The aim of the present article was to assess inflammatory response caused by implantation of Bioceramic material in rats’ subcutaneous tissue. Nine male Wistar rats were used (Rattus Norvegicus) to which four dentin tubes filled with Bioceramic sealing cement material and one empty tube (control group) were implanted. Results were analyzed in three time periods (96hours, 10 and 21 days). Animals were sacrificed by anesthetic overdose. Obtained samples were processed by hematoxylin and eosin staining in order to be analyzed with microscope. Results after 96hours revealed moderate inflammation in 75% of all cases and severe inflammation in 25% of all cases. Ten days later, inflammation decreased from moderate (67%) to mild (25%). At the final period of 21 days, moderate to mild inflammation was observed (50%). It was concluded that there was presence of moderate to severe inflammation at initial periods which decreased to mild inflammation at the final period. «Bioceramic» brand material exhibits acceptable biological response in rats’ subcutaneous tissues.
El propósito fue evaluar la respuesta inflamatoria a la implantación del material Bioceramic en tejido subcutáneo de ratas. Se utilizaron nueve ratas machos Wistar (Rattus norvegicus) a las que les implantaron cuatro tubos de dentina rellenos con cemento sellador Bioceramic y un tubo vacío como grupo control. Se analizaron en tres periodos de tiempo (96 horas, 10 y 21 días). Los animales fueron sacrificados por sobredosis y las muestras obtenidas se procesaron mediante tinción con hematoxilina y eosina para ser analizadas microscópicamente. Los resultados mostraron a las 96 horas inflamación moderada en 75% y severa en 25%. 10 días después disminuyó la inflamación de moderada (67%) a severa (25%). En el periodo final de 21 días se observó inflamación moderada a leve (50%). Se concluye que existió inflamación de moderada a severa en los periodos iniciales, disminuyó a leve en el último periodo. Bioceramic presenta una aceptable respuesta biológica en tejido subcutáneo de ratas.
In the field of dentistry, introduction of bioceramic materials has triggered new interactions in studies of original reference materials: in the field of endodontics, biomaterials play an important role since they directly contact periodontal tissue and alveolar bone through the apical foramen, root fractures root perforations or retrograde fillings. Biomaterials are composed of calcium silicate, zirconium oxide, calcium monobasic phosphate, calcium hydroxide as well as thickeners and filling agents.1,2
«Bioceramic» brand cement is a pre-mixed bioceramic material designed as silicate cement.1 It possesses adequate radio-opacity, 12.93 pH, dimensional stability, minimal contraction, and worktime of four hours. Additionally it is not resorbable at the interior of the root canal.4 It is presented in powder/ liquid form, paste/paste form, and more recently premixed in a syringe. Bioceramic cements’ biological, physical and chemical properties as well as their citotoxicity, pH, radio-opacity and calcium ion release have been researched in some projects.5–12
Zhang et al (2009) demonstrated that iRoot SP cement, known as Bioceramic, eliminated all bacteria after a two minute contact. These authors considered that this potent anti-bacterial effect could be due to a combination of its elevated pH, hydrophilic nature as well as its active diffusion of calcium hydroxide.6 Candeiro et al (2012) proved that Bioceramic presented radio-opacity lower than AH-Plus, as well as higher pH and more abundant release of calcium ions.10 Moreover, both sealers met with requirements of norm ISO 6876/2001. Han and Okiji (2013) compared white Pro-Root MTA, Biodentine and Bioceramic with respect to their ability to produce apatite and release calcium ions. Biodentine and white Pro-Root MTA released more abundant calcium ions when compared to Bioceramic.11 Zhang & Peng (2010) cultivated mice fibroblasts and applied three sealing cements: Bioceramic, white Pro-Root and Bioceramic MTA. AH Plus proved to be more toxic for fibroblasts when compared to white Pro-Root MTA and Bioceramic, with intermediate toxicity.7
Zoufanet et al, (2011) in their cell culture studies, showed that GuttaFlow and Bioceramic possessed lesser cytotoxity than AH Plus and Tubli-Seal.8 Loushine et al (2011) showed that AH-Plus and Bioceramic exhibited severe cytotoxicity after 24hours. Nevertheless, cytotoxicity gradually decreased with AH-Plus, while Bioceramic remained moderately toxic during a period of six weeks.9
In scientific literature, few studies have been found on assessment of bioceramic cements¿ biocompatibility in conjunctive tissue. Therefore, the aim of the present study was to assess inflammatory response elicited by Bioceramic in rats’ subcutaneous tissue.
