In recent years, total androgen blockade therapy for advanced prostate cancer has been a reasonable option to castration due to the discovery and development of new pharmacologic agents. In this study, our aim was to evaluate the efficacy of the combined treatment of nilutamide plus buserelin and to describe the occurrence of adverse events associated with this treatment.
Material and methodsA descriptive, prospective study was conducted. Patients with advanced prostate cancer receiving nilutamide plus buserelin were evaluated at 3, 6, and 9 months. The primary endpoint was the reduction of serum levels of prostate-specific antigen (PSA).
ResultsOne hundred and four patients were included in the study, but only 67 patients had complete information and thus were evaluated in the efficacy analysis: 65 (97.0%) achieved a 50% reduction in PSA level, compared with the baseline value, and 2 patients achieved a decrease <10ng/ml. The combination therapy was well tolerated, given that only 7 patients (6.7%) presented with mild adverse events that did not require treatment suspension or other specific maneuvers.
ConclusionsTreatment with nilutamide plus buserelin appears to be safe and effective in controlling tumor activity in advanced prostate cancer patients.
En años recientes la terapia de privación máxima de andrógenos para el cáncer avanzado de próstata constituye una opción razonable a la castración debido al descubrimiento y desarrollo de nuevos agentes farmacológicos. En este estudio nuestro objetivo fue describir la eficacia del tratamiento combinado de nilutamida más buserelina y la ocurrencia de efectos adversos asociados con este tratamiento.
Material y métodosSe realizó un estudio descriptivo, prospectivo, en pacientes con cáncer de próstata avanzado, los cuales recibieron nilutamida más buserelina y fueron evaluados a los 3, 6 y 9 meses. El desenlace primario fue el descenso del nivel sérico de antígeno prostático específico.
ResultadosSe incluyeron 104 pacientes, pero solo 67 fueron considerados porque tuvieron información completa: 65 (97%) lograron un descenso del 50% del antígeno prostático específico en comparación con el inicial; 2 pacientes lograron un descenso <10ng/mL. El tratamiento fue bien tolerado ya que solo 7 reportaron efectos adversos (n=104); estos fueron leves y no requirieron suspender el tratamiento u otras maniobras específicas.
ConclusionesEl tratamiento con nilutamida más buserelina fue seguro y efectivo para controlar la actividad tumoral en pacientes con cáncer de próstata avanzado.
Prostate cancer (CaP) is the most common cancer in men.1 In Mexico, it is the second cause of cancer-related mortality.2 Even though prostate-specific antigen (PSA) screening has led to some over-diagnosis and over-treatment of CaP in younger men, older men tend to have more aggressive tumors and few receive curative therapy, thus explaining the high mortality rate.3 Furthermore, 26–34% of patients will be diagnosed at an advanced stage of the disease.4
Androgen-deprivation therapy (ADT) is the standard approach for the first-line treatment of advanced CaP.5 ADT can be achieved with the use of hormonal therapy or through surgical castration.
Androgens have a stimulating effect on the androgen receptors within the epithelial cells of the prostate. Once the hormones are located intracellularly, they are converted into dihydrotestosterone (DHT) by 5-alpha reductase. DHT is the most active form of testosterone and carries most of the hormonal functions, including cell replication.6 Thus, ADT induces apoptosis in susceptible CaP cells by reducing the synthesis of androgens and their interaction with the androgenic receptor.7
ADT is usually achieved through the use of luteinizing hormone-releasing hormone analog or a gonadotropin-releasing hormone agonist, such as buserelin.8 These drugs must be combined with antiandrogens, such as bicalutamide or nilutamide (which block the binding of DHT to the androgen receptor in the nucleus of CaP cells), to completely block the effect of testosterone.9
Whenever these drugs are used in combination, the resulting therapy is called maximum androgen blockade (MAB).10
Nilutamide is a nonsteroidal androgen receptor antagonist that shows affinity for androgen receptors, but not for other steroid receptors. It has a low adverse event profile consistent with androgen depletion (hot flushes, gynecomastia, impotence, and gastrointestinal disturbances), and has been shown to exert a significant PSA response in advanced CaP patients.11
Buserelin is a synthetic luteinizing hormone-releasing hormone analog. Its intermittent administration stimulates the release of luteinizing hormone and follicle-stimulating hormone, thus increasing testosterone concentrations in males, whereas its continuous administration suppresses secretion of both LH and FSH, resulting in a drop in testosterone concentrations. Its adverse event profile is also low and has been widely studied in advanced patients.12
Due to our interest in the use of MAB on PSA levels in advanced CaP patients, we conducted a study to evaluate the efficacy of the combination treatment with nilutamide plus buserelin and to describe the occurrence of adverse events (AEs) associated with that treatment.
