Oral squamous cell carcinoma (OSCC) is a malignant neoplasia originated in the epithelium's keratocytes. Early biopsies reveal dysplastic mitotic keratocytes and sub-epithelial inflammatory infiltrate which progress towards a loss of basal membrane. Nevertheless, hematoxillin and eosin (H&E) histological diagnosis does not show suitable clinical correlation; this aspect improves when molecular markers such as Ki-67 and cyclin D1 are used. The aim of the present study was to correlate histological description of OSCC cases with Ki-676 and cyclin D1 expression.
Material and methodsTwelve patients with lesions suggestive of oral cancer were analyzed, patients with OSCC diagnosis were included in the study. Single biopsies were taken, observing histological and technical processing for paraffin cuts, coloration with H&E and immunohistochemical coloration with cyclin D1 and Ki-67. Three patients met inclusion criteria, they were named C1, C2 and C3. OSCC type was classified according to the American Joint Committee on Cancer.
ResultsHistologically, C1 case was classified as type II OSCC. Cases C2 and C3 were classified as type I OSCC. Immunohistochemical analysis for C1 revealed Ki-67 positive and cyclin D1 negative; C2 exhibited Ki-67 negative and cyclin D1 positive, and C3 showed Ki-67 positive and cyclin D1 negative.
ConclusionsSearch for markers such as Ki-67 and cyclin D1 in pre-established OSCC diagnoses can influence histological results, contributing thus to more accurate diagnosis and treatment for the patients.
El carcinoma oral de células escamosas (COCE) es una neoplasia maligna originada en los queratinocitos del epitelio. Biopsias tempranas, evidencian queratinocitos mitóticos displásicos e infiltrados inflamatorios subepiteliales que progresan a pérdida de la membrana basal. Sin embargo, en el diagnóstico histológico con hematoxilina y eosina (H&E) no se muestra adecuada correlación clínica, aspecto que mejora cuando se utilizan marcadores moleculares como Ki-67 y ciclina D1. El objetivo de este estudio es correlacionar la descripción histológica de casos de COCE con la expresión de Ki-67 y ciclina D1.
Material y métodosSe analizaron 12 pacientes con lesiones sugestivas de cáncer oral; fueron incluidos en el estudio pacientes con diagnóstico de COCE. Se tomaron biopsias únicas, procesamiento histotécnico para cortes en parafina, coloración con H&E y coloración inmunohistoquímica con ciclina D1 y Ki-67. Tres pacientes cumplieron con los criterios de inclusión, llamados C1, C2 y C3. Se clasificó el tipo de COCE según el American Joint Committee on Cancer.
ResultadosHistológicamente el caso C1 fue clasificado como COCE tipo II, los casos C2 y C3 tipo I; en la inmunohistoquímica encontramos en C1 Ki-67 positivo y ciclina D1 negativo, para C2, Ki 67 negativo y ciclina D1 positivo y para C3 Ki-67 positivo y ciclina D1 negativo.
ConclusionesLa búsqueda de marcadores como Ki-67 y ciclina D1 en diagnósticos de COCE preestablecidos, pueden influenciar los resultados histológicos, contribuyendo a un diagnóstico más acertado así como los tratamientos a realizar en el paciente.
