Urinary tract infections are a leading cause of medical consultations in Mexico and the growth of antimicrobial resistance results in increased morbidity and rising costs.
AimTo make an economic evaluation of ceftibuten as treatment for uncomplicated urinary tract infections in adults, from the perspective of the Mexican private health system.
MethodsA cohort-based decision-making model was developed to compare ceftibuten with TMP-SMX, ciprofloxacin, and cefalexin. Effectiveness was measured using local susceptibility rates of Escherichia coli. Costs were obtained from official market value data and converted to 2014 USD values. Incremental analysis was employed to determine if ceftibuten was a worthwhile investment on the part of the private health system in Mexico.
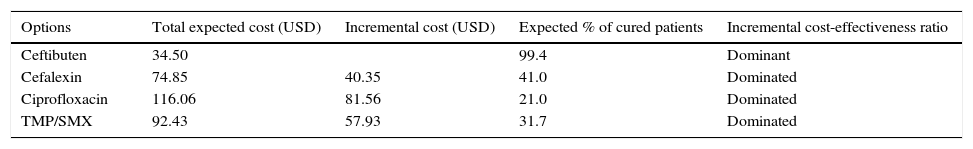
ResultsThe total expected cost per patient for ciprofloxacin was $116 USD and the corresponding costs for TMP/SMX and cefalexin were $92.40 USD and $74.80 USD, respectively. Ceftibuten had a lower expected cost ($34.50 USD) and a higher percentage of therapeutic success (99.4%), compared with ciprofloxacin 21%, cefalexin 41%, and TMP/SMX 31.7%.
ConclusionsEven though ceftibuten has a higher market price than other antimicrobials in Mexico, it can represent possible savings by avoiding the costs associated with undesirable results due to antimicrobial resistance to E. coli.
Las infecciones urinarias son una causa principal de consultas médicas en México. La resistencia antimicrobiana creciente incrementa la morbilidad y los costos.
ObjetivoRealizar una evaluación económica del ceftibuteno como tratamiento de infecciones urinarias no complicadas en adultos desde la perspectiva del sistema de salud privado en México.
Material y métodosCohorte de modelo de decisión comparando ceftibuteno con TMP-SMX, ciprofloxacina y cefalexina. Medición de efectividad usando rangos de susceptibilidad locales para Escherichia coli. Costos obtenidos de datos oficiales de valores de mercado y convertidos en valores en dólares americanos en 2014. Análisis incremental utilizado para determinar la inversión en ceftibuteno desde la perspectiva del sistema de salud privado en México.
ResultadosLos costos totales por paciente con ciprofloxacina fueron US$116 mientras que los costos correspondientes con TMP/SMX y cefalexina fueron US$92.4 y US$74.8 respectivamente. El ceftibuteno resultó con valor más bajo (US$34.50) y un alto porcentaje de éxito terapéutico (99.4%) comparado con ciprofloxacina (21%); cefalexina (41%) y TMP/SMX (31.7%).
ConclusionesEl ceftibuteno aun teniendo el valor más alto por unidad en México provee de posibilidad de obtener ahorros evitando costos asociados a resultados indeseables por la resistencia antimicrobiana de Escherichia coli.
Urinary tract infections (UTIs) are one of the leading causes of medical consultations by adults in Mexico, especially in sexually active women.1 The incidence of UTIs has increased more than 500% in Mexico, becoming a public health problem that has put pressure on the national healthcare system.1 A report published by the Mexican Epidemiology Surveillance System revealed a rising incidence of UTIs from 2004 to 2010. The age group of 25–44 years was the most affected with 1,090,886 cases, followed by the group of 50–59 years of age with 386,584 cases. There were 345,152 cases reported for the population 65 years of age and older.1
Common symptoms of cystitis are dysuria, urinary frequency and/or pollakiuria,2 but inappropriate management can result in the complication of pyelonephritis. The impact of UTIs on patient work activities and quality of life makes them a matter of public health.3
UTI treatmentAntimicrobial therapy is central in UTI management and essential for preventing the involvement of the renal parenchyma.4 Choosing antibiotics entails factors that must be considered, such as their in vitro activity against the most prevalent uropathogens, urinary concentrations of the chosen antibiotics, the possibility of sub-optimal concentrations, effects of the antibiotic on the vaginal and gastrointestinal flora, adverse events, and the cost of the therapy.