Materials and methodsA descriptive, prospective study evaluated patients with CaP receiving nilutamide and buserelin, at baseline and at 3, 6, and 9 months.
Six centers participated in this study: 5 in Mexico City and one in Morelia. All the centers are community-based hospitals.
The attending urologists were the investigators, and were directly responsible for the treatment of the CaP patients at each center.
The Mexican Urological Association Ethics Committee assessed and approved the study.
Inclusion criteria- 1.
Males with biopsy-proven CaP
- 2.
Age>18 years
- 3.
Signed Informed consent
- 4.
Measurable or evaluable disease
- 5.
No previous surgery to remove the testes
The treatment scheme consisted of 300mg of nilutamide (150mg P.O. every 12h) for 4 weeks, and then 150mg P.O. daily for 8 months plus 9.45mg of buserelin bi-monthly in the form of a subcutaneous implant.
Efficacy criteriaPrimary endpoints
- 1.
At least 50% reduction of serum PSA from the baseline value
- 2.
Total PSA levels <10ng/ml
Secondary endpoints
- 1.
No increase of PSA levels at 9-month follow-up
- 2.
Undetectable PSA values at 9-month follow-up
- 3.
No clinical evidence of disease progression during the study period
A case report form (CRF) for data collection was designed. Information was manually collected by the attending urologist. At the end of the study, all CRFs were gathered and submitted for data compilation and analysis by INNOVAL, a data management company. The variables registered and analyzed were: age (in years), body height (in centimeters), body weight (in kilograms), body mass index, general demographic variables, CaP family history, co-morbidities, Gleason score, prostate-specific antigen value (PSA, in ng/ml), Karnofsky score, and adverse events. During each visit, the primary endpoints were determined, as well as the Karnofsky score and the presence of adverse events. Previously reported adverse events associated with nilutamide and buserelin were specifically investigated, but spontaneous reports were also registered in the CRF.
Data management, review, and validationAll medical records were handled and kept at each participating center. A study monitor performed 2–4 site visits for data review and validation during the study period. The study monitor evaluated data accuracy, and gathered the CRF sheets containing all the information at the end of the study.
The final PSA values were compared with pre-treatment PSA values with a paired two-sample t-test after each visit. When the outlier values affected the t-test, a Friedman test was used to adjust the extreme values. The Karnofsky scale was analyzed using the Friedman test3.
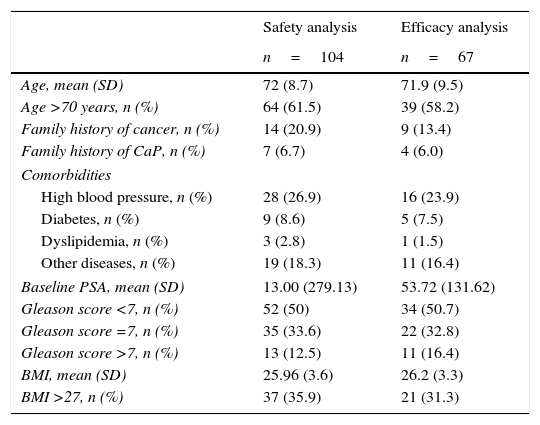
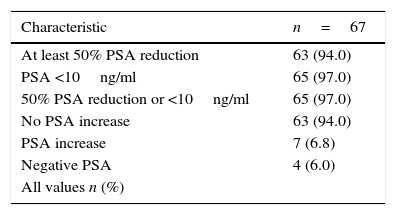
ResultsA total of 104 patients with biopsy-proven CaP that received nilutamide plus buserelin were identified and their data were used for the safety analysis. However, 37 patients were excluded from the final analysis: 15 were lost to follow-up (total follow-up: 21 months), 20 had incomplete PSA information or a non-measurable effect (undetectable serum PSA at the baseline), and two patients died due to causes unrelated to the drug intervention. Therefore, for efficacy analysis purposes, data from 67 patients were analyzed (Table 1): 65 (97.0%) reached the combination primary outcome of 50% reduction in PSA levels from the baseline (n=63, 94.0%) or PSA levels <10ng/ml (n=2, 97.0%). Sixty-three (94.0%) patients achieved the secondary outcome of no increase in PSA levels at the follow-up at 9 months (visit 4). Thirty-five (52.2%) of the patients achieved undetectable PSA values at the 9th-month follow-up. Disease progression (i.e., increased PSA levels from the baseline values) occurred in 4 (6.0%) patients (Table 2).
