Retinopathy of prematurity (ROP), the leading cause of childhood blindness around the world, is potentially avoidable. The incidence of ROP varies between countries due to a variety of factors. The aim of this study is to assess the effectiveness of screening criteria in Mexico valid in March 2015 as an example of a middle-income country.
MethodsThe medical records of 261 patients from a single center covering a period of 42 months (October 2011–March 2015) were retrospectively analyzed to identify infants with ROP that did not fall within screening criteria set forth by regional health authorities.
ResultsOf the 261 infants in our study group, 55 (21.1%) weighed more than 1500g (ranging from 466 to 2910), 129 (49.4%) had a GA >30 weeks (ranging from 22 to 36), and 47 (18%) patients presented both. Overall, the mean birth weight for infants with ROP was 1270.6±365.3g. The mean gestational age was 30.4±2.3 weeks. Following actual AAO/AAP guidelines for ROP screening, 17 infants (6.5%) in our study group would have gone undiagnosed.
ConclusionsThese findings show that the valid guidelines at the time of the screening were based on a different population and were not sufficient to detect all ROP cases in a middle-income country. With the update of the Mexican guidelines established in July 2015, the patients from this study would have been screened. Therefore, review and modification of the current screening guidelines in other middle-income countries should be considered to include all babies at risk for ROP.
La retinopatía del prematuro (ROP), la principal causa de ceguera infantil del mundo, es potencialmente prevenible. La incidencia de ROP varía entre países debido a múltiples factores. El propósito de este estudio es analizar la efectividad de las guías de tamizaje vigentes en México durante marzo de 2015 como país en vías de desarrollo.
MétodosLos expedientes de 261 pacientes de un único centro, cubriendo un periodo de 42 meses (octubre 2011–marzo 2015), fueron analizados identificando pacientes con ROP que no entraban en los criterios de tamizaje establecidos por las autoridades de salud.
ResultadosDe los 261 lactantes del estudio, 55 pacientes (21.1%) pesaron más de 1,500g (intervalo de 466–2910), 129 (49.4%) tuvieron una edad gestacional mayor a 30 semanas al nacer (intervalo de 22–36) y 47 (18%) presentaron ambos. El peso promedio de los pacientes con ROP fue de 1,270±365.3g. La edad gestacional al nacimiento fue de 30.4±2.3 semanas. Siguiendo las guías de tamizaje de la AAO/AAP, 17 pacientes (6.5%) del estudio no hubieran sido diagnosticados.
ConclusionesEstos hallazgos demuestran que las guías de tamizaje vigentes en México durante el estudio estaban basadas en una población diferente y no eran suficientes para detectar todos los casos de ROP. Con la actualización de las guías mexicanas en julio 2015, esta población sí hubiera estado cubierta, por lo que la revisión y modificación de estas guías en otros países emergentes debe ser considerada para incluir a todos los bebés en riesgo de presentar ROP.