Most antibiotics reach high concentrations in urine, above the minimum inhibitory concentration for common uropathogens, which will influence treatment effectiveness.5
Patterns of antimicrobial resistanceAntimicrobial resistance is a growing global concern because of increased morbidity and mortality, and the consequent rising cost. It is a multifactorial phenomenon caused by the unnecessary use of antibiotics and treatment discontinuation.6
In Mexico, Escherichia coli is the most prevalent bacteria in ambulatory patients, causing 70–95% of the cases, followed by Staphylococcus saprophyticus (5–20%), Klebsiella pneumoniae and Proteus mirabilis (5–20%).7
Studies published over the last decade have reported patterns of in vitro bacterial resistance of E. coli strains to amoxicillin-clavulanate, ampicillin-sulbactam, cefuroxime, trimethoprim sulfamethoxazole (TMP-SMX), and fluoroquinolones. This resistance pattern involves most of the oral antimicrobials available in Mexico, limiting treatment to ambulatory UTI patients.7,8 Other studies have found that TMP-SMX administered 3–6 months prior to the occurrence of a new UTI increases the risk of antibacterial resistance 2.5- to 5-fold, similar to the risk of other factors such as recent hospitalization and diabetes.9,10
Within the framework of these rates of antimicrobial resistance, recent studies have shown that the percentage of resistance of E. coli to TMP-SMX in a given community is greater than 22% and empirical therapy with fluoroquinolones would be less expensive than TMP-SMX. However, resistance to fluoroquinolones has increased in the last decade in other countries.11 A specific concern for the present authors is that the Mexican Clinical Guidelines include fluoroquinolones for UTI treatment.1 Empirical treatment with TMP-SMX and/or fluoroquinolones would cause 50–60% in vitro antimicrobial resistance with a great potential for clinical failure.12
Treatment duration has been another concern in relation to antimicrobial coverage in UTIs. For example, TMP-SMX treatment has been recommended for a 3-day period in some studies, but it would be unhelpful due to the high level of bacterial resistance.13,16
The antimicrobial activity of ceftibuten14 was originally determined for a wide variety of bacterial species selected for their resistance to oral and parenteral β-lactam antibiotics. Ceftibuten turned out to be an active β-lactam antibiotic against Enterobacteriaceae strains because it inhibits 81.6% of the strains to ≤8.0μg/mL, compared with 75% and 54.8% of the strains inhibited by cefixime and cefuroxime, respectively.
A study in a Mexican population on in vitro antimicrobial susceptibility of gram negative microorganisms related to UTI showed that ceftibuten and netilmicin had the greatest activity against E. coli, Klebsiella spp., Proteus spp., Serratia spp., Morganella morganii, Enterobacter, and Citrobacter spp. Antimicrobials with the lowest activity were TMP-SMX, ciprofloxacin, amikacin, and levofloxacin.15
Economic impact of antimicrobial resistanceThe irrational use of antibiotics threatens public health safety because of an increased rate of drug resistance. Before 2008, only 15% of the treatments in Mexico involved the prescription of antibiotics by a physician and it was a known fact that around 80% of the prescriptions were given out by pharmacy employees.16
Since 2008, the Mexican health authorities highlighted the problem of antimicrobial resistance, regulating the antibiotic markets by making the purchase of antibiotics possible only when prescribed by a physician.16
Antimicrobial resistance translates into substantial financial burden for the healthcare system, as well as significant morbidity for the patients.16 Ceftibuten is a good option for the treatment of UTIs due to its lack of resistance. On the other hand, it has a higher market price, compared with other options, making it necessary to assess its cost-effectiveness. Thus, the aim of this study was to perform an economic evaluation of ceftibuten for the treatment of uncomplicated UTIs in adults, from the perspective of the private healthcare system in Mexico.