Demographic data of the patients included in this study.
| Safety analysis | Efficacy analysis | |
|---|---|---|
| n=104 | n=67 | |
| Age, mean (SD) | 72 (8.7) | 71.9 (9.5) |
| Age >70 years, n (%) | 64 (61.5) | 39 (58.2) |
| Family history of cancer, n (%) | 14 (20.9) | 9 (13.4) |
| Family history of CaP, n (%) | 7 (6.7) | 4 (6.0) |
| Comorbidities | ||
| High blood pressure, n (%) | 28 (26.9) | 16 (23.9) |
| Diabetes, n (%) | 9 (8.6) | 5 (7.5) |
| Dyslipidemia, n (%) | 3 (2.8) | 1 (1.5) |
| Other diseases, n (%) | 19 (18.3) | 11 (16.4) |
| Baseline PSA, mean (SD) | 13.00 (279.13) | 53.72 (131.62) |
| Gleason score <7, n (%) | 52 (50) | 34 (50.7) |
| Gleason score =7, n (%) | 35 (33.6) | 22 (32.8) |
| Gleason score >7, n (%) | 13 (12.5) | 11 (16.4) |
| BMI, mean (SD) | 25.96 (3.6) | 26.2 (3.3) |
| BMI >27, n (%) | 37 (35.9) | 21 (31.3) |
BMI, body mass index; CaP, prostate cancer; PSA, prostate-specific antigen; SD, standard deviation.
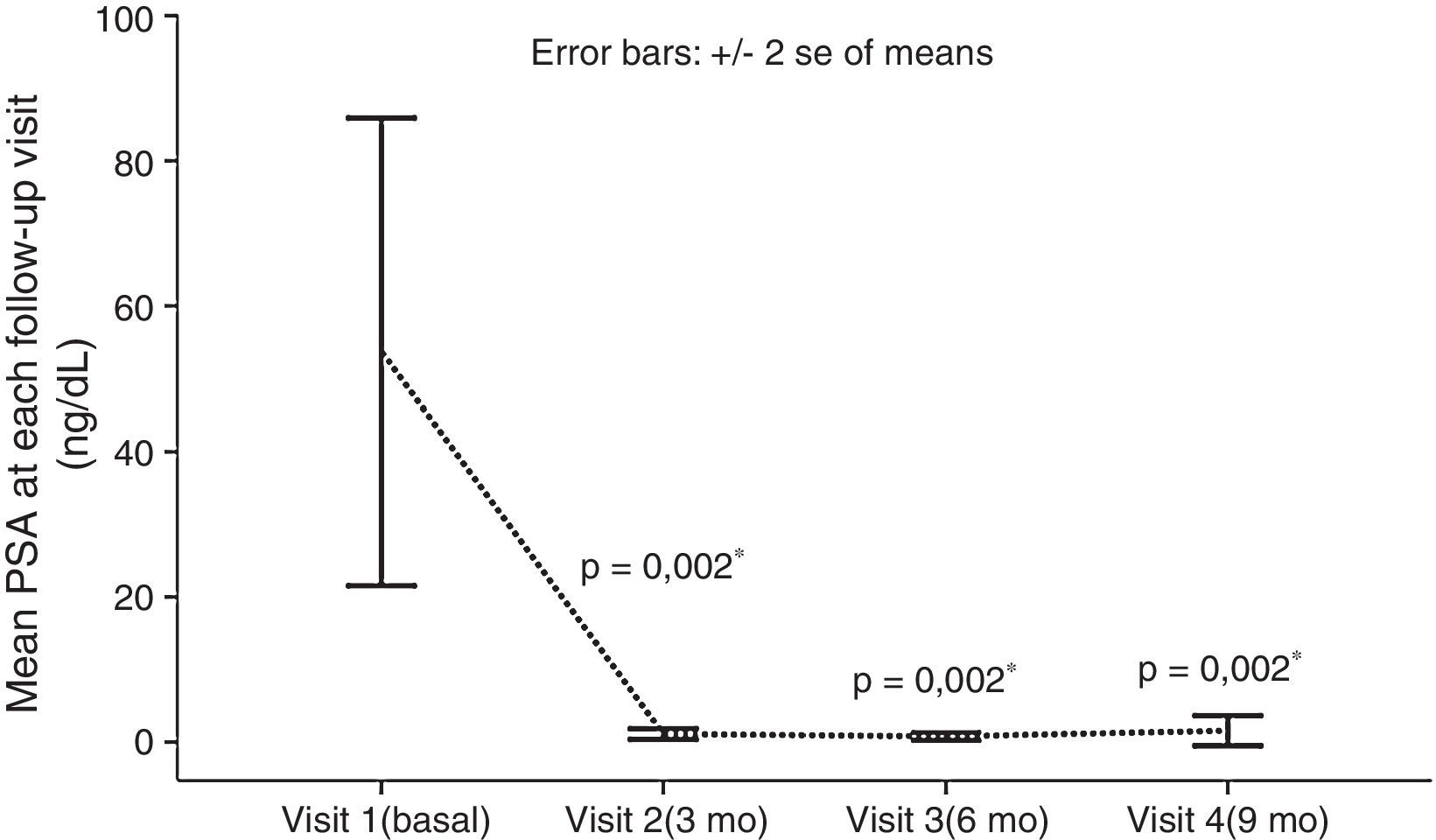
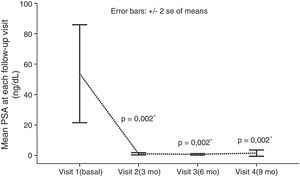
None of the registered variables appeared to influence treatment response in the univariate analyses. A highly significant difference was observed in the mean baseline PSA values and those reached at visit 4 (at follow-up at 9 months): 53.72±131.62ng/ml vs. 1.58±8.47ng/ml, respectively; p=0.002 (Fig. 1). The mean Karnofsky performance score improved significantly from the baseline to visit 4 (92.69 vs. 97.61, respectively; p=0.02).
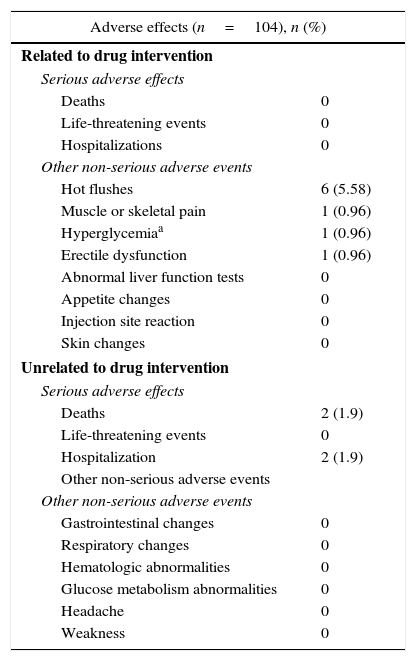
The combination therapy was well tolerated, with only 7 patients reporting adverse events. These events were mild, and did not require treatment suspension or any additional specific treatment. Six patients (5.8%) reported hot flushes. Muscle/skeletal pain, mild hyperglycemia (<7mmol/l), and erectile dysfunction were each reported by one patient (0.9%).