Retinopathy of prematurity (ROP) is a serious condition found in newborns and is characterized by abnormal vascular growth in the immature retina. This abnormal vessel development can lead to blindness in severe cases. It is now widely recognized that ROP is the leading cause of childhood blindness.1 As neonatal mortality around the world decreases, ROP is no longer seen only in the most developed countries. Developing countries are now seeing a spike in ROP prevalence often referred to as the “third epidemic,” due to the higher premature birth rates, decreased access to neonatal resources, and possibly due to lack of awareness or training of healthcare professionals.2,3 Of the estimated 50,000 children to have ROP, over half are located within Latin America.1,2,4 The main risk factors for developing ROP are inherent to premature infants, namely gestational age (GA) and birth weight (BW).1,5 Both of these have been accepted as the two most important risk factors for developing ROP. However, continued monitoring has helped us to understand that other risk factors play an important role in ROP development including artificial ventilation, sepsis, necrotizing enterocolitis, postnatal glucocorticoids, and cardiopathy.6–8
Due to the subclinical nature of ROP, the diagnosis can be missed during the initial hospital stay. This can be detrimental to the child mainly because early detection and treatment is vital to avoid blindness in serious cases. As such, screening guidelines have been put into place across the globe to help detect and treat this disease early. This is especially relevant to middle-income countries as the rate of premature infant survival is rapidly improving, followed closely by an increase in ROP prevalence.1,2,9 Mexico, a middle-income country, has witnessed this increase first hand. Even with national screening guidelines, ROP continues to be the leading cause of childhood blindness in Mexico.4,10 The criteria that most institutions in Mexico recognize and follow was released by the American Academy of Pediatrics and the American Academy of Ophthalmology in 1990 and revised in 1995, 2008 and 2013.11 These guidelines state that any infant of GA of less than 30 weeks or with BW of less than 1500g should be screened. The 2013 revision also included select infants with BW 1500g and GA greater than 30 weeks with an unstable clinical course that should be screened. This includes infants who are on cardiorespiratory support or at high risk for developing ROP according to the neonatologist.11 However, the use of these guidelines is meant for developed countries with excellent prenatal care.5 Because of the vast diversity in neonatal care around the world, the AAO/AAP guidelines may not be applicable to middle income countries.2–5,9,12–14 Mexico, a middle income country, does not have the same facilities or neonatal care to allow adequate implementation of the AAO/AAP guidelines. The Mexican Secretary of Health (SSA – Spanish acronym) released its own guidelines in 2010 that followed the 2008 AAO/AAP recommendations closely.10 As such, healthcare workers mainly follow the guidelines put into place by the AAO/AAP, the only difference being that they recommend screening babies of GA≤32 weeks instead of 30 weeks. In the Clinical Practice Guidelines released by the SSA, an unstable clinical course is defined as the need to use vasopressor drugs or mechanical ventilation to keep the vital signs within the normal range. Babies born outside of these criteria may develop ROP and not receive much needed treatment. Therefore, in July 2015, The SSA released an update in the screening guidelines in which they established that all babies born of GA≤34 weeks or less that 1750g, with oxygen exposure or with any associated risk factor should be screened. Institutions in middle-income countries should be aware of additional screening criteria that would allow identification of all ROP patients in need of care.
The purpose of this study is to report the incidence of ROP in infants who do not fall within the AAP/AAO retinopathy of prematurity screening criteria in order to better help identify infants at risk for ROP in Mexico and other middle-income countries.
MethodsThis study was authorized by the IRB and Research Committee of the Asociación para Evitar la Ceguera en México “Dr. Luis Sánchez Bulnes” I.A.P. (APEC). A retrospective study was performed by analyzing patient's charts that were screened for ROP between the dates of October 2011 and March 2015. Patients who were included were all from the APEC, a tertiary care hospital of ophthalmology. Records of prenatal and postnatal care are included in this study. Patients were grouped based on GA, BW, and stage of ROP for analysis. The study mainly focused on infants that were outliers to the general screening guidelines (GA>30, BW>1500) in which a diagnosis of ROP was made.
Patients were generally screened between 4 and 6 weeks of age and were followed until complete vascularization of the retina was achieved. The child's demographic information was documented including BW, GA, medical history and any previous interventions. Systematic risk factors that could be associated with ROP development were also recorded including sepsis, cardiopulmonary support, respiratory distress syndrome, hyaline membrane disease, intraventricular hemorrhage (IVH), necrotizing enterocolitis, pneumonia, surfactant treatment, transfusions, glucocorticoid administration, cardiopathy, or supplemental oxygen use.