According to World Health Organization (WHO) criteria oral squamous cell carcinoma (OSCC) is a neoplasia originated in the keratocytes belonging to oral squamous stratum of the oral epitheliumi. It is considered malignant due to its infiltrative capacity and early tendency to metastasis.1,2 It constitutes over 90% of malignancies found in the mouth and oropharynx,1,2 it is more frequently found in males, and its incidence is considered high in the western world.2,3 In Colombia, official figures revealed that oral cancer was considered the seventh most frequent cancer, 2,000 new cases were detected each year between 1989 and 1997. The tongue is the most common location, representing 26.8% of all cases.4,5 In local studies conducted at the San Vicente de Paul Hospital (Medellin-Colombia), between 1990-1996, 228 oral neoplasia cases were found, of which the most frequent location was the tongue with 30% of cases.6 In the same hospital, between 1996-2000, 41% of oral cancer cases were related to alcohol consumption.7 OSCC is commonly found in the anterior third and lateral dorsum of the tongue.1,2,8 At early stages it appears as reddish or ulcerated areas which evolve into indurated masses with high borders, later they convert to large masses with necrotic foci and infiltration to ganglia, bone muscle and skin.1 From the histological point of view, incipient-stage OSCC frequently reveal sub-epithelial inflammatory infiltrate which progresses to stages where there is loss of basal membrane, with evidence of hypertrophic dysplastic squamous keratinocytes of irregular shape, in atypia and presence of mitotic figures even lamina propia infiltrate.2 In final diagnosis it is accepted that the histological degree does not show adequate correlation with the patient's evolution,9,10 and this aspect improves when the depth and infiltration of the invasive margins are assessed,11–15 or molecular markers are used.16 Among these markers we find Ki-67, a cellular proliferation marker expressed in the «S» phase of the cell cycle;17,18 it is significantly correlated to lingual OSCC proliferation rates.19 Cyclin D1 is a marker related to malignancy20 when expressed in the «G1» phase of the cell cycle.21,22 The objective of the present study was to correlate histological description of squamous cell carcinoma cases in the mouth with expression of Ki-67 and cyclin D1.
MATERIAL AND METHODSTwelve patients were analyzed by means of a retrospective study from August 2011 to April 2012. Patients exhibited lesions that suggested the existence of oral cancer. The present study included patients with diagnosis of squamous cell carcinoma and who attended the San Rafael Dental Clinics, Bogota DC. Endorsement of the Institution's Ethical Committee was secured as well as patients’ informed consent. Patients who authorized use of information, but objected to photographs of the procedures, patients with data in the clinical history deemed insufficient to complete analyses and patients lacking histological slides were excluded.
Single 5mm3 biopsies were taken from the lesions’ central areas. Processing protocol observed at the Luis A Granobles Histology Laboratory, La Sabana University, Bogota, Colombia for specimens harvested from the oral cavity are the following: sample fixation with 10% formalin, histological-technical processing for paraffin cuts at 4μ and coloring with hematoxillin and eosin (H&E). A new histological reading was conducted with Zeiss Axiostar microscope for cases which met inclusion criteria, later, paraffin blocks were sent for immunohistochemical coloration or cyclin D1 and Ki-67; positive control was obtained, pictures were taken with a PC adapted camera with image analyzer software, Motic license, Version 4.61.
RESULTSThree patients met with inclusion criteria. All patients were male, ages ranging 31-62 years, average age 46 years (SD ± 12.6). In all three cases lesions were located in the lingual region.
