species)*, Cercopithecus mitis** | Bleher and Böhning-Gaese (2000) |
According to von Teichman (1989), once the origins of exocarp and endocarp have been defined, including that of its derivatives, both in Bursera and Commiphora, the remaining tissue layers located between the 2 epidermal derivatives correspond to the mesocarp; and, according to our results and to comparisons with van der Walt's (1975) study, in both genera the pseudaril arises from one of the layers of the mesocarp, strongly suggesting a homology. Nevertheless, it is still necessary to perform an ontogenic study of the pseudaril in species from genera of the tribe Protieae such as Protium, Tetragastris and Crepidospermum (Daly et al., 2011) and evaluate the homology using a phylogenetic analysis.
To A. I. Bieler of the Microcine Laboratory, Facultad de Ciencias, Universidad Nacional Autónoma de México for helping with photographs. To C. Esquivel, R. Wong, M. Pérez-Pacheco, M. Espinosa-Sánchez and S. Gómez-Sánchez for their technical assistance. We thank C. Machuca and M. López-Carrera for assistance in the field. To an anonymous reviewer for the revision of an earlier version of the manuscript. English version was revised by S. Mooney. Financial support was provided by DGAPA-PAPIIT No. IN217511. This work is part of postdoctoral research project of MFR-O in the DGAPAUNAM Postdoctoral Fellowships Program.