Diagnosis of ROP was performed by ophthalmologists according to the International Classification of Retinopathy of Prematurity and described according to grade and stage.15 ROP was subsequently treated using antiangiogenic therapy with a single dose of Bevacizumab or Ranibizumab if needed.16,17 For cases of ROP that did not regress or were too advanced for antiangiogenic treatment, laser therapy or vitrectomy was performed. Indications for treatment followed the guidelines of ETROP.18
Data was collected and compiled according to GA, BW and ROP diagnosis. Special attention was given to infants weighing more than 1500g at birth or with GA greater than 30 weeks as determined by a neonatologist.
ResultsFrom October 2011 to March 2015, 268 patients were screened for ROP. of these patients, 7 (2.61%) were not included in the study due to incomplete follow-up or missing data. The data from 261 individuals (97.39%) who were included in the study was collected and compiled for examination. For all individuals in the study, the mean GA was 30.2±2.34 weeks while the mean BW was 1270.6±365.68g. Of the 261 infants screened, 216 (82.76%) were diagnosed with ROP in either eye, with 71 (32.87%) of those patients requiring treatment. The study included patients ranging from 22 to 36 weeks GA and 466 to 2932g BW. Tables 1 and 2 describe the demographic data of all patients screened for ROP.
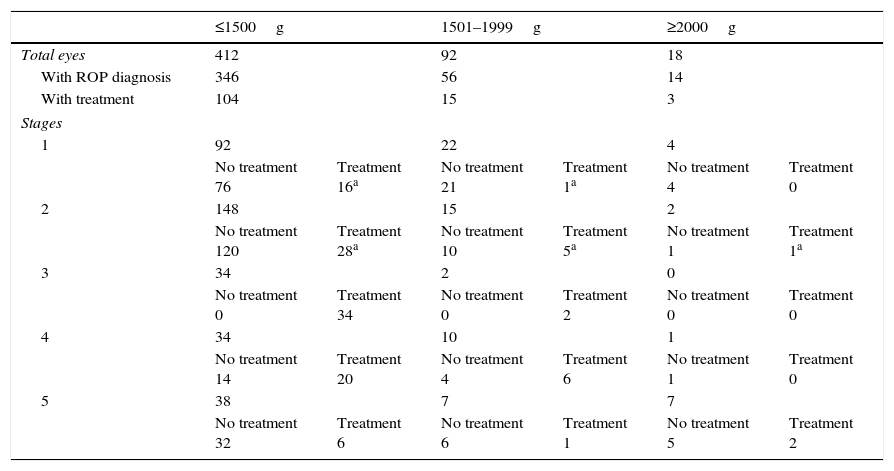
Demographic data presenting birth weight in relation to ROP diagnosis.
| ≤1500g | 1501–1999g | ≥2000g | ||||
|---|---|---|---|---|---|---|
| Total eyes | 412 | 92 | 18 | |||
| With ROP diagnosis | 346 | 56 | 14 | |||
| With treatment | 104 | 15 | 3 | |||
| Stages | ||||||
| 1 | 92 | 22 | 4 | |||
| No treatment 76 | Treatment 16a | No treatment 21 | Treatment 1a | No treatment 4 | Treatment 0 | |
| 2 | 148 | 15 | 2 | |||
| No treatment 120 | Treatment 28a | No treatment 10 | Treatment 5a | No treatment 1 | Treatment 1a | |
| 3 | 34 | 2 | 0 | |||
| No treatment 0 | Treatment 34 | No treatment 0 | Treatment 2 | No treatment 0 | Treatment 0 | |
| 4 | 34 | 10 | 1 | |||
| No treatment 14 | Treatment 20 | No treatment 4 | Treatment 6 | No treatment 1 | Treatment 0 | |
| 5 | 38 | 7 | 7 | |||
| No treatment 32 | Treatment 6 | No treatment 6 | Treatment 1 | No treatment 5 | Treatment 2 | |
Of the 110 eyes belonging to the 55 infants with BW >1500g, 70 were diagnosed with ROP: 26 (37.1%) eyes were diagnosed with stage 1 ROP, 17 (24.3%) eyes with stage 2, 2 (2.9%) with stage 3, 11 (15.7%) with stage 4, and 14 (20%) with stage 5. Of the 70 eyes, 18 required treatment.