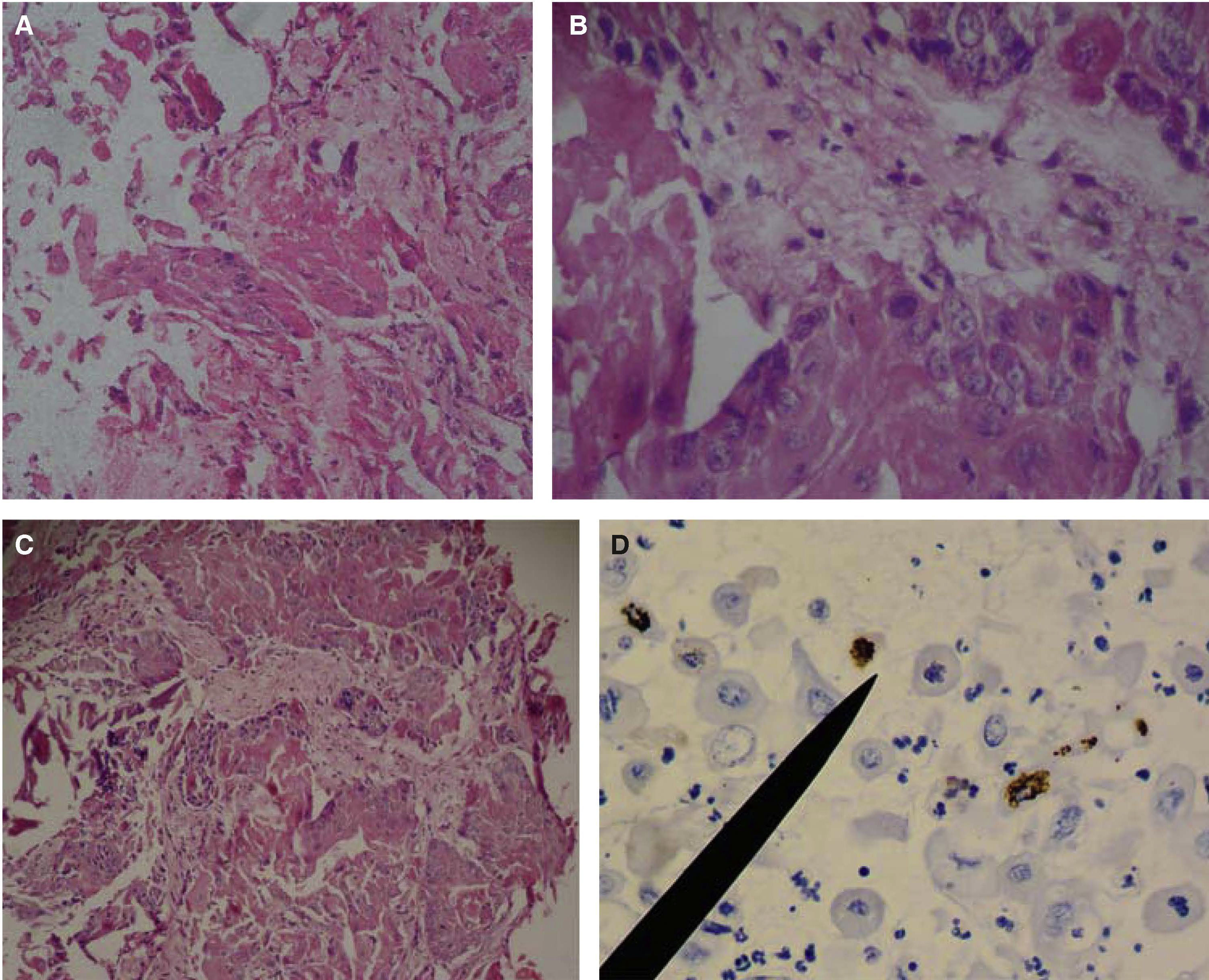
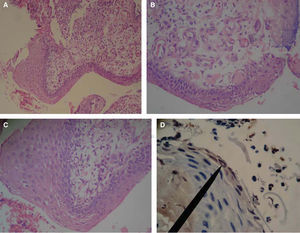
Case 1: a 45 year old male sought treatment for a tongue lesion of four month evolution. The patient did not report history of tobacco use, alcohol consumption or systemic conditions. Patient informed that algid area was located at the left hemi-mandibular region, with ipsilateral adenopathy centered at the submaxillary triangle of this area. Intra-oral examination revealed a mass in the left lateral anterior border of the lingual mucosa, the mass was papular, of whitish hue, oval-shaped, with slightly circumscribed borders, measuring 38 x 25mm. Greyish reticulated foci could be observed in the described mucosa. Incisional biopsy was conducted at the central area of the lesion. Figures 1A-D show histopathological findings.