MethodsStudy designThis study was conducted following the international Good Research Practices recommendations for cost-effectiveness analysis in healthcare.17 The analysis was developed simulating a hypothetical cohort of adult men and women with uncomplicated UTI belonging to the Mexican private healthcare system. Four treatment strategies were considered for the management of uncomplicated UTIs in an outpatient setting according to relevant options for decision makers in private local markets: (1) TMP-SMX 160/800mg; (2) ciprofloxacin 500mg; (3) cefalexin 500mg, and (4) ceftibuten 400mg. Treatment duration was decided by the authors according to drug presentations, which were a box with 14 tablets (1 BID for 7 days), a box with 12 capsules (1 BID for 6 days), a box with 20 tablets (1 BID for 10 days), and a box with 5 capsules (1 QD for 5 days), respectively.
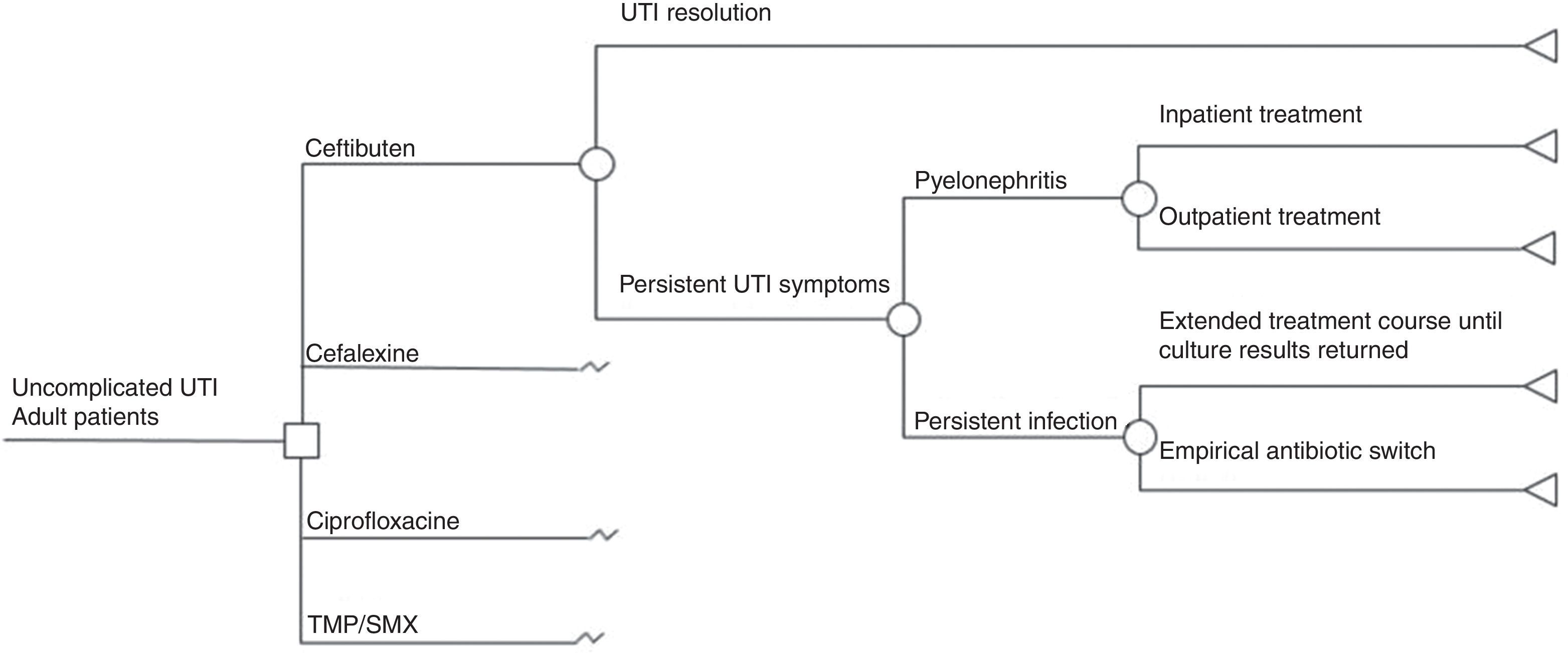
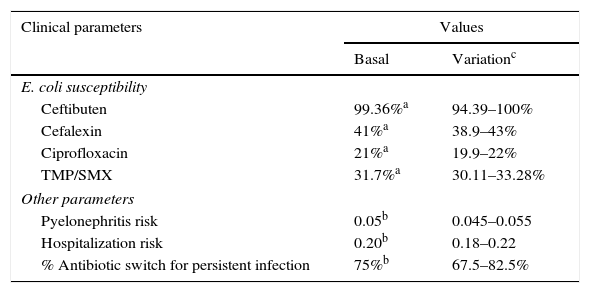
Study setting and populationIt was assumed that once a diagnosis of uncomplicated UTI was made, outpatient therapy with the mentioned antimicrobials would be considered, and one of the therapeutic options must be chosen. Since E. coli is the main causal agent of UTIs (>80%) in the Mexican population, it was assumed that the therapeutic response of the antibiotics under comparison was a function of E. coli susceptibility to them. Susceptibility rates of E. coli to different antimicrobials reported by Barriga et al.15 were used to model the expected UTI resolution or the persistence of UTI symptoms. That trial included 1200 bacterial isolations obtained sequentially during six months from adult outpatients with clinical symptoms of uncomplicated UTI and positive urine culture, having received ambulatory care. Table 1 shows all the clinical parameters used in the model and the corresponding data sources.18