Of the 104 study patients, two died after visit 1: one patient with metabolic syndrome had myocardial infarction, and one patient with high blood pressure, apparently under control, had intracerebral hemorrhage. Both events required hospitalization and were finally considered unrelated to the drug intervention by the investigator in charge of each case. We observed no other major adverse events, including cardiovascular effects (Table 3).
Safety analysis: adverse events reported during the study period.
| Adverse effects (n=104), n (%) | |
|---|---|
| Related to drug intervention | |
| Serious adverse effects | |
| Deaths | 0 |
| Life-threatening events | 0 |
| Hospitalizations | 0 |
| Other non-serious adverse events | |
| Hot flushes | 6 (5.58) |
| Muscle or skeletal pain | 1 (0.96) |
| Hyperglycemiaa | 1 (0.96) |
| Erectile dysfunction | 1 (0.96) |
| Abnormal liver function tests | 0 |
| Appetite changes | 0 |
| Injection site reaction | 0 |
| Skin changes | 0 |
| Unrelated to drug intervention | |
| Serious adverse effects | |
| Deaths | 2 (1.9) |
| Life-threatening events | 0 |
| Hospitalization | 2 (1.9) |
| Other non-serious adverse events | |
| Other non-serious adverse events | |
| Gastrointestinal changes | 0 |
| Respiratory changes | 0 |
| Hematologic abnormalities | 0 |
| Glucose metabolism abnormalities | 0 |
| Headache | 0 |
| Weakness | 0 |
CaP control was successfully achieved in most of the patients evaluated in the efficacy analysis (67 out of 104 patients) during the 9-month study period, as evidenced by the very low levels of PSA found in our patients at all study visits. Significant and sustained reduction in PSA values was quickly achieved from the first 3 months of treatment with nilutamide plus buserelin.