Histological pictures of OSCC with H&E. A) 10x objective, epithelial dysplasia with connective anaplasia can be observed. B) 40x objective: keratinocytes with euchromatic, poikylocariotic nuclei, with megacariotic predominance; telophasic mitotic shape is observed. C) 40x objective: conspicuous conjunctive tissue with epithelial cell infiltrate is observed, megacariotic, euchromatic, poikylocariotic. D) 40x objective, the marker shows mild positive immunohistochemistry for K-667 in keratinocytes infiltrated en lamina propria, and negative for cyclin D1.
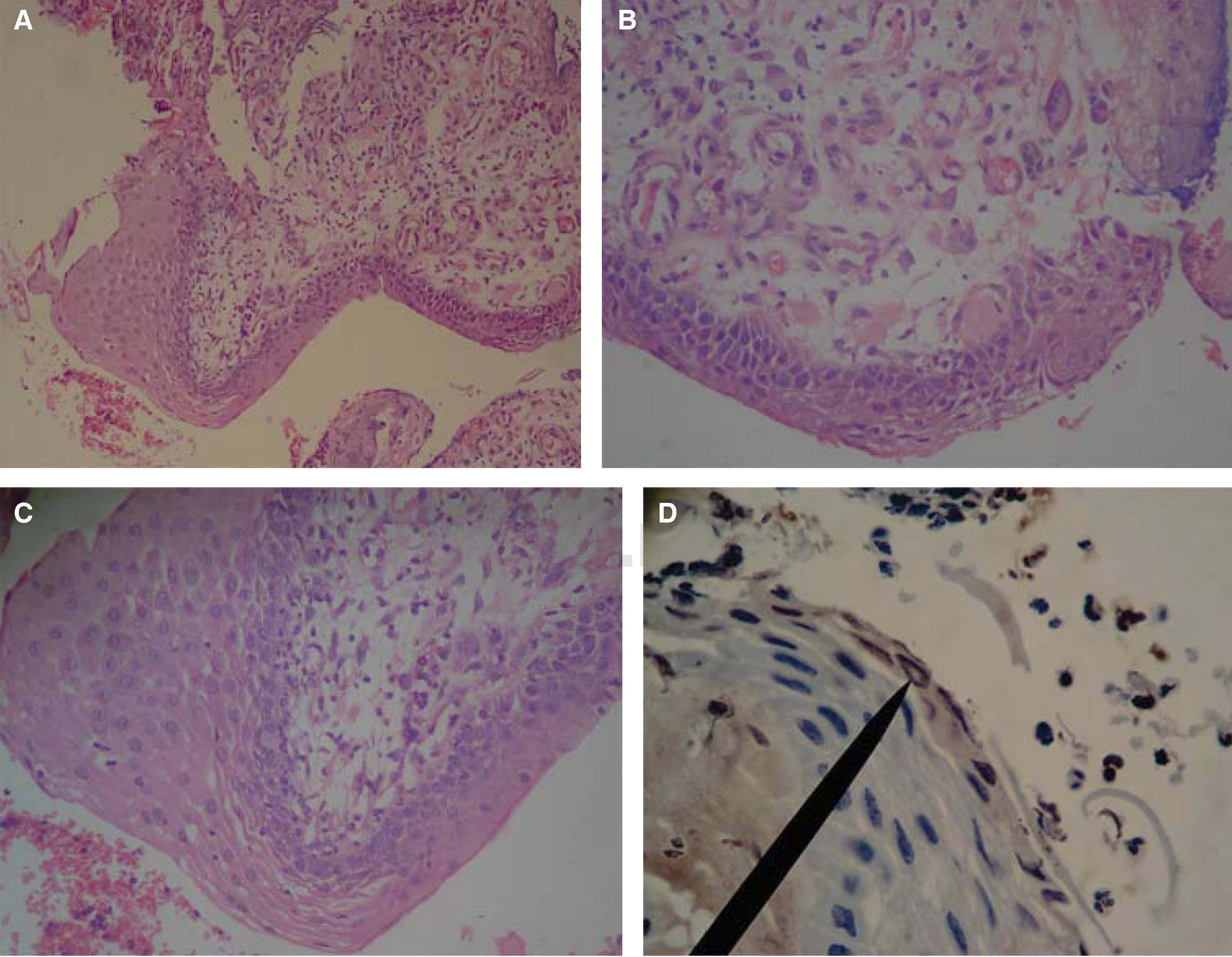
Case 2: a 62 year old male patient sought treatment for a tongue lesion of two and a half month evolution. The patient reported no tobacco consumption habit but habitual alcohol intake. As pathological history he reported high blood pressure. Intraoral examination revealed an opaque, whitish, papular lesion; the lesion was homogeneously flat, of irregular shape, with leukoplastic aspect, circumscribed borders, measured 29 x 14mm and was located at the right lateral border of the tongue. Surgical description reported a 5mm3 incisional biopsy. Figures 2A-D show histopathological and immunohistochemical findings.