MATERIAL AND METHODSNine Wistar male rats (Rattus Norvegicus) were used. Rats weighed 200-300 grams and were distributed in three groups of three rats each. All animals were implanted with three dentin tubes measuring 5mm diameter, which were obtained from palatal and distal roots of molars previously filled with Bioceramic (Endosequence BC Sealer) according to manufacturer's instructions. The empty tube was used as a control group.
Before surgical procedure, dentin tubes were disinfected in 2.2% glutaraldehyde for 12hours and then sterilized in an autoclave. Tubes were filled with Bioceramic and divided into three groups, according to analyzed time period (96hours, 10 and 21 days). Animals were anesthetized with a dosage of 0.001mg/kg weight. Drug used to this effect was Ketamine Hydrochloride (Cheminova, Mexico-D.F.): it was administered through intra-peritoneal approach. The animals’ fur had been removed from the dorsum and isodine (Dermodine, Morelos Mexico) asepsis had been previously conducted. Three approximately 5mm incisions were performed with number 15 scalpel blade (Denti-Lab, Mexico City, Mexico) in the dorsum's anterior and posterior sections. Divulsion was undertaken and tubes filled with bioceramic and empty tubes were longitudinally implanted in animals. Sutures were executed with 6.0 nylon thread (Ethicon, Mexico D.F.). Animals were sacrificed at 96hours, 10 and 21 days with anesthetic overdose (ketamine hydrochloride, Cheminova, Mexico D.F.). Study tissue was obtained with excision biopsy of areas surrounding the implants. Samples were fixated in 10% formalin. Tubes were removed with an incision on the longitudinal axis and were dislodged without touching tissue extremities, 48 samples were thus