Clinical parameters used in the decision model.
| Clinical parameters | Values | |
|---|---|---|
| Basal | Variationc | |
| E. coli susceptibility | ||
| Ceftibuten | 99.36%a | 94.39–100% |
| Cefalexin | 41%a | 38.9–43% |
| Ciprofloxacin | 21%a | 19.9–22% |
| TMP/SMX | 31.7%a | 30.11–33.28% |
| Other parameters | ||
| Pyelonephritis risk | 0.05b | 0.045–0.055 |
| Hospitalization risk | 0.20b | 0.18–0.22 |
| % Antibiotic switch for persistent infection | 75%b | 67.5–82.5% |
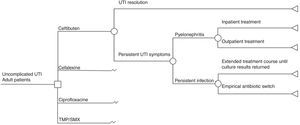
We developed a decision tree model (Fig. 1) to simulate possible outcomes of uncomplicated UTI treated either with TMP-SMX, ciprofloxacin, cefalexin, or ceftibuten, based on a model previously published by Le and Miller.18 We modeled the following clinical outcomes: UTI resolution or persistent UTI symptoms. Unresolved UTI could derive into pyelonephritis requiring either inpatient or outpatient treatment, or only persistent infection which could give rise to two possible situations: extended treatment according to urine culture or an empirical antibiotic switch. Successful treatment meant that a patient was clinically cured. A time frame less than one year was used in the model, based on the natural history of disease in UTIs.
Model assumptionsThe model assumed that there were no fatal events from acute pyelonephritis following a real-life scenario and that there were no recurrences of acute pyelonephritis either. When patients were treated for uncomplicated UTI with TMP-SMX, ciprofloxacin, or cefalexin, and there was persistent infection, the antibiotic was empirically switched to ceftibuten. In turn, if infection persisted after the initial treatment with ceftibuten, the change was made to another option.