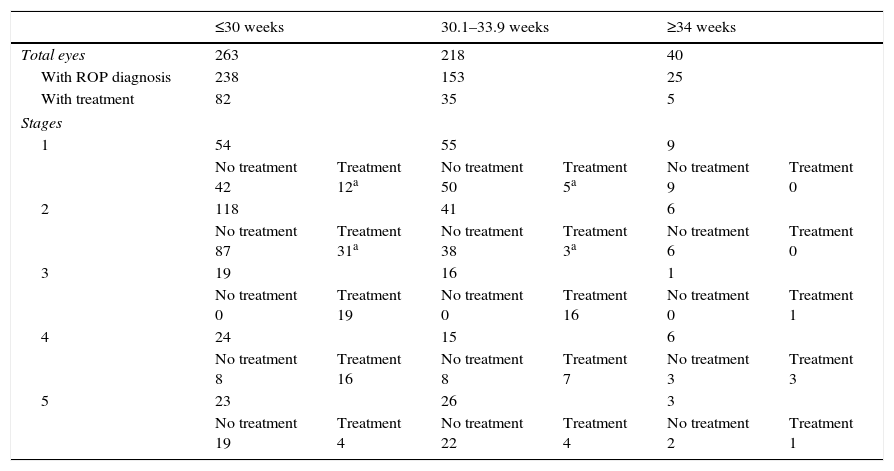
Demographic data presenting gestational age at birth in relation to ROP diagnosis.
| ≤30 weeks | 30.1–33.9 weeks | ≥34 weeks | ||||
|---|---|---|---|---|---|---|
| Total eyes | 263 | 218 | 40 | |||
| With ROP diagnosis | 238 | 153 | 25 | |||
| With treatment | 82 | 35 | 5 | |||
| Stages | ||||||
| 1 | 54 | 55 | 9 | |||
| No treatment 42 | Treatment 12a | No treatment 50 | Treatment 5a | No treatment 9 | Treatment 0 | |
| 2 | 118 | 41 | 6 | |||
| No treatment 87 | Treatment 31a | No treatment 38 | Treatment 3a | No treatment 6 | Treatment 0 | |
| 3 | 19 | 16 | 1 | |||
| No treatment 0 | Treatment 19 | No treatment 0 | Treatment 16 | No treatment 0 | Treatment 1 | |
| 4 | 24 | 15 | 6 | |||
| No treatment 8 | Treatment 16 | No treatment 8 | Treatment 7 | No treatment 3 | Treatment 3 | |
| 5 | 23 | 26 | 3 | |||
| No treatment 19 | Treatment 4 | No treatment 22 | Treatment 4 | No treatment 2 | Treatment 1 | |
Of the 258 eyes belonging to the 129 infants with GA >30 weeks, 178 were diagnosed with ROP: 64 eyes (36%) were diagnosed with stage 1, 47 eyes (26.4%) with stage 2, 17 eyes (9.6%) with stage 3, 21 eyes (11.8%) with stage 4, and 29 eyes (16.3%) with stage 5. Of the 178 eyes with ROP, 40 required treatment.
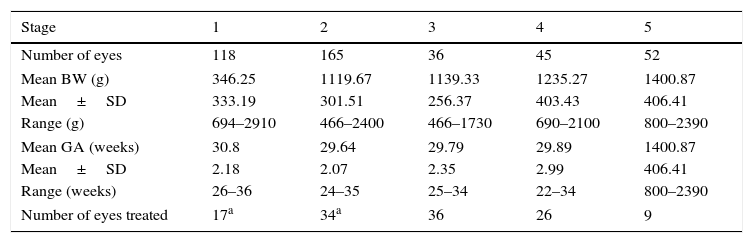
Of the 216 patients diagnosed with ROP, 200 (92.59%) of them had bilateral ROP involvement. Staging ROP is the best indicator as to the severity of the disease. Among the 522 eyes, 416 eyes were diagnosed with ROP: 118 (28.37%) were stage 1, 165 (39.66%) were stage 2, 36 (8.65%) were stage 3, 45 (10.82%) were stage 4, and 52 (12.5%) were stage 5. Table 3 shows the stages of ROP along with their respective mean BW and GA.