These results are consistent with other published studies. In the study by Choo et al.,13 in which androgen suppression consisted of oral nilutamide 100mg three times daily for 4 weeks and buserelin acetate 6.3mg subcutaneous depot every 2 months for 2 years for patients with pathologic T3 disease and/or positive surgical margins after radical prostatectomy, they found that 49 out of 78 patients had undetectable PSA (<0.2ng/ml) and a 5-year survival rate of 96%.
In another study by Castañeda et al.,14 27 patients with adenocarcinoma of the prostate in locally advanced and metastatic stages were initially treated with an antiandrogen plus a LHRH analog, leaving this last drug as monotherapy once the nadir of the PSA was reached. They found that in all but 2 patients, the PSA level reached its nadir point after the third application of the LHRH analog. In the study by Ricardez-Espinoza et al.,15 30 patients were treated with intermittent ADT with goserelin and bicalutamide and 90% of them showed PSA levels <0.2ng/ml after 6 months of treatment.
Kassouf et al.,16 showed that up to 64% of patients with advanced CaP and failed androgen ablation achieved a significantly sustained PSA response and favorable toxicity profile with nilutamide administration. They also found that patients with a previous antiandrogen withdrawal response had a significantly greater chance of responding to nilutamide. This drug has also been associated with a 50% or greater decrease in PSA in about one third of patients, with a typical median response duration of 4–7 months17 in patients with failed flutamide use or upon antiandrogen withdrawal.18
In addition, data from a retrospective analysis show that a 50% or greater decrease in PSA at 12 weeks, regardless of the therapy employed, more than doubled patient survival.19 These data have also been supported by prospective studies20 and are currently included in Mexican guidelines.21
A study by Rodriguez et al.,22 evaluated QoL in patients with advanced CaP treated with nilutamide once a day and subcutaneous buserelin every two months. They proposed this combination therapy as an alternative to surgical castration, advocating its effectiveness in the control of advanced cases and the improvement in patient quality of life, given that they found no increase in the frequency of specific adverse events with either drug.
Despite the treatment efficacy we found in our patients, our study had certain limitations, the most important of which was the short follow-up period, which prevented us from performing a survival analysis. Nevertheless, based on the abovementioned studies, we theorize that survival curves would not be dissimilar to those reported in the international literature.
Another drawback was the high number of patients that had to be excluded from the efficacy analysis: 14.4% (15 patients) were lost to follow-up, 20 more patients had to be excluded due to lack of PSA information, and there were two deaths, producing a total patient loss of 35.6% in our study. This fact was most likely a source of bias in our final study results, because the reasons for patient data unavailability are clearly associated with the outcome of interest (PSA serum levels).
On the other hand, our findings support a good response to MAB achieved through the use of nilutamide and buserelin by using PSA as a marker for disease progression. We chose PSA as an endpoint because of its simplicity, repeatability, reproducibility, and clear association with the disease time course. However, it is to be noted that sometimes changes in PSA values may or may not solely reflect treatment.23 Therefore, we view our present work as a proof-of-concept study to facilitate a new project that includes long-term follow-up.
Finally, we observed two deaths in this study: one case of myocardial infarction (MI) and one case of stroke. In this regard, we are confident that these deaths were not related to the treatment. First of all, the attending physician of each case carried out a thorough patient evaluation, which revealed the presence of vascular risk factors that made both patients high-risk profile carriers for cardio and cerebrovascular disease. Moreover, despite the fact that an increased risk of cardiovascular disease has been previously documented, a recent meta-analysis found that ADT use, at least for buserelin,24 was not associated with an increased risk of cardiovascular death, and was associated with a lower risk of prostate cancer-specific mortality and all-cause mortality.25 This has led to the proposal that until the direction of the association between ADT and cardiovascular risk is confirmed in a suitable experimental study, the effect of ADT on cardiovascular risk should be cautiously interpreted within the context of the available information.26
ConclusionIn this study, treatment with nilutamide plus buserelin was safe and effective in controlling disease activity in advanced prostate cancer patients. The high number of patients that reached the primary endpoint in our efficacy analysis supports this affirmation. Based on our findings, we aim to perform a larger randomized placebo-controlled clinical trial to evaluate the role of MAB on patient survival, using the combination therapy of nilutamide plus buserelin.
Ethical responsibilitiesProtection of people and animalsThe authors state that for this investigation have not been performed experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare that this article does not appear patient data.
FinancingSanofi-Aventis contract an independent company for statistical processing of this study, as well as providing scientific and technical advice.
Conflict of interestsThe authors declare no conflict of interest.
The authors wish to thank Dr. Fernando Mendoza-Peña, Head of the Urology service of the Hospital Regional Licenciado Adolfo López Mateos for his support in allowing the present study to be conducted in said Service.