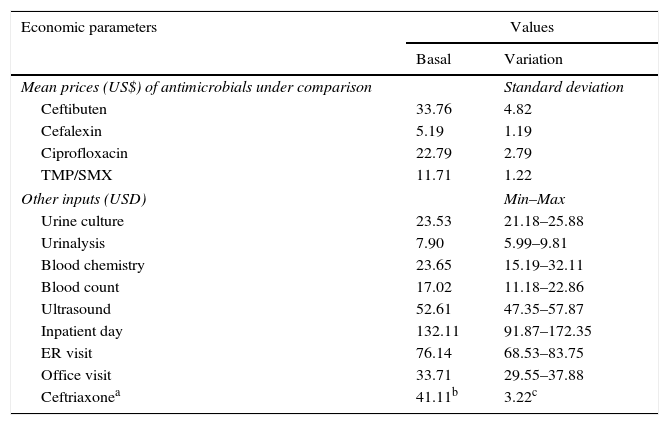
CostsFrom the perspective of the Mexican private healthcare system, the data on direct medical costs were collected and converted to 2014 U.S. dollars (USD) (Table 2), including the costs of antibiotic therapies, laboratory tests, ultrasounds, hospital stay/day, emergency room (ER) visits, and office visits. The costs of antibiotic therapies were obtained from the market wholesale drug prices. The unit costs of other medical inputs were obtained from the National Institute of Statistics and Geography (INEGI for its Spanish acronym) website.19 The estimation of inpatient treatment cost of pyelonephritis was made assuming the use of the following resources: ceftriaxone IV 2g daily (unit price: $41.11 USD; 10-day treatment: $822.22 USD), 5 hospital days ($660.56 USD), one ER visit ($76.14 USD), one office visit ($33.71 USD), and the following tests: urinalysis, urine culture, chemistry test, blood count, and ultrasound ($124.71 USD). The total cost of inpatient treatment of pyelonephritis was $1717.35 USD. The cost of outpatient treatment of pyelonephritis ($946.93 USD) was estimated using the same amount of resources as with the inpatient scenario, except for hospital stay and ER visit. Costs were not discounted in our analysis because the time horizon used was less than one year.
Costs data used in the decision model.
| Economic parameters | Values | |
|---|---|---|
| Basal | Variation | |
| Mean prices (US$) of antimicrobials under comparison | Standard deviation | |
| Ceftibuten | 33.76 | 4.82 |
| Cefalexin | 5.19 | 1.19 |
| Ciprofloxacin | 22.79 | 2.79 |
| TMP/SMX | 11.71 | 1.22 |
| Other inputs (USD) | Min–Max | |
| Urine culture | 23.53 | 21.18–25.88 |
| Urinalysis | 7.90 | 5.99–9.81 |
| Blood chemistry | 23.65 | 15.19–32.11 |
| Blood count | 17.02 | 11.18–22.86 |
| Ultrasound | 52.61 | 47.35–57.87 |
| Inpatient day | 132.11 | 91.87–172.35 |
| ER visit | 76.14 | 68.53–83.75 |
| Office visit | 33.71 | 29.55–37.88 |
| Ceftriaxonea | 41.11b | 3.22c |
Since therapeutic response was based on susceptibility rates of E. coli to the antimicrobials being compared, the effectiveness measure modeled was the expected percentage of patients cured of uncomplicated UTI with the antimicrobials analyzed.
Base-case analysisTo determine whether ceftibuten would be a worthwhile investment for the treatment of uncomplicated UTI, we performed the standard incremental analysis of cost-effectiveness ratios using spreadsheets. As a decision rule to know if the treatment with ceftibuten was cost-effective in relation to the Mexican private healthcare system, we used the cost-effectiveness threshold of one gross domestic product (GDP) per capita per additional health benefit unit, which is generally accepted in Mexico by the health authorities.
Sensitivity analysisIn order to embody the uncertainty of the parameters used in the decision model, we conducted several one-way sensitivity analyses. Basal values of clinical and economic parameters were varied throughout plausible ranges as shown in Tables 1 and 2.
ResultsBase-case resultsThe total expected costs per treated patient for each therapeutic option, as well as the expected percentage of cured patients, are shown together in Table 3. We can see that ceftibuten exhibits the lower cost and also the higher percentage of therapeutic success compared with the rest of the antibiotics. Using the jargon of pharmacoeconomics, we can state that ceftibuten is a ‘dominant’ option compared with other options, since it is more effective and less costly. Furthermore, under a dominant scenario like that shown for ceftibuten, decision makers do not need any additional analysis, such as the calculation of incremental cost-effectiveness ratios, to know that the dominant therapy (ceftibuten) represents ‘good value-for-money’.