Stages of ROP along with their respective mean BW and GA.
| Stage | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Number of eyes | 118 | 165 | 36 | 45 | 52 |
| Mean BW (g) | 346.25 | 1119.67 | 1139.33 | 1235.27 | 1400.87 |
| Mean±SD | 333.19 | 301.51 | 256.37 | 403.43 | 406.41 |
| Range (g) | 694–2910 | 466–2400 | 466–1730 | 690–2100 | 800–2390 |
| Mean GA (weeks) | 30.8 | 29.64 | 29.79 | 29.89 | 1400.87 |
| Mean±SD | 2.18 | 2.07 | 2.35 | 2.99 | 406.41 |
| Range (weeks) | 26–36 | 24–35 | 25–34 | 22–34 | 800–2390 |
| Number of eyes treated | 17a | 34a | 36 | 26 | 9 |
Other risk factors for the development of ROP outside of GA and BW are vast. Many other studies have shown a correlation between ROP and a variety of comorbidities. Some of the most notable comorbidities noted in our study included sepsis, interventricular hemorrhage of all grades, necrotizing enterocolitis, pneumonia, respiratory distress syndrome, hyaline membrane disease, bronchopulmonary dysplasia, surfactant usage, corticosteroid usage, and transfusions.7,19 In this study, 240 (92%) infants diagnosed with ROP suffered with at least one comorbidity.
Using the current AAO/AAP screening guidelines (screen infants BW≤1500g or GA≤30 weeks),10 47 infants (94 eyes) in our study group fell outside of the screening criteria and would not have been screened. Of these 94 eyes, 56 (59.6%) had any grade of ROP while 23 (24.5%) eyes went on to develop threshold ROP, with 9 (9.6%) of them requiring treatment. Even with the new qualification included in the 2013 revision of AAO/AAP guidelines (screen infants 1500–2000g or >30 weeks with an unstable clinical course), the criteria would still exclude 58 eyes (29 infants) from the screening. Of these 58 eyes, 34 eyes (58.6%) were diagnosed with any stage of ROP, 17 (29.3%) of which developed stage 3 ROP or worse with 5 (8.6%) of them requiring treatment.
DiscussionThis study retrospectively reviews the diagnosis and treatment of 216 infants diagnosed with ROP over the course of 42 months. Our main purpose was to analyze the effectiveness of the AAO/AAP screening guidelines for our population in a middle-income country. Our data shows that in our population, diagnosis of ROP is not uncommon in infants who fall outside the AAO/AAP ROP screening guidelines, namely those with birth weight >1500g and/or gestational age >30 weeks.