harvested to be processed according to histological and technical procedure. To follow procedure, 5μm thick serial sections were dyed with hematoxylin and eosin so as to be observed in optic light microscope at 40x. Assessment of inflammatory response revealed presence of inflammatory cells such as polymorphonuclear neutrophils, lymphocytes, macrophages, eosinophils, plasmatic and giant cells close to the tube opening; attributed scores were 0- absence, 1- mild, 2- moderate, and 3- severe. Samples were interpreted by a pathology specialist and were subjected to statistical analysis.
RESULTSA total of 48 samples were examined; 32 with Bioceramic cement and 16 with empty tubes.
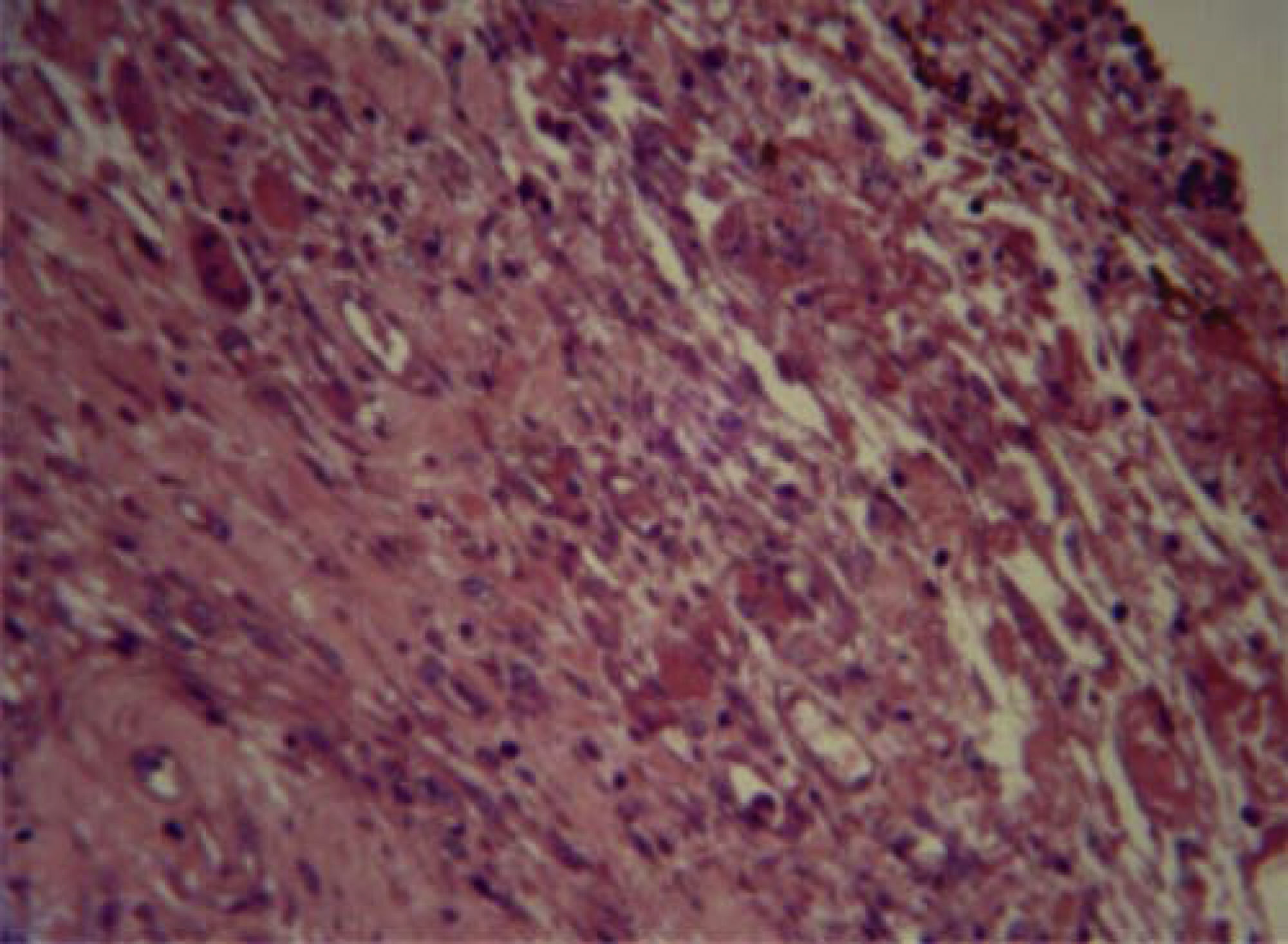
Tissue response with Bioceramic tube at 96hours revealed neutrophils, macrophages, plasma cells and moderate vascular congestion (Figure 1).
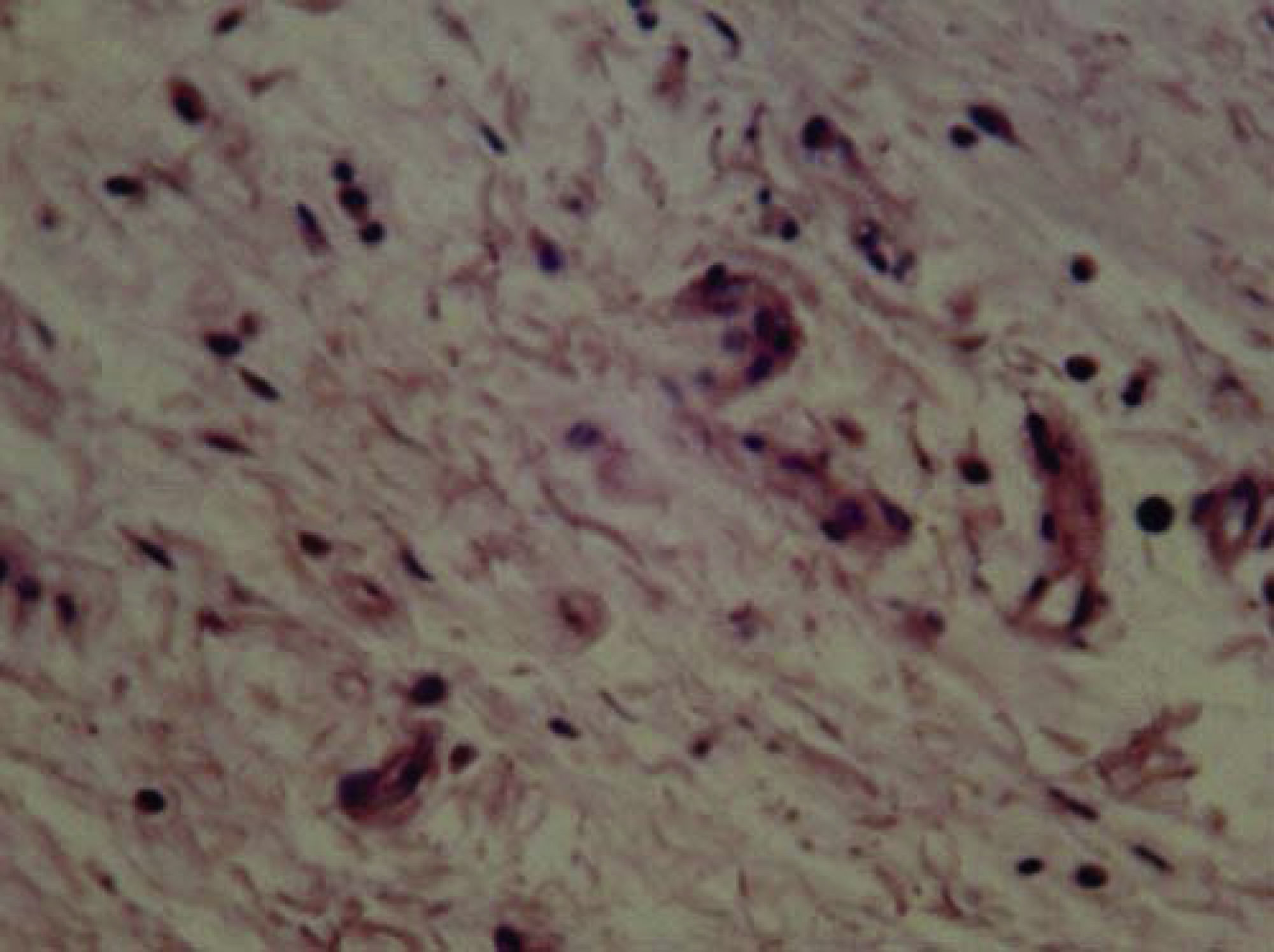
Study of the 10-hour samples revealed lymphocytes, plasma cells, scarce eosinophils and fibroblasts (Figure 2). Study of the 21-day samples revealed fibroblasts, collagen and scarce inflammatory cells, most of them lymphocytes (Figure 3).
It is proposed that there is a tendency for inflammation to decrease the longer the material remains implanted. This is sustained on the fact that at 96hours and 10 days, moderate and severe inflammation was observed, whereas at 21 days observed inflammation was moderate and mild. Control group did not exhibit tissue damage associated to dentin tube; inflammatory response was considered normal.