Base-case results per treated patient.
| Options | Total expected cost (USD) | Incremental cost (USD) | Expected % of cured patients | Incremental cost-effectiveness ratio |
|---|---|---|---|---|
| Ceftibuten | 34.50 | 99.4 | Dominant | |
| Cefalexin | 74.85 | 40.35 | 41.0 | Dominated |
| Ciprofloxacin | 116.06 | 81.56 | 21.0 | Dominated |
| TMP/SMX | 92.43 | 57.93 | 31.7 | Dominated |
Since ceftibuten turned out to be a dominant strategy for the treatment of uncomplicated UTI, the economic analysis must focus on the expected savings per patient treated with ceftibuten, instead of the other three options. Comparison between ceftibuten and ciprofloxacin resulted in a higher savings per patient treated (Table 3), followed by the comparison between ceftibuten and TMP/SMX.
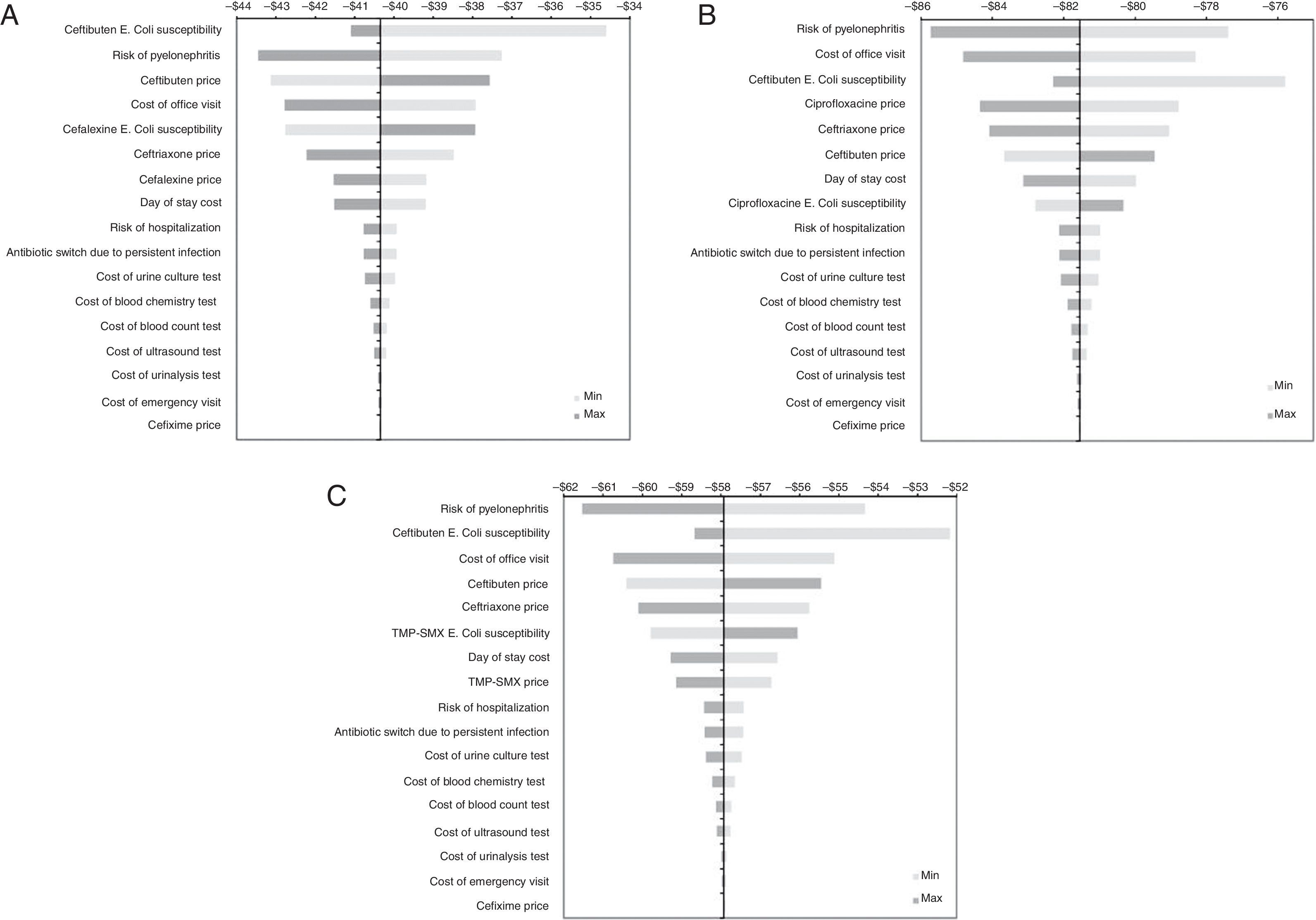
Sensitivity analysis resultsGiven that the relevant base-case results were focused on savings gained with ceftibuten, hence the objective of the one-way sensitivity analyses was to assess the robustness of the model in terms of expected savings. Fig. 2 shows a series of tornado diagrams represented by means of horizontal bars, from the widest to the narrowest, expressing different degrees of uncertainty from the parameter values into the base-case results. We can see that although some parameters embodied a high degree of uncertainty, the results did not change the dominant profile of ceftibuten.
Results of univariate sensitivity analysis of the economic evaluation of ceftibuten. Results of univariate sensitivity analyses are shown by means of tornado diagrams formed by horizontal bars. The name of these types of diagrams comes from the fact that parameters with high uncertainty are located at the top of the diagram, so the parameter with less uncertainty is located at the bottom. Tornado A shows the results for the comparison between ceftibuten and cefalexin. Tornados B and C show the results for the comparison between ceftibuten and ciprofloxacin, and ceftibuten and TMP/SMX, respectively.
The pharmacoeconomic analysis informs key decision makers about the economic bearings associated not only with the price of the drugs, but also with all the relevant outcomes expected from the use of either therapeutic options. These results enabled us to conclude that despite the fact that ceftibuten has the highest unit market price in Mexico, it can still represent savings, when compared with the other drugs analyzed, by avoiding the costs associated with undesirable outcomes caused by the antimicrobial resistance of E. coli, the most prevalent causal agent of uncomplicated UTI in Mexico.