Histological pictures of OSCC with H&E. A) 10x objective shows non keratinized, stratified flat lingual epithelium with hyperplastic foci at the expense of the spinous stratum, alternating with highly dysplastic tissue foci. B) 40x objective reveals in the lamina propria abundant basophilic nuclei predominantly rounded and ovoid shaped, megakariotic and euchromatic, alternating with spaces coated with cells of epithelioid aspect compatible with blood vessels. C) 40x objective reveals lamina propria infiltrated with poly-segmented, heterochromatic nuclear shapes, polymorphonuclear compatible over fabric de eosinophilic thin fibers compatible with reticular fibers. D) 40x objective. The marker shows mildly positive immunohistochemistry for cyclin D1 in spinous stratum keratinocytes, and negative to Ki-67.
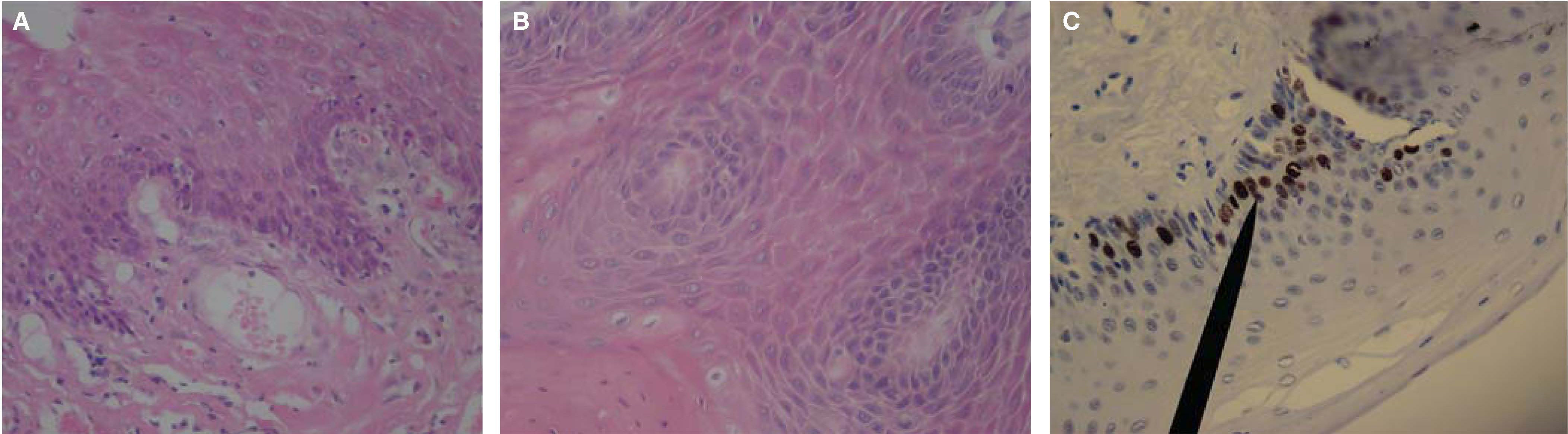
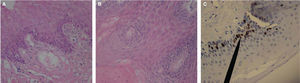
Case 3: a 31 year old patient sought treatment for an eight month evolution lesion in the tongue. During history taking, the patient did not report habits of tobacco use, alcohol consumption or systemic condition. Clinical examination of head and neck revealed a slight bilateral, symmetrical submaxillary ganglionar increase, with no algidity. Oral cavity examination revealed generalized gingivitis and a mass over the anterior-lateral border of the lingual mucosa due to fractured mesiolingual cusp of the lower first molar. The mass was of whitish hue, not elevated, circumscribed to the trajectory of the fractured molar, approximately measuring 11 x 7mm. Clinical history reported previous incisional biopsy in the central area of the lesion. Figures 3A-C show histopathological and immunohistochemical findings.