Chi-square (χ2) statistical analysis allowed to determine that there was statistically significant difference among all three periods of time (χ2=14.31: 4 gl, p < 0.05), this allowed the assertion that inflammation tended to significantly decrease with time. Presence of the dentin tube did not cause any reaction which might be related to its presence. Chisquare (χ2) statistical analysis did not reveal significant differences (χ2=0.356: 2 gl; p > 0.05) among the three measurement times. Mild and moderate inflammation was present with same frequency at the three measured times (Figure 4).
DISCUSSIONBiocompatibility expresses the ability of a material or substance to coexist with host's tissues. This test can be obtained in vitro by analyzing its toxicity by means of cell culturing.13 These cells can be odontoblasts, fibroblasts, macrophages or osteoblasts. This test is simple to conduct, it is affordable and possible to standardize. A material is considered cytotoxic when it prevents cell adherence, causes certain amount of morphological and drastic alterations in the cell which will in turn decrease cell viability.13 According to ISO 10993-5 recommendation, the surface or medium to be tested must have 1.25-6.0cm2. This causes greater surface contact of the material when compared to the diameter of a foramen which is 0.2-0.3mm. For this reason, in vitro cytotoxicity studies must be carefully interpreted. Nevertheless, according to clinical protocols, these studies are used for biomaterials’ toxicity tests.13,14In vivo studies include use of experimental animals such as monkeys, rats and dogs. In studies conducted on rats’ subcutaneous tissue with silicate-originated materials such as MTA, these materials were less irritant,15–17 this allows us to consider that this research model is adequate to conduct toxicity primary tests such as those used in the present study, nevertheless in different time periods.