In order to aid the decision-making process with good evidence, the limitations of this study should be mentioned. The bacterial resistance data were obtained from only one source that analyzed in vitro strains from uncomplicated UTI patients from a single healthcare center in Mexico. In addition, information about the resources used was obtained from a review of the Mexican Clinical Guidelines for UTI in order to model the usual treatment path. Another limitation was the simplification of the decision model under a local frame of limited information, although it was driven by broader assumptions in order to facilitate the assessment of therapies. However, the international principles of good practice in pharmacoeconomic research were always followed to enhance the transparency and reproducibility of the results.
Future research will be needed to know if the use of ceftibuten for the treatment of uncomplicated UTI in the Mexican population will modify the antimicrobial resistance patterns of E. coli. This will also have economic consequences, since the expected rate of therapeutic success or failure under a new environment of antimicrobial resistance could represent a different disease burden for the patients, healthcare providers, and payers. For now, the management of uncomplicated UTIs with ceftibuten is a good value-for-money from the perspective of the patients directly paying for the treatment, but also for third party payers, such as the private insurance companies.
ConclusionsPrivate healthcare systems in Mexico should evaluate current interventions for treatment of uncomplicated UTI that are cost-effective from the resistance perspective. We believe our analysis shows that ceftibuten, previously not fully considered, could be cost-effective. We support the performance of prospective new interventions that would challenge and compare current practice strategies.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Financial disclosureAn unrestricted grant was received from Merck Sharp & Dohme Mexico.
Conflict of interestsAlfonso Reyes-Lopez has participated as a speaker bureau member and as a consultant for Merck & Co. He works at RCEI Consulting SC, a consulting business, as Research Director.
Virginia Blandón-Vijil is an employee of Merck & Co. Inc. and can own stock or stock options in the company.