Histological pictures of OSCC with H&E. A) 10x objective revealed thickened hyperplastic epithelium infiltrated towards the lamina propria. B) 40x objective reveals lingual epithelium squamous stratum with dysplastic foci, where mega to microkaryotic keratinocytes can be observed, with chromatin predominance. C) 40x objective: the marker shows moderate positive immunohistochemistry for Ki-67 in kerantinocytes of spinous and basal stratum, negative for cyclin D1.
From a macroscopic and morphological perspective, the American Joint Committee on Cancer (AJCC) classified epithelial carcinoma into type I, II, III and IV; criterion considered is size of the neoplasia; OSCC type I and II are smaller than 4mm and types III and IV are larger than 4mm.23 From the histological perspective, it is accepted that carcinoma be classified according to cellular differentiation degrees into the following categories: well differentiated, moderately differentiated and undifferentiated.24
According to OSCC already discussed criteria, clinical cases would be classified as clinical case 1, type II, moderately differentiated, with a size of 38 x 25mm, anaplastic histology with keratinocytes recognition (Figures 1A-C), clinical cases 2 and 3 exhibited, type I well differentiated, measuring 29 x 14mm and 11 x 7mm respectively, histologically recognizing non keratinized stratified epithelium (Figures 2A-C), nevertheless, when conducting Ki-67 immunohistochemical demonstrations, in the histological aspect of clinical cases, greater expression was found in clinical case 3 which showed lesser dysplasia (Figure 3C) and lesser expression in case 1 which exhibited greater dysplasia (Figure 1D), this can be considered a contradictory aspect since literature reports a direct relationship between expression of cells in phase «S» and dysplasia.18 If we only consider the immunomarking here stated, diagnosis of OSCC could be reconsidered for a diagnosis of oral basal cell carcinoma, which shows Ki-67 expression limited to spinous and basal keratinocytes (Figure 3C), this concurs with other immunohistochemiocal studies conducted on Ki-67 expression in basal cell carcinoma.19
With respect to immunohistochemistry with cyclin D1, only a mild expression was observed, which was limited to squamous stratus in clinical case 2 (Figure 2D); a case classified as type I due to the size, and histologically well differentiated. Nevertheless, scientific literature is consistent in showing that biological value of cyclin D1 marker in OSCC cases, indicates worse prognosis and recurrence.25 In clinical case 2, no nodal involvement was observed notwithstanding the fact that the patient had reported habitual alcohol consumption, a risk factor which does not establish direct relationship with cyclin D1, but nevertheless contributes to a poor prognosis with possible metastasic outcome.
CONCLUSIONSWe consider that search of markers such as Ki-67 and cyclin D1 in pre-established OSCC diagnoses can influence and optimize final histological results, and in this sense, it is suggested they must be considered as part of the tools used to perfect final diagnosis as well as treatments reported to the patient.
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
School of Dentistry, Nueva Granada Military University, Medical Morphology Group, Bogota, Colombia. School of Dentistry, La Sabana University, Proseim Group, Bogota DC, Colombia.
Maxillofacial and Oral Surgery Department, Central Military Hospital, School of Dentistry, Nueva Granada Military University, Bogota DC, Colombia.