As noted in various other articles, the screening criteria used in more developed nations may not be applicable for middle-income countries.2–5,9,12–14 In India, reports show that the incidence of infants >1500g treated for threshold ROP with cryotherapy was 15.3%.20 A study in Vietnam reports that out of the 21 babies with threshold ROP, 13 were >1250g at birth.21 In Lithuania, 54% of the infants needing treatment for ROP were >1500g at birth.22 These findings were not similar to those in more developed countries. Various studies in the United States indicate that threshold disease is not found above 30 weeks GA or >1500g BW23,24 which is definitely not the case with our population. One study done in the United States in 1996 did indicate the presence of ROP in infants with BW>2000g when suffering hemorrhagic shock after birth.25
Risk factors other than birth weight or gestational age may contribute to the prevalence of ROP amongst heavier and older babies. There are many systemic illnesses that can contribute to the development of ROP regardless of age, but especially applicable for infants who are not included in the general screening criteria based on BW and GA. They include sepsis, pneumonia, respiratory distress, hyaline membrane disease, and cardiac abnormalities.7,19 Medical interventions also play a role in developing ROP. As shown by Wagner, infants who were treated with supplemental oxygen or the administration of CPAP had a higher rate of occurrence for ROP.26 Transfusions during the neonate period also increase drastically the risk of developing threshold ROP.27 Surfactant therapy, while extremely beneficial for premature lungs, has been shown to be an independent risk factor for developing ROP.28
The 2013 revision of the AAO/AAP ROP guidelines state that infants with BW 1500–2000g or with GA>30 weeks with a severe clinical course should be screened under the discretion of the attending neonatologists.11 Of the 261 individuals screened in our study, 55 (21.1%) weighed more than 1500g and 129 (49.4%) had a GA >30 weeks. Furthermore, 47 (18%) infants had both GA >30 weeks and BW >1500g, 29 of which had a stable clinical course which would have left them out of the screening criteria. If these infants had not been screened, 17 patients would have gone undiagnosed and 5 would have been left untreated. The surprising statistics in our study was the prevalence of grade 3 or higher ROP in heavier and more mature infants.
Previous studies in Mexico performed in 2005 and 2008 have shown a prevalence of ROP to be around 24–28% amongst newborns, with about 10–11% requiring treatment.29–31 These studies increased their screening criteria to include babies with GA of <32 weeks. Our study contained infants with ROP up to 36 weeks GA and up to 2910g BW. At the time of our study, the valid screening guidelines were the Clinical Practice Guidelines released by the Mexican Secretary of Health in 2010 (screen infants BW≤1500g, GA≤32 weeks, or GA>32 weeks with an unstable clinical course). Following these criteria, 12 infants (24 eyes) in our study group would have not gone through the screening and 9 patients would have gone undiagnosed. Of these 24 eyes, 18 had any grade of ROP while 13 went on to develop threshold ROP, with 4 of them requiring treatment. Using the current guidelines released in July 2015, these patients would have been screened and appropriately treated. This shows that in our population, older and heavier babies are at risk for developing ROP, with or without the presence of associated risk factors.
In our population, almost all children with ROP had either a GA of ≤34 weeks or a BW of ≤1750g. Only one child (0.003%) in our study fell outside of this increased screening criteria who developed stage 1 zone III ROP in both eyes which did not require treatment and regressed spontaneously. With this increased criteria established in the Clinical Practice Guidelines of July 2015 of either GA≤34 weeks or BW≤1750g, we would effectively screen the pediatric population at risk in Mexico.
The limitations of this study include its retrospective nature, the urban location of participating hospitals, and variable duration of follow-up visits. Also, as a tertiary care center, all patients were referrals for ROP screening and none were born in house. Also, some infants were treated before arriving at our facility. As such, there were some that were not treated in accordance to our standards, mainly those treated for stage 1 or stage 2 ROP. Further studies are recommended to analyze the prevalence and characteristics of ROP in a more rural setting and to assess the efficacy and cost of the proposed increase in screening.
In conclusion, ROP diagnosis among infants weighing >1500g at birth and/or with gestational age of >30 is not uncommon in Mexico and other middle-income countries. As this study shows, the current screening guidelines, including those recommended by AAO/AAP are not completely applicable due to the exclusion of some patients who go on to develop severe ROP. With the criteria established by the SSA in the Clinical Practice Guidelines of July 2015, we would effectively screen the pediatric population at risk in Mexico. Review and modification of the current screening guidelines in other middle-income countries should be considered in order to include all babies at risk for ROP. Our recommendations include increasing the screening criteria to incorporate newborns with GA≤34 weeks or BW≤1750g regardless of any other diagnosis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Funding supportNone.
Conflict of interestAuthors have nothing to disclose.