Dentin and polyethylene tubes are used to implant materials in rats’ subcutaneous tissues. In the present study tolerance to dentin tube was observed, since no tissue damage related to its presence was observed; inflammatory response was deemed normal.13,18
Tissue response after 96hours revealed neutrophils, macrophages, plasma cells and vascular congestion in moderate amounts, all of which decreased after 10 days. After 21 days fibroblasts, collagen and scarce inflammatory cells, mostly lymphocytes were observed. This allowed the assertion that inflammation exhibited tendency to decrease the longer the implanted material stayed into place. This was proposed after observing that after 96hours and 10 days, moderate to severe inflammation predominated, whereas after 21 days there was mild to moderate inflammatory response.
Bioceramic cement in its pre-mixed paste presentation was studied in the present study. Loushine et al (2011) assessed setting time and micro-hardness of pre-mixed silicate cement (Bioceramic Endo Sequence Sealer) in the presence of different mixtures (0-9 wt%) in contact with MC3T3 cells. Both sealers exhibited severe cytotoxicity after 24hours. AH-Plus toxicity gradually decreased and showed to be non cytotoxic while Bioceramic remained moderately cytotoxic during the six week period. Nevertheless, further studies are needed to assess correlation between Bioceramic material's setting time and toxicity degree.9
Ma et al (2011) assessed cytotoxity of pre-mixed EndoSequence Root Repair Material (ERRM), MTA, IRM and Cavit in gingival fibroblasts’ culture. They found similar results for MTA and ERRM.19 In another study Zhang & Peng (2010) implanted Bioceramic, ProRoot MTA and AH-Plus cements in fibroblast cultures. They observed that AH Plus was more toxic when compared to other silicate-based materials.7
Bioceramic exhibited intermediate toxicity, this reveals it might be related to type of employed cell, since sealing cements release cytotoxic sub-products which cause tissue irritation before full setting, thus causing delays and preventing healing. Loushine et al (2011) observed severe cytotoxity after 24hours in a study conducted on cultivated mice osteoblasts (MC3T3) exposed to AH-Plus and Bioceramic cements.9 Bioceramic material remained moderately toxic for six weeks and inflammation decreased from moderate to mild in periods ranging from 96hours to 21 days. From the aforementioned facts it can be inferred that when patients¿ teeth are filled, there will be acute inflammation such as that found with exposition to materials, and that it will decrease even when found in periapical tissues.
Periapical and pulp response was assessed in dogs’ teeth after conducting pulpotomies and direct protection with Biodentine and compared with MTA by means of radiographic, histological and histomicrobiological analyses. Biodentin exhibited compatibility and allowed formation of mineralized tissue after pulpotomy in all samples with similar morphology and integrity as those formed with MTA.20 This shows that Bioceramic is compatible in the presence of connective tissue and that, even though it exhibits initial inflammation it presents tissue repair and induces mineralization. In another study, Mori et al (2014) implanted Bidentine, MTA and IRM in rats's subcutaneous tissue. Animals were sacrificed at 7, 14, and 21 days. Biodentine showed moderate inflammation at seven days. At 14 and 30 days, inflammatory process was lesser and insignificant, thus showing Biodentine's biocompatibility21 In the present study, Bioceramic caused moderate and severe inflammation at 10 days, and moderate and mild inflammation at 21 days.
In another study, Liuet et al (2015) assessed the effects of Bioceramic (iRoot BP Plus- Innovative Bioceramic Inc. Vancouver, Canada) as in vivo and in vitro pulp protector. They observed that iRoot BP Plus showed suitable compatibility with pulp tissue and induced pulp cell proliferation and repair with mineralized tissue. iRoot BP could be used in pulp therapy.22 The present study revealed that implantation of dentin tubes filled with Bioceramic allowed tolerable compatibility in the body. Since we can consider that pulp is connective tissue, it is possible to infer that it will cause suitable response in human pulp tissue.
CONCLUSIONPre-mixed Bioceramic cement elicited acceptable inflammatory response with biocompatibility characteristics in rats¿ subcutaneous tissue.
Professor, University Center for Health Sciences, University of Guadalajara, Mexico. Private Practice.
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
Endodontics spsecialists, Military School of Health Graduate, Air Force and Army University, Mexico.
Oral Pathology specialist, attached to Dental specialty Unit of the National Defense Ministry, Mexico.
Professor of Endodontics at the Graduate and Research School, National School of Dentistry, National University of Mexico (UNAM). Private practice in Endodontics.